Abstract
To further establish species determination using the muscle attachment site (MAS) pattern method, third instar larvae of five forensically important species of Sarcophaga Meigen were investigated: Sarcophaga argyrostoma (Robineau-Desvoidy), Sarcophaga caerulescens Zetterstedt, Sarcophaga melanura Meigen, Sarcophaga albiceps Meigen and Sarcophaga similis Meade. As in the previously investigated Calliphoridae, patterns were found to be species specific. The main feature of the Sarcophaga patterns is the divided central horizontal row of segment four. A genus pattern was established to be used as base for comparison in further species determination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fly family Sarcophagidae, also known as flesh flies, is globally distributed and comprises almost 3000 species, subdivided into only three subfamilies (Pape 1996). The taxon is monophyletic, although internal relationships among subfamilies are still unresolved (Piwczyński et al. 2014). Most species of medical and veterinary importance belong to the traditionally recognized subfamilies Sarcophaginae and Paramacronychiinae and are scavengers, coprophages, or myiasis agents. The third subfamily, Miltogramminae, contains predominantly kleptoparasites of solitary wasps and bees, with a few scavengers, reptilian egg predators, and true parasites of adult insects (Pape 1996; Szpila et al. 2010).
Most flesh flies considered of high forensic importance are species belonging to the genus Sarcophaga Meigen, 1826 (Povolný and Verves 1997; Szpila et al. 2015a). In Central Europe, a large carrion attracts a relatively high number of species of adult flesh flies but only few are successful in colonizing this breeding substrate (Szpila et al. 2015a). Successional experiments and forensic case reports indicate Sarcophaga argyrostoma, S. caerulescens, S. melanura, and S. similis to be the most successful species in colonizing a large animal carrion and human corpses (Anton et al. 2011; Cherix et al. 2012; Grassberger and Frank 2004; Mądra et al. 2015; Matuszewski et al. 2013; Pohjoismäki et al. 2010; Szpila et al. 2015a). In Mediterranean parts of Europe, this list would include several additional species (Szpila et al. 2015a).
Unfortunately, species determination for Sarcophaga is especially difficult in larvae (Szpila et al. 2015b) as they are typical maggots with a narrow morphological diversity (Pape 1987). Forensic entomology manuals recommend mass rearing of collected larvae to adults or the use of molecular methods (Byrd and Castner 2009; Smith 1986). Two recent publications partly solve this problem at least for the European ecozone by providing a large amount of new information about larval stages of flesh flies (Richet et al. 2011) and a key to third instar larvae of forensically important species (Szpila et al. 2015b). Characters proposed for determination of Sarcophaga larvae, however, are often subtle and may pose problems for unexperienced users. Therefore, new and easy methods for species identification in larval Sarcophagidae are still required.
The method of muscle attachment site (MAS) patterns proved to be powerful in species determination of forensically important Calliphoridae larvae (Niederegger and Spieß 2012; Niederegger and Spieß 2014; Niederegger et al. 2015). The aim of this study was to further establish the method for five of the most frequent visitors and successful colonizers of large carrions: S. argyrostoma, S. caerulescens, S. melanura, S. albiceps, and S. similis.
Materials and methods
Animals
Adult females were attracted to decomposing pig or chicken liver and collected by sweep net. Detailed data regarding collecting locations in Poland are presented in Szpila et al. (2015b). The first instar larvae were obtained by gently squeezing the abdomen of living females. Larvae from different females were reared separately at room temperature in plastic containers (150 ml) with small portions of pig or chicken liver (20–30 g) as feeding medium. When the larvae reached the post-feeding stage of the third instar, they were killed by dousing with boiling water and stored in 70% ethanol.
Unambiguous species identification was possible by breeding some larvae of each species to the adult form. Obtained male specimens were identified using keys of Pape (1987), Povolný and Verves (1997), and Richet et al. (2011). All maternal females and laboratory-bred adult specimens were labeled and are available as voucher specimens in the insect collection of the Chair of Ecology and Biogeography, Faculty of Biology and Environmental Protection, Nicolaus Copernicus University in Toruń, Poland.
Preparation
The larvae were measured to the nearest 0.1 mm using a dissecting microscope (Zeiss Stemi 2000C) with digital camera (Zeiss AxioCam ICc1) and measuring software (AxioVision). All preparation and evaluation steps leading to the condensed patterns were performed as given in our previous publications (Niederegger et al. 2013; Niederegger and Spieß 2012).
Data evaluation
All rows in segments 2–4 were labeled according to our previous publication on Lucilia Robineau-Desvoidy species (Niederegger et al. 2015), except for 4.1. This part of the MAS pattern is doubled in comparison to Lucilia and was thus labeled 4.1a and 4.1b (Fig 1). The patterns were evaluated using Inkscape (freeware) and Adobe Photoshop; means and standard deviations were calculated using Microsoft Excel 2010.
Results
Transversal muscles of brachyceran larvae are attached to the inside of the cuticula in bilaterally symmetrical rows. These muscle attachment site (MAS) rows can be stained and subsequently easily distinguished. The range of MAS for all three hemi segments was quite vast (Table 1). The lowest total number of MAS was found in an individual of S. albiceps with 127 MAS, and the highest number was found in S. caerulescens with 202 MAS. The smallest individuals investigated belonged to S. melanura with an average size of 13.04 mm, closely followed by S. similis with 13.31 mm; S. albiceps had a mean size of 15.56 mm and S. caerulescens with 17.08 mm and S. argyrostoma with an average length of 18.43 mm were the largest (Table 1).
The average number of MAS per hemi segment over all individual larvae investigated was 44 (±8) for segment 1, 54 (±8) for segment 2, and 62 (±10) for segment 4 (Table 2). Because the numbers of MAS in pattern rows are very similar in most cases, deviations (framed in Table 2) can be of important assistance for species determination.
Most deviations showed three or four MAS above the average genus count. Predominantly, they were found in S. argyrostoma, which comprised the largest larvae investigated. Interestingly, one such deviation was found in S. melanura, comprising the smallest larvae. Only two deviations with three MAS less than the average genus count were found (Table 2).
The genus pattern, generated for the five species of the genus Sarcophaga investigated in this study, is the base of comparison for the researcher in order to further determine the species once the genus is defined.
Genus pattern (n = 50)
The genus pattern (Fig. 1) contains no MAS at position 2.1 at the center of segment 2. Rows 2.2, 2.3, 2.4, and 2.5 are short vertical rows with slight curves in the middle like closing brackets. Rows 2.4 and 2.5 are positioned more anteriorly, giving the pattern a step-like appearance.
In segment 3, the central rows 3.1 are pointing their convex parts at each other like inverted brackets while rows 3.2 enclose the two small ones like big closing brackets. Rows 3.3 are almost straight with an angle of about 30° to the midline. 3.4 is z-shaped on the left hemi segment and s-shaped on the right hemi segment, whereas 3.5 again follows the bracket-like shaping of the rows in segment 2. The step-like appearance is maintained as in segment 2.
Compared to the genus pattern found for Lucilia in a previous study (Niederegger et al. 2015), the pattern for central horizontal row of segment 4 is divided; the parts where therefore named 4.1a and 4.1b. 4.1a comprises two short mirrored horizontal rows at the center. 4.1b is composed of two short mirrored horizontal rows divided by a small horizontal row in the middle. 4.2 is the only divided vertical row in the genus pattern, where the top part is more curved than the bottom part. 4.3 is an L-shape positioned in the lower part of the segment. 4.4 and 4.5 are almost mirrored bended vertical rows but 4.4 is positioned higher in the segment than 4.5. The step-like appearance is maintained in this segment as well.
Sarcophaga (Parasarcophaga) albiceps Meigen, 1826 (n = 10)
All rows of segment 2 were shorter in S. albiceps (Fig. 2) compared to the genus pattern. Rows 2.2 were bent rather than just curved, as were rows 3.2. The other rows of segment 3 were more or less concordant to the genus pattern when both hemispheres were taken into account. In segment 4, rows 4.3 were less curved than the L-shapes of the genus pattern and rows 4.4 were longer throughout all larvae investigated.
Sarcophaga (Pandelleisca) similis Meade, 1876 (n = 10)
At the first glance, several rows seemed to differ in S. similis (Fig. 3) compared to the genus pattern. Over all larvae investigated, however, most differences were not consistent on both hemispheres and were therefore omitted. Only one difference was present in both hemispheres and in all larvae investigated: rows 4.2 were not divided in S. similis which is a distinctive discrepancy compared to the genus pattern.
Sarcophaga (Liopygia) argyrostoma (Robineau-Desvoidy, 1830) (n = 10)
S. argyrostoma (Fig. 4) had 2.1 rows, or dots rather. In 9 of 10 larvae 2.1, on each side consisted of one MAS point and one larva lacked them entirely. Furthermore, there were divisions in rows 2.4 in both hemispheres. Similar disruptions could be found in rows 3.4, where the upper part of the row corresponded to the 3.4 row of the genus pattern, but an additional small row part was found below. Overall, most rows were longer than in the genus pattern.
Sarcophaga (Helicophagella) melanura Meigen, 1826 (n = 10)
In S. melanura, (Fig. 5) most of the rows seem a tad shorter than in the genus pattern. In the second segment, rows 2.3 had an additional small piece above the main row in half of the larvae. Rows 3.4 lacked the bending into an s- or z-shape and presented themselves as short straight rows instead.
Sarcophaga (Robineauella) caerulescens Zetterstedt, 1838 (n = 10)
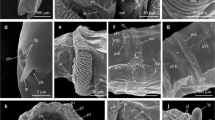
Condensed MAS pattern for Sarcophaga caerulescens (solid structures) superimposed with outlines of the genus pattern (dotted lines). Numbers indicate rows of transversal muscle patterns according to the location on and affiliation with a segment. Arrows indicate differences compared to genus pattern
S. caerulescens (Fig. 6) showed the most conspicuous deviations from the genus pattern as there were divisions as well as additions in the rows. Rows 2.3 were divided in the upper middle part and had an additional piece on top of the divided row. Rows 3.3 had divisions but lacked an additional piece. Furthermore, the upper parts of rows 4.2 and 4.4 were bent more than corresponding rows in the genus pattern. This, however, should not be taken into account as indicative discrepancy.
Discussion
Sarcophaginae larvae, including the genus Sarcophaga, can easily be recognized from the large and deep spiracular cavity on the last segment (Szpila et al. 2015b). Species identification however is much more difficult. Previous studies on MAS patterns for Calliphoridae species (Niederegger et al. 2013; Niederegger and Spieß 2012; Niederegger and Spieß 2014; Niederegger et al. 2015) motivated us to exploring the method for this forensically important genus.
As was previously shown for Calliphoridae larvae, MAS patterns only expand in size during growth and development of the larvae, while maintaining their shape and numerical characteristics (Niederegger et al. 2013). Even though behavior of the larvae changes within the third instar from feeding to migration, their muscular and cuticular equipment remains the same as a further ecdysis would be necessary to alter it. These assumptions were adapted for Sarcophagidae larvae. For this study, therefore, only third instar larvae were investigated.
Five species of the genus Sarcophaga from five different subgenera were examined, and a characteristic genus pattern as well as species-specific pattern variations was found. The previously defined field of analysis comprising segments 2–4 of the third-stage larvae did not have to be extended. Size differences between species were only rarely reflected in the patterns: if deviations were found, the number of MAS per row was never more than four above or below the number for the genus.
Sarcophaga argyrostoma, S. caerulescens, and S. similis are known mostly as carrion breeders with potential facultative parasitism or predation (Pape 1987; Povolný and Verves 1997; Richet et al. 2011). Sarcophaga albiceps and S. melanura are ubiquitous and can develop successfully in carrion but their preferred breeding substrates are feces (S. albiceps) or undefined remains with probable facultative predation on other fly larvae (S. melanura) (Bänziger and Pape 2004; Povolný and Verves 1997; Richet et al. 2011). Larval behavior of these species was not studied in detail, but similarity can be assumed. Only obvious differences in behavior or habitat would be expected to lead to dramatic alterations in the larval muscular equipment and thus in MAS numbers or patterns. Obligatory parasitic larvae for instance might have a reduced number of muscles due to diminished motility. Confirmation of this statement however needs further studies of larvae representing different breeding behaviors.
As the genus Sarcophaga comprises a large number of species (Pape 1996), this study of five species can only be preliminary. These first results however are encouraging for the continuation of this method within the genus Sarcophaga and other genera of the vast family of Sarcophagidae. In further studies, it would be interesting to investigate whether the genus pattern found here is characteristic for all or most species of Sarcophaga or if MAS patterns from species with different food preferences or habitats deviate from necrophagous species.
With expansion of the MAS method for more species and genera, it becomes apparent that it is a useful and applicable tool for species determination in brachyceran larvae. The first concept of “key features” (Niederegger and Spieß 2012), however, had to be abandoned with the examination of more species. The risk of false determinations when relying solely on “key features” rose with the broadening of the field of analysis to include all rows of three segments. The consideration of the entirety of features is necessary for correct species determination.
A previous experiment in species determination using MAS patterns performed by impartial and inexperienced test subjects showed good results (Niederegger and Spieß 2012). As with all determination keys and methods however, some level of experience and practice is necessary for successful identifications. A long-term aim for further facilitation of the method and elimination of researcher bias is the development of recognition and comparison software for MAS patterns.
References
Anton E, Niederegger S, Beutel RG (2011) Beetles and flies collected on pig carrion in an experimental setting in Thuringia and their forensic implications. Med Vet Entomol 25:353–364
Bänziger H, Pape T (2004) Flowers, faeces and cadavers: natural feeding and laying habits of flesh flies in Thailand (Diptera : Sarcophagidae, Sarcophaga spp.). J Nat Hist 38:1677–1694
Byrd JH, Castner JL (2009) Forensic entomology. The utility of arthropods in legal investigation. CRC Press, Boca Raton
Cherix D, Wyss C, Pape T (2012) Occurrences of flesh flies (Diptera: Sarcophagidae) on human cadavers in Switzerland, and their importance as forensic indicators. Forensic Sci Int 220:158–163
Grassberger M, Frank C (2004) Initial study of arthropod succession on pig carrion in a central European urban habitat. J Med Entomol 41:511–523
Mądra A, Frątczak K, Grzywacz A, Matuszewski S (2015) Long-term study of pig carrion entomofauna. Forensic Sci Int 252:1–10
Matuszewski S, Szafałowicz M, Jarmusz M (2013) Insects colonising carcasses in open and forest habitats of Central Europe: search for indicators of corpse relocation. Forensic Sci Int 231:234–239
Niederegger S, Miroschnikow A, Spieß R (2013) Marked for life: muscle attachment site patterns in blowfly larvae are constant throughout development. Parasitol Res 112:347–355
Niederegger S, Spieß R (2012) Cuticular muscle attachment sites as a tool for species determination in blowfly larvae. Parasitol Res 110:1903–1909
Niederegger S, Spieß R (2014) Muscle attachment sites of Phormia regina (Meigen). Parasitol Res 113:4313–4314
Niederegger S, Szpila K, Mall G (2015) Muscle attachment site (MAS) patterns for species determination in European species of Lucilia (Diptera: Calliphoridae). Parasitol Res 114:851–9
Pape T (1987) Diptera: the Sarcophagidae of Fennoscandia and Denmark. Fauna Entomologica Scandinavica, Vol 19. Brill, Leiden-Copenhagen.
Pape T (1996) Catalogue of the Sarcophagidae of the world (Insecta: Diptera). Mem Entomol Int 8:1–558
Piwczyński M, Szpila K, Grzywacz A, Pape T (2014) A large-scale molecular phylogeny of flesh flies (Diptera-Sarcophagidae). Syst Entomol 39:783–799
Pohjoismäki JLO, Karhunen PJ, Goebeler S, Saukko P, Sääksjärvi IE (2010) Indoors forensic entomology: colonization of human remains in closed environments by specific species of sarcosaprophagous flies. Forensic Sci Int 199(1–3):38–42
Povolný D, Verves Y (1997) The flesh-flies of central Europe (Insecta, Diptera, Sarcophagidae). Spixiana, Supplement 24 München
Richet R, Blackith RM, Pape T (2011) Sarcophaga of France (Diptera: Sarcophagidae), Pensoft Series Faunistica, vol 97, Sofia
Smith KGV (1986) A manual of forensic entomology. The Trustees of the British Museum (Natural History), London.
Szpila K, Mądra A, Jarmusz M, Matuszewski S (2015a) Flesh flies (Diptera: Sarcophagidae) colonising large carcasses in Central Europe. Parasitol Res 114:2341–2348
Szpila K, Richet R, Pape T (2015b) Third instar larvae of flesh flies (Diptera: Sarcophagidae) of forensic importance—critical review of characters and key for European species. Parasitol Res 114:2279–2289
Szpila K, Voss JG, Pape T (2010) A new dipteran forensic indicator in buried bodies. Med Vet Entomol 24:278–283
Acknowledgments
Parts of this work were financially supported by the Polish National Science Centre (grant no. 2012/07/B/NZ8/00158).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niederegger, S., Szpila, K. & Mall, G. Muscle attachment site (MAS) patterns for species determination in five species of Sarcophaga (Diptera: Sarcophagidae). Parasitol Res 115, 241–247 (2016). https://doi.org/10.1007/s00436-015-4740-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4740-4