Abstract
The aim of this study was to examine helminths and protozoans in cynomolgus macaques (Macaca fascicularis) imported from registered breeding facilities in China and their relation to health risks for non-human primate handlers in biomedical research centers and in breeding facilities. Fresh fecal samples were collected from a total of 443 M. fascicularis and analyzed by copromicroscopical analysis, immunoenzymatic, or molecular assays. As to helminths, whose eggs were shed in 2.03% of the samples, Trichuris and Oesophagostomum were the only two taxa found, with low prevalence and low eggs per gram (EPG) values. Protozoans were more frequently detected (87.40%), with Entamoeba coli (85.19%) and Endolimax nana (79.26%) as the most prevalent species shed. Other parasites found by fecal smear examination were uninucleated-cyst-producing Entamoebas (78.52%), Iodamoeba bütschlii (42.96%), and Chilomastix mesnili (24.44%), while cysts of Balantidium coli (22.2%) were only observed by sedimentation. No coproantigens of Giardia duodenalis, Cryptosporidium spp., and Entamoeba histolytica complex were detected. Blastocystis sp. infection was noticed in 87.63% of macaques by PCR. These cynomolgus monkeys were infected with many subtypes (ST1, ST2, ST3, ST5, and ST7), where the predominant Blastocystis sp. subtypes were ST2 (77.5%), followed by ST1 (63.5%). Data collected confirmed the presence of potentially zoonotic parasites and a high parasite diversity, suggesting the need for appropriate and sensitive techniques to adequately control them and related health risks for handlers of non-human primates in biomedical research centers and in breeding facilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastrointestinal parasitism in colonies of non-human primates (NHPs) used for research is a common occurrence (Sano et al. 1980; Takano et al. 2005; da Silva Barbosa et al. 2015). Strongyloides spp., Oesophagostomum spp., and Trichuris trichiura were previously considered among the most common pathogens causing poor development, anemia, and diarrhea in macaques and in other NHPs (Honjo et al. 1963; Wong and Conrad 1978; Abbott and Majeed 1984). Regular deworming and high-standard hygienic measures currently adopted in breeding facilities and research centers should lower helminthic infections. Nevertheless, Trichuris could persist in NHPs for biomedical research due to their egg high resistance and to the long life span of their adults, which requires a specific treatment strategy to eradicate them (Reichard et al. 2007). Recently, captive NHPs were also demonstrated as frequently affected by several species of intestinal protozoans that resulted pathogenic for their hosts (Lee et al. 1990; Muriuki et al. 1997; Vogel et al. 1996; Zanzani et al. 2014). Moreover, most parasites recorded in NHPs represent a potential zoonotic risk for researchers and caretakers in breeding centers (Loomis 1983; Roperto et al. 1985; Muriuki et al. 1998; Pedersen et al. 2005; Yoshikawa et al. 2009; Meloni et al. 2012). This investigation aimed at evaluating the following in cynomolgus macaques, a non-human primate species: (i) the prevalence of gastrointestinal parasites (helminths and protozoans), (ii) the degree of infection (helminths), and (iii) the prevalence of Blastocystis genotypes in order to improve both health management and welfare of this species of monkey and to prevent the transmission of zoonotic parasites to researchers and staff.
Material and methods
The study was approved by the Animal Care and Use Committee of the University of Milan.
Sampling
Fresh fecal samples from 443 cynomolgus macaques (Macaca fascicularis) imported from registered breeding facilities in China (F2 purpose-bred) were obtained on four occasions throughout 2013. The feces were stored at +4 °C and, under refrigerated condition, were sent for examination to a laboratory within 48 hours.
Fecal examination
Fecal samples (n = 443) were microscopically analyzed; feces were previously added with formol-ether and centrifuged and their sediment analyzed by FLOTAC® basic technique using a flotation solution with sucrose and sodium nitrate (specific gravity: 1.20) for the detection of nematode eggs, and their eggs per gram (EPG) number was calculated. Cysts of Balantidium coli and trematode eggs were detected by sedimentation. Following a few cases of severe diarrhoeic diseases, the analysis focused also on protozoan infections. Fecal smears stained with Lugol’s solution were performed in 135 samples out of 443 for protozoan detection; the nomenclature for protozoans partially used for Entamoebas was the one proposed by Stensvold et al. (2011). Eventually, two aliquots were stored at −20 °C until immunoenzymatic and molecular analysis was carried out.
Immunoenzymatic assay
One hundred and thirty-five fecal samples were screened by commercial available kits (RIDASCREEN® Cryptosporidium, RIDASCREEN® Giardia and RIDASCREEN® Entamoeba, R-Biopharm, Darmstadt, Germany) for the antigens of Cryptosporiudm parvum, Giardia duodenalis, and Entamoeba histolytica complex.
PCR assay
A group of 97 samples were processed by a commercial kit (QIAamp DNA Stool Mini Kit, QIAGEN®, Valencia, CA, USA) for DNA extraction. A PCR protocol was applied to amplify a fragment of the nucleotide SSU rDNA of Blastocystis. For external PCR, the forward primer Blast 505–532 (5′ GGA GGT AGT GAC AAT AAA TC 3′) and the reverse primer Blast 998–1017 (5′ TGC TTT CGC ACT TGT TCA TC 3) were used. A ca. 500 (479)-bp fragment, containing a variable region that allows subtyping of Blastocystis specimens was amplified (Santín et al. 2011). Amplification products were run on 1% ethidium bromide agarose gels and visualized under ultraviolet light. Bands were excised from agarose gels and purified using a QIAquick Gel Extraction Kit (QIAGEN®, Valencia, CA, USA). Amplification products were sequenced using the seven pairs of STS primers (SB83, SB155, SB227, SB332, SB340, SB336, and SB337) to genotype Blastocystis sp. as employed by Yoshikawa et al. (2004) and Stensvold (2013).
Results
Parasitological analysis
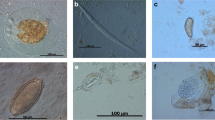
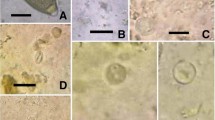
Prevalence of gastrointestinal parasites is reported in Table 1. Copromicroscopic analysis demonstrated helminth eggs shedding in 2.03% of 443 fecal samples from cynomolgus monkeys; both found taxa, Trichuris and Oesophagostomum, showed low prevalence and low EPG values (Table 1). Protozoans (87.40%) were more frequently detected than helminths. Entamoeba coli (85.19%) and Endolimax nana (79.26%) were the most prevalent species shed. Other parasites found by examination of fecal smears were uninucleated-cyst-producing Entamoebas (78.52%), Iodamoeba bütschlii (42.96%), and Chilomastix mesnili (24.44%), while cysts of B. coli (22.2%) were only observed by sedimentation. No coproantigens of G. duodenalis; Cryptosporidium spp., and E. histolytica complex were detected.
Genotyping of Blastocystis sp.
Blastocystis sp. infection was noticed in 87.63% of the macaques (85/97) under investigation. The most predominant Blastocystis sp. subtype was ST2 (77.5%), followed by ST1 (63.5%), ST7 (41.2%), ST3 (38.8%), and ST5 (1.2%). ST4 and ST6 were not isolated. A mixed infection with two or more subtypes (76.5%) occurred more frequently than an infection sustained by a single subtype (23.5%). Combinations of ST1 and ST2 or ST1, ST2, and ST3 were the most common forms of mixed infection (16.47 and 11.76%, respectively). A mixed infection with ST1, ST2, ST3, and ST7 showed a percentage of 11.76% (Table 2).
Discussion
Eight distinct taxa of enteric parasites were detected by microscopic examination, showing a faunal diversity consistent with previously published data (Bezubik and Furmaga 1960; Matsubayashi et al. 1992; Tachibana et al. 2001; Lee et al. 2010; MacIntosh et al. 2010). Captive non-human primates are frequently infected with parasites having a direct life cycle and show a lower number of parasitic species in comparison with wild NHPs; besides, the majority of their enteric parasites are protozoans, as recently demonstrated (Lane et al. 2011; Ye et al. 2014; da Silva Barbosa et al. 2015).
In our study, helminth infections were represented only by nematodes belonging to Trichuris spp. and Oesophagostomum spp. Trichuris spp. inhabits the ceca and colons of a variety of simians. T. trichiura, a species infecting humans (Dinh 2002; Melfi and Poyser 2007), is thought to be present in M. fascicularis, M. mulatta (Taylor et al. 1994), and in other Old and New World primate species. Further, Trichuris is highly pathogenic for monkeys, as previously observed in M. fascicularis and in other monkeys (Janagi 1981; Loomis and Wright 1986; Emikpe et al. 2002).
Oesophagostomum is a parasitic nodular worm whose larvae can encyst in the wall of the large intestines of macaques (Honjo et al. 1963). Among captive monkeys, infections with Oesophagostomum show lower frequency, likely due to the epidemiology of its infective larvae (Abbott and Majeed 1984) than Trichuris, its larvae being protected by egg shells. Though both Trichuris and Oesophagostomum infections showed low prevalence and EPG values, the relevance of these parasites in monkeys managed for biomedical purposes should be carefully considered. In fact, even light infections could somehow interfere with biomedical research despite the fact that, as recently observed, Trichuris infections can improve colitis in rhesus monkeys by restoring their mucosal barrier functions, reducing the overall bacterial attachment, and altering the community of attached bacteria (Broadhurst et al. 2012). It should be considered that available drugs cannot be equally effective in eradicating Trichuris infections in monkeys; thus monitoring of these parasites is crucial to their control and should be warranted in animals not individually caged (Reichard et al. 2007). As recently revealed by our unpublished data, infections with Trichuris were heavier in cynomolgus monkeys housed in groups where EPG varied from 6 to 184 and whose prevalence values (9/20, 45%) were higher than those in the present survey.
Regarding protozoans, Entamoebas cysts are more frequently shed by monkeys than other parasitic species. Non-pathogenic Entamoeba species, such as E. coli and uninucleated-cyst-producing Entamoebas, were the most prevalent in our samples as observed also in previous surveys on captive and wild-trapped non-human primates (Rivera et al. 2010; Tachibana et al. 2001; Regan et al. 2014). Particularly, in cynomolgus monkeys from Japan, higher prevalence of non-pathogenic Entamoeba species rather than of E. histolytica was detected both by microscopy and by PCR (Takano et al. 2005; Feng et al. 2011). However, E. histolytica NHP variant was detected in captive non-human primates, including the macaques, with moderate prevalence (36%) (Levecke et al. 2010). In the present study, cynomolgus monkeys harbored E. nana, I. bütschlii, and E. polecki; these are generally considered harmless for NHPs even though some pathological consequences were reported (Loomis 1983; Vogel et al. 1996). Also in humans, these parasites species are regarded as nonpathogenic intestinal protozoans, and when detected in symptomatic people, other etiologies should be considered (http://www.cdc.gov/parasites/nonpathprotozoa/).
Additionally, our survey revealed the presence of B. coli, a cosmopolitan ciliate colonizing the intestine of many animals, humans included, with pigs serving as reservoir hosts (Schuster and Ramirez-Avila 2008). In humans, B. coli infections are considered zoonotic and are generally associated with close proximity to swine. Nevertheless, NHPs are often infected with this ciliate showing large variations in the prevalence values (Nakauchi 1999; Schuster and Ramirez-Avila 2008; da Silva Barbosa et al. 2015). Recently, a broad genetic diversity of isolates of B. coli from several species of NHPs was found; however, the high risk for humans from these ciliates inhabiting the intestines of NHPs seems to be confirmed (Pomajbíkova et al. 2013).
In the current study, tested NHPs were G. duodenalis, Cryptosporidium spp., and E. histolytica free. Recently, Ye et al. (2014) detected G. duodenalis and Cryptosporidium spp. in laboratory macaques at very low prevalence by molecular tools. Particularly, G. duodenalis infection was detected in 5 of 205 animals mainly young and housed in groups; in contrast, macaques from the present survey were adult and located in single cages where the risk of infection could be low. E. histolytica prevalence of infection varied in different studies: 26 % of M. fascicularis harbored in a Philippinian facility were infected (Rivera et al. 2010), while it was not detected in Macaca mulatta and M. fascicularis reared in China (Feng et al. 2011). The latter finding agrees with results of the current study that were obtained from M. fascicularis imported from China.
Finally, molecular analysis performed in our study revealed that cynomolgus monkeys were infected with Blastocystis, a well-known microeukaryote infecting the large intestine of possibly more than one billion people from both developed and developing countries (Boorom et al. 2008). Human-to-human transmission of B. hominis frequently occurs, but most Blastocystis isolates from animals and humans are shown to be genetically similar or identical indicating a possible animal-to-human transmission, a hypothesis also supported by the occurrence of Blastocystis infections with high prevalence in people in close contact with animals (Yoshikawa et al. 2009). Non-human primates are often infected with Blastocystis, and they host subtypes (ST) identified also in human feces. In a recent survey on the distribution of Blastocystis subtypes in 260 NHPs, the major groups of NHPs were found infected with eight subtypes; six of these (ST1, ST2, ST3, ST4, ST5, and ST8) were also found in humans (Alfellani et al. 2013a, b). According to previous studies, ST1 and ST2 were the most frequent subtypes of Blastocystis commonly identified in fecal samples of cynomolgus monkeys (Abe et al. 2002). However, cynomolgus monkeys were found infected with a number of ST (ST1, ST2, ST3, ST5, and ST7) higher than in other investigations where only ST1, ST2, and ST3 had been found in macaques and ST5 in Gorilla gorilla and Pan troglodytes (Alfellani et al. 2013a, b; Stensvold 2013). In NHPs, ST7 had not yet been identified. It is a subtype rarely occurring in humans; being considered of avian origin, both NHPs and humans could be infected through bird feces exposure. Humans are natural hosts of nine subtypes (ST1 through ST9), of which ST1 to ST4 are by far the most common; all subtypes found in macaques are likely to infect people. While humans and NHPs can host the same ST with the same alleles, a few ST alleles appeared to be NHPs specific (Stensvold et al. 2012; Alfellani et al. 2013a, b). Blastocystis sp. infection can cause acute and chronic intestinal diseases both in humans and in monkeys (Cekin et al. 2012; Zanzani et al. 2014); nevertheless, several single and mixed Blastocystis STs infections were detected in healthy humans and the possibility that Blastocystis could be a member of the normal gut microbiota should be evaluated also in NHPs (Scanlan et al. 2014; Scanlan et al. 2015). Acquisition of the infection seems to be more likely due to the conditions of animals in breeding farms rather than in research centers, where they are kept in single cages. The occurrence of asymptomatic Blastocystis sp. infections that in presence of stressors (i.e., transport, separation of animals from a group into single cages, dietary changes) can turn symptomatic should also be considered (Liu et al. 2008).
Conclusion
This study reports the prevalence of helminth and protozoan infections of the gastrointestinal tract in macaques employed in biomedical research. Data collected showed a high parasite diversity and the presence of potentially zoonotic parasites that can pose health risks to people involved in the care and use of non-human primates in biomedical research centers or to staff managing them in breeding facilities. The rich parasite fauna detected suggests the importance of a periodic coprological examination in order to minimize related health risks. Further, the panel of such fecal examination should include protozoans; in fact, they resulted to be more frequent parasites than helminths. In particular, protozoans can be zoonotic or cause acute diarrhea when NHPs are delivered to research centers. Finally, to enhance the control of helmintic infections, the evaluation of EPG by a highly sensitive technique, e.g., the FLOTAC technique, should be recommended, mainly in captive monkeys bred in groups. In fact, parasitic infections can be more frequent as the number of animals in social groups increases (Phillippi and Clarke 1992) and even latest regulations underline that research centers had better consider small NHP groups. As a consequence, minor health risks for humans would derive.
Notably, this is the first study to report a high prevalence of Blastocystis sp. infection in monkeys involved in biomedical research and to confirm the presence of zoonotic subtypes in NHPs.
References
Abbott DP, Majeed SK (1984) A survey of parasitic lesions in wild-caught, laboratory-maintained primates: (rhesus, cynomolgus, and baboon). Vet Pathol 21:198–207
Abe N, Nagoshi M, Takami K, Sawano Y, Yoshikawa H (2002) A survey of Blastocystis sp. in livestock, pets, and zoo animals in Japan. Vet Parasitol 106:203–212
Alfellani MA, Aner-Mulla D, Jacob AS, Atim Imeede C, Yoshikawa H, Stensvold CR, Clark CG (2013a) Genetic diversity of Blastocystis in livestock and zoo animals. Protist 164:497–509
Alfellani MA, Jacob AS, Ortíz Perea N, Krecek RC, Taner-Mulla D, Verweij JJ, Levecke B, Tannich E, Clark CG, Stensvold CR (2013b) Diversity and distribution of Blastocystis sp. subtypes in non-human primates. Parasitology 140:966–971
Bezubik B, Furmaga S (1960) The parasites in Macaca cynomolgous from Indonesia. Acta Parasitol 8:335–344
Boorom KF, Smith H, Nimri L, Viscogliosi E, Spanakos G, Parkar U, Li LH, Zhou XN, Ok ÜZ, Leelayoova S, Jones MS (2008) Oh my aching gut: irritable bowel syndrome, Blastocystis, and asymptomatic infection. Parasite Vector 1:40
Broadhurst MJ, Ardeshir A, Kanwar B, Mirpuri J, Gundra UM, Leung JM, Wiens KE, Vujkovic-Cvijin I, Kim CC, Yarovinsky F, Lerche NW, McCune JM, Loke P (2012) Therapeutic helminth infection of macaques with idiopathic chronic diarrhea alters the inflammatory signature and mucosal microbiota of the colon. PLoS Pathog 8:e1003000
Cekin AH, Cekin Y, Adakan Y, Tasdemir E, Koclar FG, Yolcular BO (2012) Blastocystosis in patients with gastrointestinal symptoms: a case-control study. BMC Gastroenterol 12:122
da Silva Barbosa A, Pissinatti A, Verdan Dib L, Perlingeiro de Siqueira M, Lessa Cardozo M, Monteiro Fonseca AB, de Barros OA, Alves da Silva F, Antunes Uchȏa CM, Machado Pereira Bastos O, Reis Amendoeira MR (2015) Balantidium coli and other gastrointestinal parasites in captives non-human primates of the Rio de Janeiro, Brazil. J Med Primatol 44:18–26
Dinh SV (2002) Intestinal parasites of Macaca fascicularis in a Mangrove Forest, Ho Chi Minh City, Vietnam. Laboratory Primate Newsletter 41:9–10
Emikpe BO, Ayoade GO, Ohore OG, Olaniyan OO, Akusu MO (2002) Fatal trichuriosis in a captive baboon (Papio anubis) in Ibadan Nigeria: a case report. Trop Vet 20:36–39
Feng M, Yang B, Yang L, Fu Y, Zhuang Y, Liang L, Xu Q, Cheng X, Tachibana H (2011) High prevalence of Entamoeba infections in captive long-tailed macaques in China. Parasitol Res 109:1093–1097
Honjo S, Muto K, Fujiwara T, Suzuki Y, Imaizumi K (1963) Significance of the natural infection of Oesophagostomum sp. in cynomolgus monkeys (Macaca irus) used as experimental animals. Jpn J Med Sci Biol 16:225–227
Janagi TS (1981) A report of gastro-intestinal helminth parasites found in Macaca fascicularis in peninsular Malaysia. Malaysia Applied Biol 10:99–100
Lane KE, Holley C, Hollocher H, Fuentes A (2011) The anthropogenic environment lessens the intensity and prevalence of gastrointestinal parasites in Balinese long-tailed macaques (Macaca fascicularis). Primates 52:117–128
Lee JI, Kang SJ, Kim NA, Lee CW, Ahn K, Kwon H, Park C, Kim S (2010) Investigation of helminths and protozoans infecting old world monkeys: captive vervet, cynomolgus, and rhesus monkeys. Korean J Vet Res 50:273–277
Lee RV, Prowten AW, Anthone S, Satchidanand SK, Fisher JE, Anthone R (1990) Typhlitis due to Balantidium coli in captive lowland gorillas. Rev Infect Dis 12:1052–1059
Levecke B, Dreesen L, Dorny P, Verweij JJ, Vercammen F, Casaert S, Vercruysse J, Geldhof P (2010) Molecular identification of Entamoeba spp. in captive non-human primates. J Clin Microbiol 48:2988
Liu YW, Suzuki S, Kashima M, Tokado H, Fukuzaki K, Miyajima H (2008) Clinical pathology data from cynomolgus monkeys from China in which diarrhea was observed during quarantine. Exp Anim 57:139–143
Loomis M (1983) Hepatic and gastric amebiasis in black and white colobus monkeys. J Am Vet Med Assoc 183:1188–1191
Loomis MR, Wright JF (1986) Gastric trichuriasis in a black and white colobus monkey. J Am Vet Med Assoc 189:1214–1215
Matsubayashi K, Gotoh S, Kawamoto Y, Watanabe T, Nozawa K, Takasaka M, Narita T, Griffiths O, Stanley MA (1992) Clinical examinations on crab-eating macaques in Mauritius. Primates 33:281–288
MacIntosh AJJ, Hernandez AD, Huffman MA (2010) Host age, sex, and reproductive seasonality affect nematode parasitism in wild Japanese macaques. Primates 51:353–364
Melfi V, Poyser F (2007) Trichuris burdens in zoo-housed Colobus guereza. Int J Primatol 28:1449–1456
Meloni D, Poirier P, Mantini C, Noël C, Gantois N, Wawrzyniak I, Delbac F, Chabé M, Delhaes L, Dei-Cas E, Fiori PL, El Alaoui H, Viscogliosi E (2012) Mixed human intra- and inter-subtype infections with the parasite Blastocystis sp. Parasitol Int 61:719–722
Muriuki SMK, Farah IO, Agwiria RM, Chai DC, Njamunge G, Suleman M, Olobo JO (1997) The presence of Cryptosporidium oocysts in stools of clinically diarrhoeic and normal non-human primates in Kenya. Vet Parasitol 72:141–147
Muriuki SMK, Murugu RK, Munene E, Karere GM, Chai DC (1998) Some gastro-intestinal parasites of zoonotic (public health) importance commonly observed in old world non-human primates in Kenya. Acta Trop 71:73–82
Nakauchi K (1999) The prevalence of Balantidium coli infection in fifty-six mammalian species. J Vet Med Sci 61:63–65
Pedersen AB, Altizer S, Poss M, Cunningham AA, Nunn CL (2005) Patterns of host specificity and transmission among parasites of wild primates. Int J Parasitol 35:647–657
Phillippi KM, Clarke MR (1992) Survey of parasites of rhesus monkeys housed in small social groups. Am J Primatol 27:293–302
Pomajbíkova K, Oborník M, Horák A, Petrzálková KJ, Grim JN, Levecke B, Todd A, Mulama M, Kiyang J, Modr D (2013) Novel Insights into the genetic diversity of Balantidium and Balantidium-like cyst-forming ciliates. PLoS Neglect Trop D 7:e2140
Regan CS, Yon L, Hossain M, Elsheikha HM (2014) Prevalence of Entamoeba species in captive primates in zoological gardens in the UK. Peer J 2:e492
Reichard MV, Wolf RF, Carey DW, Garrett JJ, Briscoe HA (2007) Efficacy of fenbendazole and milbemycin oxime for treating baboons (Papio cynocephalus anubis) infected with Trichuris trichiura. J Am Ass Lab Anim 46:42–45
Rivera WL, Yason JADL, Adao DEV (2010) Entamoeba histolytica and E. dispar infections in captive macaques (Macaca fascicularis) in the Philippines. Primates 51:69–74
Roperto F, Quesada A, Pandolfi F, Izzi R (1985) Oesophagostomiasis in monkeys imported from Senegal. Atti della Societa Italiana delle Scienze Veterinarie 38:603–5
Sano M, Kino H, de Guzman TS, Ishii AI, Kino J, Tanaka T, Tsuruta M (1980) Studies on the examination of imported laboratory monkey, Macaca fascicularis for E. histolytica and other intestinal parasites. Int J Zoonoses 7:34–39
Santín M, Gómez-Muñoz MT, Solano-Aguilar G, Fayer R (2011) Development of a new PCR protocol to detect and subtype Blastocystis spp. from humans and animals. Parasitol Res 109:205–212
Scanlan PD, Stensvold CR, Cotter PD (2015) Development and application of a Blastocystis subtype-specific pcr assay reveals that mixed-subtype infections are common in a healthy human population. Appl Environ Microbiol 81:4071–6
Scanlan PD, Stensvold CR, Rajilić-Stojanović M, Heilig HG, De Vos WM, O’Toole PW, Cotter PD (2014) The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol Ecol 90:326–30
Schuster FL, Ramirez-Avila L (2008) Current world status of Balantidium coli. Clin Microbiol Rev 21:626–638
Stensvold CR (2013) Comparison of sequencing (Barcode Region) and sequence-tagged-site PCR for Blastocystis subtyping. J Clin Microbiol 51:190–194
Stensvold CR, Alfellani M, Clark CG (2012) Levels of genetic diversity vary dramatically between Blastocystis subtypes. Infect Genet Evol 12:263–273
Stensvold CR, Lebbad M, Victory EL, Verweij JJ, Tannich E, Alfellani M, Legarraga P, Clark CG (2011) Increased sampling reveals novel lineages of Entamoeba: consequences of genetic diversity and host specificity for taxonomy and molecular detection. Protist 162:525–541
Tachibana H, Cheng X, Kobayashi S, Matsubayashi N, Gotoh S, Matsubayashi K (2001) High prevalence of infection with Entamoeba dispar, but not E. histolytica, in captive macaques. Parasitol Res 87:14–17
Takano J, Narita T, Tachibana H, Shimizu T, Komatsubara H, Terao K, Fujimoto K (2005) Entamoeba histolytica and Entamoeba dispar infections in cynomolgus monkeys imported into Japan for research. Parasitol Res 97:255–257
Taylor L, Lessnau RG, Lehman SM (1994) Prevalence of whipworm (Trichuris) ova in two free-ranging populations of rhesus macaques (Macaca mulatta) in the Florida Keys. Florida Scientist 57:102–107
Vogel P, Zaucha G, Goodwin D, Kuehl K, Fritz D (1996) Rapid postmortem invasion of cecal mucosa of macaques by non pathogenic Entamoeba chattoni. Am J Trop Med Hyg 55:595–602
Wong MM, Conrad HD (1978) Prevalence of metazoan parasite infections in five species of Asian macaques. Lab Anim Sci 28:412–416
Ye J, Xiao L, Li J, Huang W, Amer SE, Guo Y, Roellig D, Feng Y (2014) Occurrence of human-pathogenic Enterocytozoon bieneusi, Giardia duodenalis and Cryptosporidium genotypes in laboratory macaques in Guangxi, China. Parasitol Int 63:132–137
Yoshikawa H, Wu Z, Kimata I, Iseki M, Ali IK, Hossain MB, Zaman V, Haque R, Takahashi Y (2004) Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol Res 92:22–29
Yoshikawa H, Wu Z, Pandey K, Pandey BD, Sherchand JB, Yanagi T, Kanbara H (2009) Molecular characterization of Blastocystis isolates from children and rhesus monkeys in Kathmandu, Nepal. Vet Parasitol 160:295–300
Zanzani S, Epis S, Bandi C, Manfredi MT (2014) What is your diagnosis? Fecal smear stained with Lugol’s solution and Giemsa from a cynomolgus macaque (Macaca fascicularis) presenting with liquid diarrhea. Vet Clin Path 43:293–294
Acknowledgments
The authors are grateful to Ms Gigliola Canepa, University of Milan, for her support in editing our manuscript.
Conflict of interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zanzani, S.A., Gazzonis, A.L., Epis, S. et al. Study of the gastrointestinal parasitic fauna of captive non-human primates (Macaca fascicularis). Parasitol Res 115, 307–312 (2016). https://doi.org/10.1007/s00436-015-4748-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4748-9