Abstract
Trichuris spp. infect the majority of captive primate species along with an estimated 1049 million people worldwide, making it an important zoonosis [Stephenson, L. S., Holland, C. V., & Cooper, E. S. Parasitology, 121(Suppl.), S73–S95, 2000]. We investigated the efficacy of methods used to evaluate the prevalence of Trichuris spp. in 2 groups (n = 12) of socially housed Abyssinian colobus (Colobus guereza kikuyensis) at Paignton Zoo Environmental Park and the factors that may affect density. We collected individual and group fecal samples over 6 mo and estimated burden (egg counts/g of feces) of Trichuris spp. via the McMaster technique. Shedding was significantly higher in the afternoon than in the morning (matched-pairs t-test: t [5] = −4.46, p < 0.01) and in dominant adult male colobus (Spearman rank: r [5] = −0.94, p < 0.01; age: r [5] = 0.89, p < 0.05). Parasitological studies of zoo-housed primates can be a useful tool to explore factors that may affect burdens of Trichuris spp. in them.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trichuris spp. are parasitic nematodes that infect the ceca and colons of a variety of mammalian species. The species that infects simians is believed to be Trichuris trichiura, which researchers have identified in Macaca fascicularis (Dinh Son 2002), Macaca mullatta (Taylor et al. 1994), and other Old and New World primate species. Trichuris trichiura is easily transmitted between humans and nonhuman primates and infects an estimated 1049 million people worldwide, making it an important zoonosis (Stephenson et al. 2000). Wild-living primates infected with Trichuris spp. are a threat to human well-being, directly and indirectly (Muriuki et al. 1998).
Trichuris trichiura has a simple, direct life cycle. After copulation, each female lays 2000–10,000 eggs/d (Urquhart et al. 1988), which are passed in the host’s feces and can be counted to give a relative burden of Trichuris. Reinfection occurs by ingestion of embryonated eggs, after which the larvae migrate to the intestine. Once mature, the anterior of the worm burrows into the intestinal mucosa, where it ingests cellular secretions. Blood loss due to damage of the intestinal epithelium may cause a variety of symptoms including anaemia, colitis, and growth retardation (Schmidt and Roberts 1985).
Researchers have studied the relationship between Trichuris spp. and a range of nonhuman primate (NHP) host species (Pan paniscus, Hasegawa et al. 1983; Papio anubis, Emikpe et al. 2002; Macaca mulatta, Phillippi and Clarke 1992; Macaca fascicularis, Janagi 1981). Fatal burdens of Trichuris spp. also occur (Colobus guereza, Loomis and Wright 1986; Papio anubis, Emikpe et al. 2002).
The study of NHP parasitology in zoos is both interesting and important, in terms of human and NHP health and welfare and also to broaden our understanding of the parasite-host relationship, which can affect nutrition and behavior. As a source of prolific zoonoses, we need to monitor incidence and density of Trichuris spp. to limit human health risks and ensure good health in our animals. Consequently, we require knowledge of individual differences in burdens of Trichuris spp. and the factors that may affect them, which we can achieve only with an accurate and reliable sampling technique.
We evaluated the parasite burden of Colobus guereza at Paignton Zoo via the McMaster technique 3 times/yr, in-house, and had it validated by an outside laboratory. The method that zoos commonly use involves collecting a group fecal sample from several feces in different areas in the enclosure (Goossens et al. 2004). We report on the periodic and individual variation in burden of Trichuris spp. and compare estimations of burden of Trichuris spp. via 2 fecal sampling methods (group vs. individual).
Methods and Results
Subjects, Housing, and Husbandry
Two groups of Colobus guereza kikuyuensis live at Paignton Zoo Environmental Park, Devon, UK. There is no physical contact between the groups. Group A consists of 8 individuals: 3 adult males (>4 yr at start of study), 3 juvenile males (<4 yr), and 2 adult females (>4 yr), one of which was lactating and pregnant. Group B comprises 4 females: 2 adults and 2 juveniles.
Both groups have 24-h access between a sheltered indoor area (A, 10.0 × 5.0 × 2.5 m; B, 5.0 × 3.0 × 2.5 m) and an enclosed outside area (A, 10.0 × 5.0 × 5.0 m; group B, 5.0 × 10.0 × 4.0 m). Both inside areas have wooden perches, Perspex feeding tables, and cement floors with a thin layer of shavings; outdoor floors consist of a deep litter system of bark for A and grass for B.
Keepers clean inside areas daily (0830 h), replace floor shavings, and wipe fixtures with Annihilate A.C.R.® (Hydra International, Milton Keynes), using the same cleaning tools in both enclosures. Outside enclosures are spot cleaned. Keepers feed all subjects a mixed diet of leafy vegetables, tubers, and occasional nuts and fruit.
Sample Collection and Analyses
We collected data between October 2002 and March 2003 opportunistically when feces were voided; hence the number of samples available varied. We marked feces voided inside that had not touched other feces on a map of the enclosure and collected them in labeled (individual’s name and date) plastic pots before storage at −5°C. We estimated mean egg count/g feces (epg) values of Trichuris spp. via the McMaster technique, using 2 slides prepared from each fecal sample (Urquhart et al. 1988). All statistical analyses are 2-tailed and non-normal data are log transformed.
Fecal Collection 1: Time of Day Effects
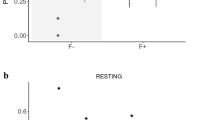
We collected feces from group A at 1100–1200 h and 1400–1500 h. Estimated mean epg are significantly lower in feces collected in the morning compared to the samples collected in the afternoon (matched-pairs t-test: t [5] = −4.46, p < 0.01; Fig. 1). To ensure consistency, we collected all future fecal samples during the afternoon.
Fecal Collection 2: Seasonal Effects
We collected feces in autumn (October–November; group A, n = 7 collected over 28 d, group B, n = 8 collected over 14 d) and spring (February–March; n = 17 collected over 50 d, n = 10 collected over 46 d).
There is no significant difference between the estimated mean epg in autumn and spring (matched pairs t-test: t [11] = 0.74, p > 0.05), so we pooled autumn and spring data sets for all further analyses.
Fecal Collection 3: Group versus Individual Sampling Technique
We collected feces from the individuals, per the method above, and from the group, per the method the zoo used. We collated the estimated daily mean epg values for all individuals to provide a collective individual mean (CI) and compared it with the mean epg estimated from the group samples.
There is no significant difference between the mean epg estimated from the CI or the group (A matched-pairs t-test: t [17] = 0.001, p > 0.05; B matched-pairs t-test: t [23] = 0.091, p > 0.05; Fig. 2). There is also no significant correlation between the 2 estimated mean epg values (A Pearson: r [17] = 0.36, p > 0.05; B Pearson: r [23] = −0.029, p > 0.05).
Individual Variation in Parasite Burden, Including Behavioral Data
There is considerable individual variation in epg for the 12 colobus. Beattie’s (adult female, group B) burden was ≥5 times greater (11,329 mean epg) than that of the other females (2780 mean epg; Fig. 2). Because Beattie’s epg was greatly different and more variable than that of the other females, we considered her an outlier.
We established that an interaction between age and sex significantly influenced mean epg (2-way ANOVA: age, F [1,10] = 12.01, p < 0.01; age*sex, F [1,10] = 7.4, p < 0.05, Fig. 3). Adult males have a significantly higher estimated mean epg, compared to adult females and juveniles. We observed the males in group A via continuous focal sampling (Martin and Bateson 1994) for 15 min (n = 6 subjects, 12 sessions/subject). All displacement behaviors are noted, where displacement is “movement of one animal (displacer) towards another stationary animal, causing the stationary animal (displacee) to move away.” Females are excluded because published literature suggests they do not form hierarchies (Grunau and Kuester 2001). We created a dominance hierarchy for the male colobus, by ordering them according to how many other males they supplant. There are significant correlations between male estimated mean epg and dominance rank, mass in kg, and age in yr (Spearman rank: r [5] = −0.94, p < 0.01; mass: r [5] = 0.93, p < 0.01; age: r [5] = 0.89, p < 0.05; Fig. 3).
Discussion
We outline some of the factors that affect the burden of Trichuris spp. in zoo-housed colobus. Our data indicate that time of day and individual differences are associated with significant variations in epg of Trichuris spp.
Previous researchers have noted periodicity in helminth transmission, e.g., with Schistosoma a circadian pattern occurs when cercariae are voided from their snail hosts and again when the matured eggs of Schistosoma are voided from their human hosts (Bogea et al. 1996; Doehring et al. 1983). For some helminths the periodicity enhances transmission under favorable conditions (Fingerut et al. 2003). For example, microfilarial numbers of Brugia malayi and Wuchereria bancrofti increase in the peripheral blood in synchrony with the biting behavior of local mosquitoes (Maizels and Kurniawan-Atmadja 2002; Shriram et al. 2005). Because Trichuris spp. require time in soil to become infective, it is unclear why shedding more eggs in the afternoon compared to the morning enhances transmission.
Equally unclear is the stimulus (zietgeber) that triggers the circadian pattern, which could be naturally occurring, including light/dark cycles or temperature (Sharma and Chandrashekaren 2005), or an artefact of the zoo environment, e.g., interactions with keepers, cleaning, or feeding times. It is unlikely that one can attribute feeding times or food composition to the pattern. Edwards and Ullrey (2001) noted that the passage rate for liquids and solids through the gut for Colobus guereza kikyuensis is >30 h and relatively unaffected by fiber content. Researchers have reported similar findings from other tripartite foregut fermenters (NRC 2003).
We noted no seasonal variation in burden of Trichuris spp., which favor wet conditions (Maipanich et al. 1998). Changes in climate may not be sufficiently different across seasons— southwest England is mild throughout the year— or captive husbandry negated any seasonal change in climate, e.g., daily cleaning.
Mean epg estimations calculated from individually identified fecal samples do not correlate with or are significantly different from group samples from around the enclosure. Both sampling methods provide comparable information about the colobus group’s mean burden of Trichuris spp., probably attributable to large intra- and interindividual epg variation. Practically, collecting group fecal samples provided adequate information about the colobus group’s burden of Trichuris spp., but information about the variability of the burden was missing.
Individual differences significantly affected the burden of Trichuris spp. Adult male colobus have significantly higher worm burdens than those of juvenile males and females. We located no comparable datum for wild Colobus guereza; however, Gillespie et al. (2005) noted that the prevalence of Trichuris spp. in wild Colobus guereza was not related to age or sex classes. Researchers in previous studies documented that burdens of Trichuris spp. are significantly higher in children than in adults, which they suggest is a consequence of age-dependent immunity response rather than decreased exposure with age (Macaca mulatta, Knezevich 1998; Homo sapiens, Bundy et al. 1991).
The burdens of Trichuris spp. we estimated may be due to social rank; male social dominance correlates strongly positively with burden of Trichuris spp. Unfortunately, male age, dominance rank, and weight are all confounding variables; older males were heavier and of higher rank. Hausfater and Watson (1976) also noted that dominant male free-ranging olive baboons (Papio anubis) and mid-ranking females shed more ova than low-ranking individuals did. Hence it is possible that in our study, burden of Trichuris spp. was not age-dependent but due to dominance.
Dominance hierarchies are associated with social stress: physiological consequences of social interactions (Sapolsky 2005). Low- and high-ranking individuals have experienced the deleterious ramifications of social stress, though their manifestations are likely to vary according to species (social system) or individual differences (Abbott et al. 2006). Researchers have associated high parasite burdens with impaired immune system function and other indications of poor health (Bradley and Jackson 2004). We suggest that worm burden provides an indication of social stress because females and juvenile males that would not be vying for dominance had low burdens of Trichuris spp. relative to those of adult males. Ecologically, one would expect dominant males to sire more offspring, which may compensate for impaired health status due to a higher worm burden (Krebs and Davis 1997).
In summary, this study outlines factors that affect Trichuris spp. burden in zoo-housed colobus. Our data suggests that time of day affected the level of eggs shed, which is likely to be a consequence of husbandry. Individual differences are also apparent in Trichuris spp. burdens, which the authors believe reflects underlying social stress; male rank is positively correlated with Trichuris spp. burden. Finally, estimations of Trichuris spp. epg from individually identified or group collected faecal samples provided comparable information for managing a preventive health programme.
Conclusion
We outlined factors that affect the burden of Trichuris spp. in zoo-housed colobus. Our data suggest that time of day affects the level of eggs shed, which is likely a consequence of husbandry. Individual differences are also apparent in burdens of Trichuris spp., which we believe reflects underlying social stress; male rank correlates positively with burdens of Trichuris spp. Finally, estimation of epg of Trichuris spp. from individually identified or group-collected fecal samples provides comparable information for managing preventative health programs.
References
Abbott, D. H., Keverne, E. B., Bercovitch, F. B., Shively, C. A., Mendoza, S. P., Saltzman, W., Snowdon, C.T., Ziegler, T.E., Banjevic, M., Garland, T. Jr., Sapolsky, R.M. (2006). Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Hormones and Behavior, 43 (1), 67–82.
Bogea, T., Favre, T. C., Rotenberg, L., Silva, H. S., & Pieri, O. S. (1996). Circadian patterns of cercarial emergence in Schistosoma mansoni (Platyhelminthes: Digenea) from isolated Biomphalaria glabriata. Chronobiology International, 13, 93–101.
Bradley, J. E., & Jackson, J. A. (2004). Immunity, immunoregulation and the ecology of trichuriasis and ascariasis. Parasite Immunology, 26(11–12), 429–441.
Bundy, D. A. P., Lillywhite, J. E., Didier, J. M., Simmons, I., & Bianco, A. E. (1991). Age-dependency of infection status and serum antibody levels in human whipworm (Trichuris trichiura) infection. Parasite Immunology, 13, 629–638.
Dinh Son, V. (2002). Intestinal parasites of Macaca fascicularis in a Mangrove Forest, Ho Chi Minh City, Vietnam. Laboratory Primate Newsletter, 41, 9–10.
Doehring, E., Feldmeier, H., & Daffalla, A. A. (1983). Day-to-day variation and circadian rhythm of egg excretion in urinary schistosomiasis in the Sudan. Annals of Tropical Medicine and Parasitology, 77, 587–594.
Edwards, M. S., & Ullrey, D. E. (2001). Effect of dietary fiber concentration on apparent digestibility and digesta passage in non-human primates. II. Hindgut- and foregut-fermenting folivores. Zoo Biology, 18, 537–549.
Emikpe, B. O., Ayoade, G. O., Ohore, O. G., Olaniyan, O. O., & Akusu, M. O. (2002). Fatal trichuriosis in a captive baboon (Papio anubis) in Ibadan Nigeria: A case report. Tropical Veterinarian, 20, 36–39.
Fingerut, J. T., Zimmer, C. A., & Zimmer, R. K. (2003). Patterns and processes of larval emergence in an estuarine parasite system. Biological Bulletin, 205, 110–120.
Gillespie, T. R., Greiner, E. C., & Chapman, C. A. (2005). Gastrointestinal parasites of the colobus monkeys of Uganda. Journal of Parasitology, 91, 569–573.
Goossens, E., Dorny, P., Vercammen, F., & Vercrysse, J. (2004). A 12-month survey of the gastro-intestinal helminths of antelopes, gazelles and giraffids kept at two zoos in Belgium. Veterinary Parasitology, 127, 303–312.
Grunau, T., & Kuester, J. (2001). Dominance style in female guerezas (Colobus guereza Ruppell). Primates, 42, 301–307.
Hasegawa, H., Takayoshi, K., & Mulavwa, M. (1983). A parasitological survey on the faeces of pygmy chimpanzees, Pan paniscus, at Wamba, Zaire. Primates, 24, 419–423.
Hausfater, G., & Watson, D. F. (1976). Social and reproductive correlates of parasitic ova emissions by baboons. Nature, 262, 688–689.
Janagi, T. S. (1981). A report of gastro-intestinal helminth parasites found in Macaca fascicularis in peninsular Malaysia. Malaysia Applied Biology, 10, 99–100.
Knezevich, M. (1998). Geophagy as a therapeutic mediator of endoparasitism in a free-ranging group of rhesus macaques (Macaca mulatta). American Journal of Primatology, 44, 71–82.
Krebs, J., & Davis, N. (1997). Behavioural ecology: An evolutionary approach. Oxford, UK: Blackwell Publishing.
Loomis, M. R., & Wright, J. F. (1986). Gastric trichuriasis in a black and white colobus monkey. Journal of the American Veterinary Medical Association, 189, 1214–1215.
Maipanich, W., Visedsuk, K., Muennoo, C., Sanguankiat, S., Yoonuan, T., Pubampen, S., et al. (1998). Soil-transmitted helminths: Source and distribution of the infective stages in Southern Thailand. Journal of Tropical Medicine and Parasitology, 21, 31–36.
Maizels, R. M., & Kurniawan-Atmadja, A. (2002). Variation and polymorphism in helminth parasites. Parasitology, 125, S25–S37.
Martin, P., & Bateson, P. (1994). Measuring behaviour: An introductory guide (pp. 74–76). Cambridge, UK: Cambridge University Press.
Muriuki, S. M. K., Murugu, R. K., Munene, E., Karere, G. M., & Chai, D. C. (1998). Some gastro-intestinal parasites of zoonotic (public health) importance commonly observed in old world non-human primates in Kenya. Acta Tropica, 71, 73–82.
NRC (National Research Council) (2003). Feeding ecology, digestive strategies, and implications for feeding programs in captivity. In: Nutrient Requirement of Nonhuman primates (2nd ed.) (pp. 5–40). Washington, DC: The National Academies Press.
Phillippi, K. M., & Clarke, M. R. (1992). Survey of parasites of rhesus monkeys housed in small social groups. American Journal of Primatology, 27, 293–302.
Sapolsky, R. M. (2005). The influence of social hierarchy on primate health. Science, 308, 648–652.
Schmidt, G., & Roberts, L. (1985). Orders Trichurata and Dioctophymata: Aphasmidian parasites. In: Foundations of Parasitology (3rd ed.) (pp. 450–452). St. Louis: Times Mirror/Mosby College Publishing.
Sharma, V. K., & Chandrashekaren, M. K. (2005). Zeitgebers (time cues) for biological clocks. Current Science, 89, 1136–1146.
Shriram, G. N., Ramiah, K. D., Krishnamoorthy, K., & Sehgal, S. C. (2005). Diurnal pattern of human-biting activity and transmission of subperiodic Wuchereria bancrofti (Filariidae: Dipetalonematidae) by Ochlerotatus niveus (Diptera: Culicidae) on the Andaman and Nicobar Islands of India. American Journal of Tropical Medical Hygiene, 72, 273–277.
Stephenson, L. S., Holland, C. V., & Cooper, E. S. (2000). The public health significance of Trichuris trichiura. Parasitology, 121(Suppl.), S73–S95.
Taylor, L., Lessnau, R. G., & Lehman, S. M. (1994). Prevalence of whipworm (Trichuris) ova in two free-ranging populations of rhesus macaques (Macaca mulatta) in the Florida Keys. Florida Scientist, 57, 102–107.
Urquhart, J. W., Armour, J., Duncan, A. M., & Jennings, F. W. (1988). McMaster’s technique. In: Veterinary Parasitology (pp. 270–271). London, UK: Longman.
Acknowledgments
We thank the staff of Paignton Zoo for facilitating the study, notably Ghislaine Sayers, Sheona McGovern, and Kelly Elford. We also thank Neil Bemment, Julian Chapman, Lisa Doran, and Tony Dobbs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melfi, V., Poyser, F. Trichuris Burdens in Zoo-Housed Colobus guereza . Int J Primatol 28, 1449–1456 (2007). https://doi.org/10.1007/s10764-007-9206-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-007-9206-9