Abstract
Background
Gastrointestinal parasites may determine diarrhea, dysentery or even death in captive mammals. These animals tend to be more susceptible to parasitic infections due to confinement and stress. Purpose To increase the information about these etiological agents in captive animals in Brazil, the gastrointestinal parasites of the captive mammals of the Rio de Janeiro Zoo were investigated.
Methods
From 2016 to 2018, 180 fecal samples were collected from animals housed in the Rio de Janeiro Zoo: 63 from animals of the order Primates, 26 of Carnivora, 78 of Artiodactyla, 9 of Perissodactyla and 4 of the order Rheiformes. The feces were processed by direct examination and by the techniques of Faust et al., Sheather, Ritchie, Lutz, and smears were stained with safranin. Immunoenzymatic assays were also performed to investigate antigens of Giardia duodenalis, Cryptosporidium spp., Entamoeba histolytica/Entamoeba dispar.
Results
Parasite positivity was identified in 68.3% of the fecal samples, with a parasite positivity rate of 68.2% among primates, 65.3% among carnivores, 69.2% among artiodactyls, 33.3% among perissodactyls, and 100% among rheiformes. The most frequently detected parasite was Entamoeba histolytica/E. dispar antigens, which showed a statistically significant positivity rate (33.3%; p = 0.000), particularly in the feces of carnivores (30.7%) and artiodactyls (53.8%). A statistically significant positivity rate of Balantioides coli (11.1%; p = 0.001) was also detected in feces from nonhuman primates, tapirs, collared peccaries and rheas. The positivity of Cryptosporidium sp. antigens in feces of the orders Carnivora, Artiodactyla and Primates was also statistically significant (7.2%, p = 0.010). Oocysts compatible with Cryptosporidium spp. were detected in 6.3% from primates. The helminths most frequently detected were thin-shelled eggs of nematodes (17.7%, p = 0.000), nematode larvae (15.5%, p = 0.000) and Trichuris trichiura eggs (6.1%, p = 0.018).
Conclusion
The positivity rate for gastrointestinal parasites demonstrates the need for a sanitation management program to be implemented in the zoo, including routine diagnostic parasitology tests followed by specific treatment for each parasitosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zoos and aquariums are sites for ex situ conservation of wild animals. These sites are essential for the protection of endangered species, given that they enable in-depth research and monitoring of such species. Zoos engage not only in species conservation but also in research, environmental education, and leisure, since a large part of the population lives in urban areas, with few opportunities to experience natural environments, which may affect the construction of values pertaining to the production of biological diversity [1]. Thus, zoos today play four essential roles: environmental education, conservation, research and leisure [2].
In nature, wild animals live in large geographic areas and are exposed to a variety of parasites such as protozoans and helminths; hence, they usually develop resistance against these infectious agents. However, when kept under human care, e.g., in zoo enclosures, animals are restricted to smaller spaces and are less frequently exposed to these agents, which somewhat diminishes their immune resistance to such infections [3]. Information about the frequency of these infectious agents is, therefore, extremely important, especially for animals to be reintroduced to their natural habitat [4].
The occurrence of parasitic diseases in captive wild animals may vary according to the health management practices employed, especially prophylactic measures, with emphasis on anthelmintic treatments [5, 6]. Confinement associated with intensive management practices can stress animals, weakening their immune system and making them more susceptible to parasitic infections. Helminth infections can also be favored by the misuse of drugs, which become ineffective over time. It should also be noted that the nutritional status of captive animals may strengthen or weaken their resistance to parasitic infections [5].
When these infections are symptomatic in these animals, they usually bring about clinical signs of diarrhea, dysentery, and in severe cases, they can lead to death [7]. In general, when animals are kept in captivity for a long time, their parasite fauna may undergo changes, presenting evolutionary forms that are also detected in humans, giving rise to cycles of zoonotic disease transmission [8]. The parasites with this potential include Balantioides coli, which has been diagnosed mainly in artiodactyls such as wild and domestic pigs and also in nonhuman primates (Schuster and Ramirez- Ávila 2008). Giardia duodenalis, Cryptosporidium spp. and Entamoeba histolytica/Entamoeba dispar complex, which also have a zoonotic potential, have been reported in different species of captive animals in zoos in Belgium and Spain [7, 9].
Given the paucity of studies published in Brazil on this theme and the importance of zoos for animals and society, the purpose of this study was to estimate the prevalence rates of gastrointestinal parasites in the animals of the Rio de Janeiro Zoo, in Brazil, correlating them with the taxonomic order of the animals and with the positivity of the diagnosed parasite structures.
Materials and Methods
This study was approved by the Ethics Committee on Animal Use of the Fluminense Federal University (CEUA–UFF), under Permit nos. 794 and 7,641,100,418. CEUA–UFF holds an annually updated permit issued by the Biodiversity Authorization and Information System (SISBIO) of the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA), under protocol number 52,578-1.
Study site
The study was conducted in the Rio de Janeiro Zoo, located in the Quinta da Boa Vista Park, in the municipality of Rio de Janeiro. The Zoo covers an area of 138,000 m2 and houses more than 1300 animals, including birds, nonhuman primates, carnivores, ungulates, proboscids, reptiles and fish. The Rio Zoo is Brazil’s oldest zoo and represents one of the Rio’s top tourist attractions, receiving an average of 948,000 Brazilian and foreign tourists per year [10].
The zoo’s captive animal facilities are arranged in distinct physical spaces, with animals allocated according to species. In general, the enclosures have a covered part and a part open to the elements, and totally or partially unfinished floors, i.e., rammed earth or sand floors, as in the case of the felines. In addition, the enclosures are equipped with tree trunks, tires, ropes, grasses for environmental enrichment. Some enclosures have water tanks for the animals to cool off, like those in the enclosures of felines and Old World nonhuman primates, including the Great Primates, where there is a waterfall, and also in the enclosures of some artiodactyls such as hippos and collared peccaries, which have a pool. The enclosures and facilities are routinely cleaned, usually in the morning, and the animals are fed daily. Routine antiparasitic treatments are dispensed according to the symptoms presented by the animals.
Sampling and Material Collection
In the period of June 2016 to July 2018, 180 fecal samples were collected from mammals and birds in the care of the Rio Zoo. The fecal samples were divided as follows: 63 from the order Primates (Supplement Table 1), 26 from Carnivora, 78 from Artiodactyla, 9 from Perissodactyla, and 4 from the order Rheiformes (Supplement Table 2). The fecal matter was collected directly from the floor of the enclosures where the animals are housed or from containers inside enclosures, such as feeders. The fecal matter on the floor was picked up by the zookeepers while they cleaned the enclosures, who prioritized the collection of individual fresh fecal pellets. The total number of fecal samples collected was equal to the number of animals housed in each enclosure. In the case of small mammals, which produced insufficient fecal matter in 1 day for the lab tests, fecal samples were collected in triplicate on three consecutive days. The fecal samples were stored in plastic bags without chemical preservatives, which were placed in isothermal containers and sent to the Laboratory of Parasitology at the Federal Fluminense University (UFF).
Laboratory Techniques
A portion of the fecal matter was immediately processed by direct examination in buffered saline solution to identify the presence of Balantioides coli trophozoites. Another part of the sample was homogenized and the filtrate was aliquoted into 15-mL conical-bottom centrifuge tubes to perform the techniques of centrifugal sedimentation described by Ritchie [11] modified by Young et al. [12], centrifugal flotation developed by [13], Sheather’s centrifugal flotation method (1923) [14] modified by Huber et al. [15], and permanent staining of coccidia with safranin solution recommended by Baxby et al. [16]. The filtrate was also aliquoted in 2-mL microtubes for the immunoenzymatic assays to detect the protozoans Cryptosporidium spp., Giardia duodenalis and the Entamoeba histolytica/Entamoeba dispar complex. Part of the filtrate was subjected to Lutz’s spontaneous sedimentation technique (1919) [17].
The slides produced by each technique and the photomicrographs were examined under an Olympus® BX41 Binocular Optical Microscope, initially under 100 × magnification, and when necessary for confirmation, 400x magnification. A Samsung® SDC415 digital camera equipped with Honestech® PVR capture software was coupled to the microscope. The only exception was the slides stained with safranin, which were examined under 400x magnification, and for confirmation, under 1000x magnification. The morphometry of the evolutionary forms of the parasite was examined using the binocular optical microscope under 400x magnification, and the safranin stained slides were examined under 1000x magnification, using an Olympus® SWH micrometer eyepiece.
Immunoenzymatic assays were performed using the following commercial kits: IVD Research®, lots LN843 and LN1242, with cut-off point at ≥ 0.08 for Cryptosporidium spp., IVD Research®, lots: LN891 and LN1067, with cut-off point at ≥ 0.08 for Giardia duodenalis, and IVD Research®, lots: LN1207 and LN120, with cut-off point at ≥ 0.15 for Entamoeba histolytica/Entamoeba dispar. The assays were performed as recommended by the kit manufacturer and the plates were read on an ELISA reader (Testline® ELx 800) at the wavelength indicated by the manufacturer’s technical standards.
Data Analysis
Fecal samples were considered positive when at least one evolutionary form of a parasite (trophozoite, egg, larva, cyst and/or oocyst) and/or of a protozoan antigen was detected. Prevalence was estimated by dividing the number of positive samples by the total number of samples collected from each group of animals under study. Fisher’s exact test with a 5% confidence level was applied, by means of the SPSS statistical program version 18, SPSS® Inc., Chicago, IL, 1999, to compare the overall parasite positivity according to the taxonomic order of the animals, and also to the evolutionary form of each detected parasite.
Results
A total of 180 fecal samples were collected, 123 (68.3%) of which were positive for evolutionary forms of gastrointestinal parasites and/or protozoan antigens. Of these, 43 (68.2%) came from nonhuman primates, 17 (65.3%) from carnivores, 56 (67.9%) form artiodactyls, 3 (33.3%) from perissodactyls and 4 (100%) from rheiformes. More evolutionary forms of helminths than of protozoa were detected in the fecal samples from nonhuman primates. However, overall, protozoan detected by the microscopy techniques associated with antigen research was found more frequently than evolutionary forms of helminths. Both helminths and protozoans showed statistically significant frequencies relative to the overall positivity of the study (p < 0.05). Amoebids stood out among the protozoa detected in the fecal matter of animals with a statistically significant frequency (p < 0.05), including antigens of the Entamoeba histolytica/Entamoeba dispar complex, followed by amoebic cysts detected by microscopy (Table 1).
In nonhuman primates, protozoans with zoonotic potential, such as Balantioides coli were detected in fecal samples collected from Old World nonhuman primates, including the Great Primates. The immunoenzymatic assay revealed other protozoans with a profile of zoonotic transmissibility, such as Giardia duodenalis in Alouatta seniculus feces, and Cryptosporidium spp. in four fecal samples, two from members of the family Atelidae and two from Cercopithecidae. Positive Entamoeba histolytica/Entamoeba dispar antigens were detected in nine samples, including the New World primate species of the families Atelidae, Callitrichidae and Cebidae and the Old World species of the families Cercopithecidae and Hominidae. Among four fecal samples from nonhuman primates which containing oocysts compatible with Cryptosporidium spp., only one fecal sample from Papio cynocephalus was positive for Cryptosporidium spp. in the immunoenzymatic assay. Among ten fecal samples (15.8%) from nonhuman primates from which amoebic cysts were recovered, only two samples from Orangutans were positive for Entamoeba histolytica/Entamoeba dispar complex by the immunoenzymatic assay (Tables 1, 2, Fig. 1).
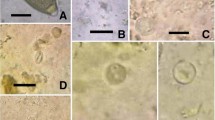
Ilustration of the evolutionary forms of protozoa detected in fecal samples collected from captive animals of Rio de Janeiro Zoo. aBalantioides coli trophozoite (400X) in chimpanzee fecal sample (Pan troglodytes) 40–150 µm × 40–90 µm (93.75 ± 31.25 × 61.25 ± 14.37). b Amoeba cyst (1000X) with one nuclei of fecal deer (Cervus unicolor) 12–13 µm (12.5 ± 0.48). c Oocyst similar to Cryptosporidium spp. (1000X) in yellow baboon fecal material (Papio cynocephalus) 4–5 μm in diameter (4.7 ± 0.37). d Thin-shelled egg of nematode in llama fecal sample (Lama glama) (400X) 118–122 μm × 60–77 μm (101.44 ± 12.05 × 65.05 ± 5.85)
The parasite structures most frequently detected among carnivores were coproantigens of the Entamoeba histolytica/Entamoeba dispar complex, which were found in 8 (30.7%) fecal samples from at least one individual among all the families included in the study. In addition, Giardia duodenalis antigens were also detected in Leopardus tigrinus feces. Cryptosporidium spp. antigens were found in the feces of Leopardus tigrinus and Chrysocyon brachyurus. Unsporulated coccidian oocysts larger than 15 μm were detected in fecal matter from Potos flavus and Procyon cancrivorus (Tables 1, 3).
Antigens of the Entamoeba histolytica/Entamoeba dispar complex were also the most frequently detected structures in the fecal samples collected from the ground in the enclosures of Cervus unicolor. Of the 43 fecal samples positive for Entamoeba histolytica/Entamoeba dispar by the enzyme immunoassay assay, amoebic cysts were detected by the microscopy techniques in 21 of the 25 samples with cysts (data not shown). These fecal samples that were positive came from Bos taurus, Bubalus bubalis, Lama glama, Cervus unicolor and Pecari tajacu. Another protozoan recovered only in fecal samples from the artiodactyls was B. coli (10.2%), which was found in all the fecal samples from P. tajacu (Table 4). Protozoan structures were detected among the fecal samples from the perissodactyls, such as unsporulated coccidian oocysts and antigens of Entamoeba histolytica/Entamoeba dispar complex in a fecal sample from Equus caballus. Evolutionary forms of the protozoan B. coli were detected in all the samples collected from the Rhea americana enclosures; this was the only parasite detected in these animals (Table 4).
In general, analyzing all the animal orders, the evolutionary forms of helminths most frequently detected in the study were thin-shelled nematode eggs, nematode larvae and Trichuris trichiura eggs, and the frequency of their detection was statistically significant (p < 0.05). In the enclosures of nonhuman primates, the highest parasite frequency detected was evolutionary forms of nematode larvae (25.3%), thin-shelled nematode eggs suggestive of hookworm (superfamily Ancylostomatoidea) or strongylids (superfamily Trichostrongyloidea or Strongyloidea) (20.6%), and Trichuris spp. eggs (17.4%) (Table 1). Nematode larvae and thin-shelled eggs were identified in fecal samples from Neotropical primates of the families Atelidae, Callitrichidae, Cebidae and from the Old World apes of the family Cercopithecidae and of the Great Primates of the family Hominidae. Trichuris spp. eggs were detected only in the fecal matter of the family Cercopithecidae (Table 2). The helminths detected in fecal matter collected from the floor of the carnivore enclosures were nematode larvae in Leopardus pardalis and Procyon cancrivorus feces, thin-shelled nematode eggs suggestive of the superfamily Ancylostomatoidea, and cestode eggs of the family Diphyllobothriidae in L. pardalis feces. In addition, eggs of the phylum Acanthocephala were detected only in Mungos mungo feces and geohelminth Toxascaris leonina in Panthera leo feces (Table 3). The helminths most frequently recovered from the fecal matter of artiodactyls were thin-shelled nematode eggs with the typical morphology of strongylids (21.8%), which were detected in Bos taurus, Lama glama, Sus scrofa and Pecari tajacu feces (Table 4, Fig. 1). Among the fecal samples of perissodactyls, an evolutionary form of nematode larvae was detected only in one fecal sample from Tapirus terrestris, and thin-shelled nematode eggs compatible with strongylids were found in fecal samples from Equus caballus. No evolutionary forms of helminths were detected in the feces of Rheiform birds (Table 4).
An association of parasitic structures was detected in 24 fecal samples from nonhuman primates, five from carnivores, 31 from artiodactyls and two from perissodactyls. The most frequent association was observed in fecal matter from primates, where 9 out of 24 fecal samples contained nematode larvae and thin-shelled nematode eggs. For carnivores, the association of parasitic structures detected did not repeat, each being observed only once. The association between amoebic cysts and antigens of the Entamoeba histolytica/Entamoeba dispar complex was the one with the highest positivity rate found in the fecal matter of artiodactyls, appearing in 21 fecal samples (Fig. 1). In the fecal samples from perissodactyls, an association was observed between Balantioides coli and nematode larvae and also between antigens of the Entamoeba histolytica/Entamoeba dispar complex, unsporulated coccidian oocysts and thin-shelled nematode eggs (Data not shown).
Discussion
An analysis of the parasite structures in the fecal samples from the various animal species in the Rio Zoo revealed an overall positivity rate of 68.3%. Other studies that analyzed the frequency of gastrointestinal parasites in captive animals in various zoos reported lower positivity rates than this one, e.g., in the state of Paraná, Brazil 38.9% [18], Malaysia 56.3% [4], India 46.2% and 58% [19, 20], Bangladesh 60% [6] and Italy 61.5% [21]. Other studies reported higher positivity rates, e.g., in the zoos of Spain 72.5% [9] and in the state of Pernambuco, Brazil 74.2% [22].
Despite the high positivity rate of parasite structures found in this study, it should be noted that the fecal samples of the animals in Rio de Janeiro Zoo were collected from the floor or ground inside their enclosures. Therefore, not all the helminths and protozoa identified are necessarily infectious to the animals but may actually be free-living protozoan and helminth species. However, the diagnosis of these biological agents indicated that the zoo animal enclosures favored the maintenance of evolutionary forms of potentially infectious organisms. Picking up fecal samples from the floor of zoo animal enclosures has been a methodology used by this research group to collect biological samples. The advantage of this form of sampling is that it avoids subjecting animals to the stress of chemical and/or mechanical restraint, thus enhancing their welfare. Few of the articles perused here provide a clear description of fecal sampling methods, but the methodology used in this study has also reportedly been employed at other zoos, including those in Lisbon—Portugal, Belgium, Croatia, and Peru, and in a nonhuman primate breeding center in Rio de Janeiro, Brazil [7, 23–26].
Protozoans were detected more frequently than helminths in the fecal matter of carnivores, artiodactyls, perissodactyls and rheiformes. The high incidence of protozoa found in the captive animals of this study may be attributed to the simplicity of the biological cycle of these parasites, which do not require intermediate hosts, and also their passive oral transmission, the low infective dose of these agents, the high resistance of their cysts and oocysts in the environment, and the infectivity of cystic forms as soon as they are excreted, enabling direct transmission between susceptible hosts [6, 7, 9]. Moreover, protozoa have low sensitivity to anthelmintics, which are the drugs routinely used in anti-parasite treatment programs for animals living in captivity [26, 27]. The higher frequency of protozoa found in Rio Zoo is not what has been demonstrated with findings described in most of the studies retrieved from the literature, which report a higher frequency of helminths, e.g., in the zoos in India [20, 28], Bangladesh [6], Malaysia [4] and Italy [21]. It should be noted that, unlike earlier studies, the frequency of protozoa found in Rio Zoo seems to have been favored by the use of commercial immunoenzymatic kits that detect protozoan antigens of Cryptosporidium sp., Giardia duodenalis and Entamoeba histolytica/Entamoeba dispar complex in fecal matter, without requiring the parasitic form to be intact in the feces, in addition to being more sensitive because they detect protozoans in low parasitic loads.
In general in this study, the parasite structures most frequently detected were antigens of the Entamoeba histolytica/Entamoeba dispar complex. The evolutionary forms of protozoa most often identified by microscopy in the feces of nonhuman primates were cysts morphologically compatible with amoebids, followed by coproantigens of the complex. Amoebic cysts were also detected in feces collected from artiodactyls. The morphology of amoebic cysts was not analyzed in-depth in this study. However, the cysts found in fresh fecal matter had an average diameter of \({\bar{x}}\) = 13 μm (± 0.5), and most of them contained only one nucleus. Such characteristics are compatible with immature cysts of the Entamoeba histolytica/E. dispar complex. Cysts with two to three nuclei were detected in only three fecal samples, while one fecal sample from a primate contained larger cysts with more than five nuclei compatible with Entamoeba coli. It is important to note that cysts identified by microscopy may belong to other species of amoebid specific to animals, including those that produce mature uninucleate cysts.
In this study was identified concomitant positivity of amoebic cysts with Entamoeba histolytica/E. dispar complex in two fecal samples from orangutans and in 21 samples from artiodactyls, including bovines, buffalos, llamas, deer and collared peccaries. The genus Entamoeba sp. has been reportedly in captive wild mammals in other zoos at a frequency of 46.1% in nonhuman primates, carnivores, perissodactyls and artiodactyls in Spain [9], 59% in Belgium, including Old World nonhuman primates [7], in captive nonhuman primates in Africa, where it has been identified at a frequency of 26.6% in baboons, 16.3% in Sykes’ monkeys and 25.4% in vervet monkeys [27], in captive Old World nonhuman primates in Rio de Janeiro, Brazil 38.3% [26], in nonhuman primates in Pernambuco, 3% (Feitas et al. 2001), and in carnivores in Nigeria, 3.8% [29]. Amoebids have been little studied in animals in general, but this group of protozoans has been reported more frequently in epidemiological studies involving nonhuman primates. It is known that the amoeba species that infect primates are similar to those that infect humans, and that they are able to harbor more specific species such as Entamoeba chattoni, which has a mature cyst with a nucleus and morphology compatible with that of the Entamoeba histolytica/Entamoeba dispar complex. Amebiasis symptoms similar to those occurring in humans, caused by the pathogenic species Entamoeba histolytica, have been reported in apes [30]. The detection of Entamoeba histolytica/Entamoeba dispar complex in zoo animals is important because this complex contains potentially pathogenic species, although the actual clinical significance of Entamoeba histolytica is unknown for the various animal species living in the Rio Zoo.
The second most frequently detected protozoan was Balantioides coli, which was identified in the feces of Old World nonhuman primates of the family Cercopithecidae, in Great Primates of the family Hominidae, in fecal matter from tapirs, and in all the fecal samples collected from the enclosure of the collared peccaries and rheas. This protozoan can parasitize several animal species, including pigs, ostriches, rheas, nonhuman primates, and even humans, and has a potential for zoonotic transmission [31]. High B. coli incidence rates have been reported in nonhuman primates, particularly among captive Old World apes and Great Primates in Belgium, Spain, Malaysia, and Bangladesh [4, 6, 7, 9] and in breeding centers in Africa and Rio de Janeiro, Brazil [26, 27]. B. coli positivity in the Old World apes and Great Primates appears to be related to their higher sensitivity to infection by this protozoan [31]. Moreover, unlike Neotropical primates, this group of animals tends to spend most of their time on the ground, which favors contact with the feces or with the floor of enclosures contaminated with B. coli cysts [26]. It is extremely important to monitor balantidiasis in simian breeding stock, given the reports of severe cases of dysentery among baboons, and of fatal cases among chimpanzees and western lowland gorillas (Gorilla gorilla gorilla) [32–34]. Comparisons could not be made with reports in the literature about the frequency of Balantioides coli in collared peccaries, since no articles were found that included this animal species. However, positivity for this parasite was expected because the collared peccary belongs to the order Artiodactyl, i.e., the same taxonomic order as pig, which is considered the main reservoir hosts of this parasite. Note that the enclosures of collared peccaries, rheas and tapirs in the Rio Zoo are actually open air pens with bare earthen floors, which makes it difficult to disinfect the environment. The rooting behavior of collared peccaries and ground scratching behavior of rheas may be favorable for reinfections by this protozoan.
Oocysts compatible with Cryptosporidium spp. were detected in four fecal samples from nonhuman primates, using permanent staining with heated safranin solution for genus-specific detection (diameter of \({\bar{x}}\) = 4.5 µm, ± 0.5). The coproantigen test of the parasite revealed a higher frequency, with the parasite detected in 13 fecal samples, including those of nonhuman primates, carnivores and artiodactyls. Similar findings have been reported in Italian and Malaysian zoos, where oocysts of this protozoan were also detected in fecal matter from carnivores, nonhuman primates and ungulates using only the Ziehl–Neelsen staining procedure [4, 21]. In Brazil, at the São Paulo Zoo, [35] analyzed 31 fecal samples from 11 captive maned wolves and found that 19.3% were positive for the protozoan. In Rio Zoo, Cryptosporidium spp. antigens were detected in only one fecal sample from this animal species. Although the immunoenzymatic assay used in this study produced a higher positivity rate than the safranin staining technique, the two techniques were not in 100% agreement regarding the positivity of the fecal samples. This finding indicates the need for further studies involving the diagnosis of this protozoan, especially in samples from wild animals. It should be noted that commercial ELISA kits are validated for human fecal samples with protozoa that frequently infect human hosts. Hence, the sensitivity of these kits to fecal samples from wild animals parasitized with different protozoan species is unknown. Therefore, the diagnosis obtained by safranin staining was described as compatible with Cryptosporidium spp., in view of the absence of 100% agreement with the results of the immunoenzymatic assay.
The frequency of G. duodenalis in this study was very low and was only diagnosed in one fecal sample from northern tiger cat and another from Colombian red howler monkey. Like what was found in this study, Giardia duodenalis has been detected in the feces of captive carnivores, i.e., jaguar, cougar, northern tiger cat and margays at a zoo in Santa Catarina, Brazil [36], in the feces of nonhuman primates such as chimpanzees in a zoo in Poland, and in feces of several ape species, mainly Great Primates, in a zoo in Belgium [7, 37]. It is worth noting that most zoos, including the Rio Zoo, are located in urban centers, which brings these animals into close contact with humans. This favors the zooanthroponotic transmission cycle of these protozoa, which has been reported in outbreaks of infection through contaminated water sources.
Other protozoan parasites were also detected in fecal matter, such as unsporulated coccidian oocysts in the feces of Golden-headed lion tamarins, orangutans, carnivores such as kinkajou and crab-eating raccoon, and in those of ungulates of the families Bovidae, Camelidae and Equidae. Although qualitative coproparasitological techniques were used here, the number of oocysts in fecal matter was small and attempts to sporulate the structures were unsuccessful, probably because they were unviable; thus, the taxonomic classification of the parasite was not possible. However, the oocysts were measured and all of them proved to be longer and wider than 15 μm, thus making them compatible with the genera Cystoisospora spp. and/or Eimeria spp., which are causative agents of coccidiosis. It is extremely important to examine protozoan parasites in the fecal matter of captive animals, particularly Giardia spp., Cryptosporidium spp., Cystoisospora spp. and Eimeria spp., because these agents are cited in the literature as the cause of gastrointestinal disorders in young captive or immunocompromised animals [23, 38].
The most frequently detected parasites in the fecal matter of nonhuman primates were evolutionary forms of helminths. What stood out was the frequency of nematode larvae and thin-shelled nematode eggs, oval, morulated and in some cases larvated, varying from 74 to 85.1 μm in length by 44.2 to 55 μm in width, compatible with the superfamily Ancylostomatoidea or superfamily Trichostrongyloidea/Strongyloidea, which were called strongylids in this study. Although the size of the eggs identified by microscopy techniques was found to be compatible with these superfamilies, these evolutionary forms may belong to as yet unidentified nematode species within the superfamily Rhabditoidea, or it may be a parasitic adaptation of the species of the superfamily Rhabditoidea to the diet supplied in captivity, which may have favored a variation in egg size. However, further studies on this theme are still needed.
Thin-shelled nematode eggs were also detected in fecal matter collected from the floor of enclosures of artiodactyls. The eggs, resembling those of strongylids, varied from 118 to 122 μm in length and 60–77 μm in width, and in only one fecal sample from an ocelot, varied from 55 to 77 μm in length by 35–48 μm in width, which is compatible with the superfamily Ancylostomatoidea. Nematode larvae were not taxonomically classified in this study and may, therefore, have been evolutionary forms of free-living soil nematodes. However, in the fecal matter of primates, a more frequent association of parasitic structures was observed between thin-shelled eggs and the larvae of nematodes, demonstrating that these evolutionary forms may originate from the hatching of thin-shelled eggs morphologically compatible with parasitic groups. The presence of these evolutionary forms may be favored by the totally or partially bare ground of the animal enclosures, which favors the migration of larvae and their development into infectious filarioid forms. Parasites such as strongylids are often detected in ruminant feces. These agents have been detected in simian feces when primates and ruminants share limited spaces in the same area [27]. Although the different animal species in Rio Zoo do not share the same area, cross contamination between animal enclosures may be favored by the slope of the land in the zoo, which may facilitate the transport of parasitic structures through rainwater.
Brown ellipsoidal eggs of nematodes of the order Enoplida, with morphology compatible to that of Capillaria spp. and Trichuris spp., were detected only in the feces of nonhuman primates. The recovered Capillaria spp. eggs presented slightly prominent opercula and striated shells, 75–96 μm in length and 66–99 μm in width, while the Trichuris spp. eggs presented prominent opercula and smooth shells measuring 74–81 μm by 37–44 μm. According to [9], Trichuris spp. have been the most frequently detected helminths in the feces of nonhuman captive primates in various countries. Lower detection rates than those in Rio Zoo have been reported in the zoos of Spain, Malaysia, Belgium, and Bangladesh, and at nonhuman primate breeding centers in Rio de Janeiro, Brazil [4, 6, 7, 9, 26]. Higher detection rates than those of this study were reported at the Pernambuco Zoo, in Brazil, and at nonhuman primate breeding centers in Africa [22, 27]. Comparisons with the literature could not be made regarding the prevalence of Capillaria spp. in captive apes in zoos, because none of articles retrieved from the literature described the detection of eggs of this parasite in the feces of these animals. It should be noted that both Trichuris spp. and Capillaria spp. eggs were larger than those of species that have previously been described parasitizing nonhuman primates, such as Trichuris trichiura, Aonchotheca (Armocapillaria) annulosa, Capillaria brochieri and Thominx platyrrhinorum. Therefore, these parasites have been diagnosed to the taxonomic category of genus, and may be another as yet undescribed nematode species, or correspond to cases of pseudoparasitism, in which animals ingest parasite eggs but do not actually become infected, possibly shedding these evolutionary forms still intact in their feces. However, the size of nematode eggs may have undergone changes due to parasitic adaptation of helminths to food fed to captive animals. Zoo animals are usually fed a pre-selected diet that includes industrialized foods, which they normally would not eat in the wild. This may be the case with the Rio Zoo primates, and the animals may actually be infected with Trichuris spp. and Capillaria spp. that have already been described infecting these animals.
Toxascaris leonina was also detected in this study, but only in the feces of a lion. Eggs of this nematode have been detected in the feces of lions in zoos located in other countries such as Italy, Bangladesh and Nigeria [6, 21, 30]. It should be pointed out that the eggs of the geohelminths, such as T. leonina, Capillaria spp. and Trichuris spp., are highly resistant to adverse environmental conditions and may remain viable for long periods in the environment. The high resistance of the eggs in the environment may be favorable for reinfections by these nematodes in the animal enclosures of the Rio Zoo. In addition, it should be noted that nematodes of the genus Trichuris are one of the most difficult gastrointestinal parasites to control in animals, since anthelmintics, which are very effective in eliminating adult forms of the parasite, are less effective against larvae, which penetrate deep into the cecal mucosa, making their exposure to the drugs difficult [39, 40].
Other helminths were also detected in the form of eggs of Physaloptera spp. in fecal matter from common marmosets, as well as eggs of the family Diphyllobothriidae in ocelot feces, of the family Dicrocoelidae in buffalo feces, and of the phylum Acanthocephala in mongoose feces. In general, the biological cycle of these parasites requires the participation of intermediate hosts. In the case of Physaloptera spp., these hosts would be insects such as crickets and beetles, and in the case of the family Diphyllobothriidae, such as the genera Spirometra or Diphyllobothrium, the cycle depends on aquatic copepods, freshwater fish, and in the case of infection by Spirometra spp., also on amphibians or snakes. Ramshorn snails and insects participate in the biological cycle of the family Dicrocoelidae; and intermediate hosts such as several arthropod species may also participate in the biological cycle of the parasites of the phylum Acanthocephala. According to [25], these intermediate hosts are not always present in zoo animal enclosures; therefore, the presence of these parasites indicates that such infections may actually have been acquired prior to captivity. As for the detection of cestodes of the family Diphyllobothriidae in the fecal matter of the ocelot, one cannot rule out the possibility that the infection may have been caused by feeding parasitized fish containing plerocercoid larvae to the animal or through the animal’s accidental ingestion of an amphibian or even a parasitized snake. The latter possibility, i.e., the accidental ingestion of an intermediate host, may also have occurred with species of the genus Physaloptera, the family Dicrocoelidae or the phylum Acanthocephala.
Most of the parasites identified in the fecal matter of captive animals have commonly been reported in zoos in various countries. Nevertheless, it should be noted that the biology of the evolutionary forms of these parasites may be directly influenced by local climate conditions; hence, it would be relevant to compare the results obtained here with those of other studies conducted in Brazilian zoos. However, unfortunately few articles were found the literature describing epidemiological studies of gastrointestinal parasites in captive animals of zoos in Brazil.
The finding of this study indicate that some animal species showed higher positivity rates and parasite diversity than others, which may have to do with their greater susceptibility to parasitic infection, the animal’s age, and the number of animals in the enclosures. Moreover, captivity may pose serious problems for animals under human care, not only because of the heightened stress caused by living in restricted spaces, which leads to the development of parasites, but also because self-infection may be constant due to the concentration of parasite evolutionary forms, making it difficult to eliminate the parasite from the animal and the environment [18, 26, 28].
Most parasitic infections in wild animals are asymptomatic, but the stress of captivity can render such infections symptomatic, leading to severe clinical conditions of diarrhea [28]. It is important to emphasize that several parasites identified in this study have a potential for zoonotic transmission, such as Giardia duodenalis, Cryptosporidium spp., the Entamoeba histolytica/Entamoeba dispar complex, and Balantioides coli, as well as helminths such as hookworms, strongylids and Trichuris spp., demonstrating that these parasites may have been transmitted to the animals due to their proximity to humans. On the other hand, they can be transmitted to the zoo animal handlers, who are not always aware of their exposure to the risk of infection by these agents [41]. Wild animals that are under human care in Zoos often have their diets modified to better meet the nutritional needs of the animals. However, if there is no sanitary care, the water and food provided can end up being a source of infection for animals. The supply of contaminated food and water should be considered one of the main causes of the infections mentioned here, since they are the sources of infection by gastrointestinal parasites most frequently reported in the literature. The environment and location of Rio Zoo generally favors the infection of animals, as it is in an urban area, near slums with poor sanitation. In addition, in the areas of the Zoo, it may be possible to observe loose animals such as rodents, bats mainly frugivorous, birds, prawns and even cats, which may also contribute to the spread of parasitic agents to captive wild animals kept in this institution. Besides that, according to [28], evolutionary forms of parasites can also be disseminated between enclosures of wild animals in captivity through footwear, clothing, hands and other fomites used by zookeepers, biologists and veterinarians. The lack of proper sanitation, i.e., the introduction of animals into enclosures that were inhabited by other animals without prior disinfection, has already been described by [36] as an important form of parasite transmission among zoo animals.
The findings of this study underscore the need to implement a zoo management program that includes routine diagnostic parasitology tests followed by specific treatment for each parasite, given that protozoans may be resistant to anthelmintic drugs. This program should prioritize the routine cleaning of animal enclosures, involving the removal of feces, sanitizing food and water containers with suitable disinfectants, and periodic sweeping with brooms. In such a program, priority should be given to effective sanitation and quarantine practices, filtered water supply, rodent control, and also rigorous hygiene of zookeeper clothing and of other fomites. In addition to sanitary management, zoos in general should enrich the environment of their animal enclosures to avoid stereotypic behaviors, such as the tendency of animals to ingest fecal matter from the floor due to boredom, favoring parasitic infections. The Rio Zoo is currently in a transitional phase involving several logistical changes, as well as the construction of buildings, etc., aimed at improving the health of its animals. It should be noted that this study was carried out with the consent of the zoo managers and of the company currently responsible for administering the Institution, who believe that the findings and discussion of this research may contribute to these improvements.
References
Iared VG, Tullio A, Oliveira HT (2012) Impressões de educadoras/es ambientais em relação à visitas guiadas em um zoológico. Revista Eletrônica Mestrado Educação Ambiental 28:258–273 (Portuguese)
Aragão OMG, Kazama R (2013) A função dos zoológicos nos dias atuais condiz com a percepção dos visitantes? Revista Educação Ambiental Ação 43:1–4 (Portuguese)
Muoria PK, Muruthi P, Rubenstein D, Oguge NO, Munene E (2005) Cross-sectional survey of gatrointestinal parasites of Grevey’s zebras in sourthen Samburu, Kenya. Afr J Ecol 43:392–395
Lim LAY, Ngui R, Shukri J, Rohela M, Naim MRH (2008) Intestinal parasites in various animals at a zoo in Malaysia. Vet Parasitol 157:154–159
Geraghty V, Mooney J, Pike K (1982) A study of parasitic infections in mammals and birds at the Dublin Zoological Garden. Vet Res Commun 5:343–348
Khatun M, Begum N, Mamun A, Hussain M, Azam S (2014) Coprological study of gastrointestinal parasites of captive animals at Rangpur recreational garden and zoo in Bangladesh. J Threatened Taxa 6:6142–6147
Levecke B, Dorny P, Geurden T, Vercammen F, Vercruysse J (2007) Gastrointestinal protozoa in non-human primates of four zoological gardens in Belgium. Vet Parasitol 148:236–246
Johnson-Delaney CA (2009) Parasites of captive nonhuman primates. Vet Clin N Am 12:563–581
Cordón PG, Prados HA, Romero D, Moreno SM, Pontes A, Osuna A, Rosales JM (2008) Intestinal parasitism in the animals of the zoological garden “Peña Escrita” (Almuñecar, Spain). Vet Parasitol 156:302–309
RioZoo. Zoológico do Rio de Janeiro (2016) Conheça, Histórico. http://www.riozoo.com.br/conheca-o-riozoo/. Acessed on Jan 26, 2018 (Portuguese)
Ritchie LS (1948) An ether sedimentation technique for routine stool examinations. Bull US Army Med Dep 8:8–326
Young KH, Bullock SL, Melvin DM, Spruill CL (1979) Ethyl acetate as a substitute for diethyl ether in the formalin-ether sedimentation technique. J Clin Microbiol 10:853
Faust EC, D’antoni JS, Odon V, Miller MJ, Perez C, Sawitz W, Thomen LF, Tobie JE, Walker JH (1938) A critical study of clinical laboratory technics for the diagnosis of protozoan cysts and helminth eggs in feces. I. Preliminary communication. Am J Tropical Med Hygiene 18:169–183
Sheather AL (1923) The detection of intestinal protozoa and mange parasites by a flotation technic. J Comp Pathol Ther 36:266–275
Huber F, Bonfim TC, Gomes RS (2003) Comparação da eficiência da técnica de sedimentação pelo formaldeído-éter e da técnica de centrífugo-flutuação modificada na detecção de cistos de Giardia sp. e oocistos de Cryptosporidium sp. em amostras fecais de bezerros. Rev Bras Parasitol Vet 12:135–137 (Portuguese)
Baxby D, Blundell N, Hart CA (1984) The development and performance of a simple, sensitive method for the detection of Cryptosporidium oocysts in faeces. J Hygiene 93:317–323
Lutz AO (1919) Schistosomum mansoni e a shistosomatose segundo observações feitas no Brasil. Mem Inst Oswaldo Cruz 11:121–155 (Portuguese)
Snak A, Agostini MK, Lenzi FP, Montanucci RC, Delgado EL, Zabott VM (2017) Perfil parasitológico de mamíferos silvestres cativos. Vet Zootecnia 24:193–200 (Portuguese)
Thawait KV, Maiti KS, Dixit AA (2014) Prevalence of gastro-intestinal parasites in captive wild animals of Nandan Van Zoo, Raipur, Chhattisgarh. Vet World 7:448–451
Varadharanjan A, Kandasamy A (2000) A survey of gastro-intestinal parasites of wild animals in captivity in the V.O.C Park and Mini Zoo, Coimbatore. Zoo’s Print J 15:257–258
Fagiolini M, Lia PR, Laricchiuta P, Cavicchio P, Manella R, Cafarchia C, Otranto D, Finotello R, Perrucci S (2010) Gastrointestinal parasites in mammals of two Italian zoological gardens. J Zoo Wildl Med 41:662–670
Freitas LFM, Oliveira BJ, Cavalcanti BDM, Oliveira AR, Sobrinho EA (2001) Perfil coproparasitológico de mamíferos silvestres em cautiverio em el estado de Pernambuco, Brasil. Parasitol al Día 25:1–6 (Spanish)
Delgado E, Fonseca PI, Fazendeiro I, Matos O, Antunes F, Cunha BM (2003) Cryptosporidium spp. in ruminants at the Lisbon Zoo. J Zoo Wildlife Med 34:352–356
Beck R, Sprong H, Bata I, Lucinger S, Pozio E, Caccio MS (2011) Prevalence and molecular typing of Giardia spp. In captive mammals at the zoo of Zagreb, Croatia. Vet Parasitol 175:40–46
Aranda RC, Serrano-Martínez E, Tantaleán VM, Quispe HM, Casas VG (2013) Identificación y frecuencia de parasitos gastrointestinales em félidos silvestres em cautiverion em el Perú. Rev Investig Vet Perú 24:360–368 (Spanish)
Barbosa SA, Pissinatti A, Dib VL, Siqueira PM, Cardozo LM, Fonseca MBA, Oliveira BA, Silva AF, Uchôa CMA, Bastos PMO, Amendoeira RRM (2015) Balantidium coli and other gastrointestinal parasites in captives non-human primates of the Rio de Janeiro, Brazil. J Med Primatolol 44:18–26
Munene E, Otsyula M, Mbaabu DAN, Mutahi WT, Muriuki SMK, Muchemi GM (1998) Helminth and protozoan gastrointestinal tract parasites in captive and wild-trapped African non-human primates. Vet Parasitol 78:195–201
Mir QA, Dua K, Singla DL, Sharma S, Singh PM (2016) Prevalence of parasitic infection in captive wild animals in Bir Moti Bagh mini zoo (Deer Park), Patiala, Punjab. Vet World 9:540–543
Adeniyi IC, Morenikeji OA, Emikpe BO (2015) The prevalence of gastro-intestinal parasites of carnivores in university zoological gardens in South West Nigeria. J Vet Med Anim Health 7:135–139
Verweij JJ, Vermeer J, Brienen EAT, Blotkamp C, Laeijendecker D, Lieshout L, Polderman AM (2003) Entamoeba histolytica infections in captive primates. Parasitol Res 90:100–103
Schuster FL, Ramirez-Ávila L (2008) Current world status of Balantidium coli. Clin Microbiol Rev. 21:626–638
Kim JCS, Abee CR, Wolf RH (1978) Balantidiosis in a chimpanzee (Pan troglodytes). Lab Animal 12:231–233
Lankester F, Mätz-Tensing K, Jensen SA, Weiss S, Leendertz FH (2008) Fatal ulcerative colitis in a western lowland gorilla (Gorilla gorilla gorilla). J Med Primatol 37:297–302
Tayib OA, Abdoum KA (2013) Balantidium coli infections in Hamadryas baboon in Saudi Arabia: a case report. J Anim Plant Sci 23:940–943
Gilioli R, Silva AF (2000) Frequency of parasites and Salmonella infection in captive maned-wolf, Chrysocyon brachyurus, kept in Zoos at the State of São Paulo, Brazil. Arqu Bras Med Vet Zootecnia 52:1–5
Müller-Graf CDM, Woolhouse MEJ, Packer C (1999) Epidemiology of an intestinal parasite (Spirometra spp.) in two populations of African lions (Panthera leo). Parasitology. 118:407–415
Peisert W, Taborski A, Pawlowski Z, Karlewiczowa R, Zdun M (1983) Giardia infection in animals in Porznan Zoo. Vet Parasitol 13:183–186
Barriga OO (1995) Veterinary Parasitology. Editora Greyden Press, Columbus
Hendrix CM, Blagburn BL, Lindsay DS (1987) Whipworms and intestinal Threadworms. Vet Clin N Am 17:1355–1375
Santos SMP, Silva NGS, Fonseca FC, Oliveira BJ (2015) Parasitos de aves e mamíferos silvestres em cativeiros no estado de Pernambuco. Pesq Vet Bras. 35:788–794
Echarte GV, Fernández YES, Augusto AM, Santos ALC, Dantas MML, Iraola RC, Amendoeira MRR (2019) Assessment professional competence and risk factors perception of Toxoplasma gondii at the Cuba National Zoo Park and Zoo Garden of Rio de Janeiro, Brazil. Rev Ciência Vet e Saúde Públ 6:235–240
Acknowledgements
We gratefully acknowledge the Rio de Janeiro Zoo for providing scientific material and technical support for this study. We are also grateful for the support provided by the Oswaldo Cruz Institute (IOC) and the postgraduate course in Tropical Medicine (IOC/Fiocruz), Office of the Vice-Chancellor for Research and Innovation of Fluminense Federal University (PROPPI-UFF).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Barbosa, A.S., Pinheiro, J.L., dos Santos, C.R. et al. Gastrointestinal Parasites in Captive Animals at the Rio de Janeiro Zoo. Acta Parasit. 65, 237–249 (2020). https://doi.org/10.2478/s11686-019-00145-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11686-019-00145-6