Abstract
First results of a new method for species determination in third instar larvae of saprophagous blowflies are introduced. Cuticular attachment sites of a limited number of transversal muscles are visualized for light microscopic analysis. After removing the muscles and staining the cuticle, the attachment sites become visible as laterally symmetrical segmental clusters of dark dots. The combined patterns of five such clusters, located in the second, third and fourth segments, show sufficient differences to allow reliable separation of externally very similar larval Lucilia sericata and Lucilia illustris as well as Calliphora vomitoria and Calliphora vicina, the most common saprophagous blowfly species in Europe. Species determination even in poorly conserved, discoloured and fragmented blowfly larvae becomes possible with this new method. The method can primarily be applied for postmortem interval (PMI) calculations in forensic entomology. Interspecific morphological similarity of the larvae and differences in growth rate make species determination an essential requisite for an exact PMI calculation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blowflies often start the biological clock for postmortem interval (PMI) calculation in forensic entomology since they approach a body for oviposition within the first hours after death (Byrd and Castner 2001; Greenberg and Kunich 2002; Gennard 2007). The age of blowfly larvae is therefore closely related to the time of death (Lane 1975; Catts and Goff 1992; Carter et al. 2007). Due to experiments in which larvae of known species were raised under controlled conditions, a given body length can be attributed to a distinct age (Reiter 1984; Anderson 2000; Kaneshrajah and Turner 2004; Donovan et al. 2006; Niederegger et al. 2010). Under field conditions however, when a conglomerate of unidentified larvae is collected from a body, reliable age determination is challenged by the facts that the larvae of different species look extremely similar but grow to different body lengths, and that temperature has a variable effect on the development of some of the most common blowflies. Grown under identical conditions, 10-mm larvae could either be addressed as a 6-day-old Calliphora vicina or a 4-day-old Sarcophaga argyrostoma (flesh fly). Also, a fully grown 14-mm Lucilia illustris (up to 9 days old) could be mistaken for a “juvenile” 6-day-old Calliphora vomitoria (Niederegger et al. 2010). A reliable species determination therefore is essential to a correct PMI calculation.
A common method is to maintain collected larvae until they hatch and determine the species by morphological features of the imago (Amendt et al. 2000; Gennard 2007), a time-consuming procedure that requires living larvae. Other methods based on light and electron microscopy rely on morphological characteristics like the shape of the cephalopharyngeal skeleton (CPS) (O’Flynn and Moorhouse 1980; Cantrell 1981; Reiter and Wollenek 1983; Erzinclioglu 1985; Queiroz et al. 1997; Szpila et al. 2008; Niederegger et al. 2011) or external structures like spiracles and spine bands (Reiter and Wollenek 1983; Wallman 2001; Sukontason et al. 2006). These methods can be hindered by fragmentation or discolouration of the specimen caused by poor preservation. A technically advanced approach aims toward DNA-based species identification using molecular biological methods (Wells et al. 2001; Wells and Sperling 2001; Boehme et al. 2010).
A method for larval species determination would ideally be fast, simple, suited for all larval stages and even applicable to a fragmented or discoloured specimen. This pilot study introduces a novel approach, promising to fulfil these requirements: the cuticular attachment sites of body wall muscles can easily be visualized and are arranged in clusters that form distinct patterns. The patterns of a cluster subset are described for C. vomitoria, C. vicina, L. illustris and L. sericata, showing that the interspecific variation allows a reliable differentiation of these four species. The application of this approach for earlier larval stages and a poorly conserved (fragmented or discoloured) specimen is discussed.
Material and methods
Animals
Adult flies were collected from pig cadavers in Thüringen (Germany) and their species determined. Several flies of each species were kept separately in 27dm3 cages (BugDorm) and supplied with water and sugar at libitum. Fifty-millilitre beakers filled with minced meat were offered for oviposition. The hatched larvae were supplied with meat according to necessity. Only third instar larvae were used in the analyses.
Preparation
Blowfly larvae move by telescopic contractions of the body wall, mediated by a complex set of muscles attached to the cuticle (compare Fig. 1c). A limited number of transversal muscles exist beneath the numerous longitudinal muscles (Fig. 1d). To visualize the attachment sites of these muscles, the larvae were killed by dousing in boiling water and transferred to 70% ethanol until the muscles detached from the cuticle (compare Fig. 1a). In 90% of the larvae this happened overnight. Before preparation, the length and diameter of the larvae were measured (accuracy ±0.1 mm) using a calibrated ocular micrometre.
Preparation and data acquisition. a Lateral view of an intact larva. White arrows indicate muscle layer detachment from the cuticle. The segments of interest are indicated (S2–S4). b A schematic drawing of a larva, dorsal view. Dashed lines indicate incisions. St anterior stigmata. c Opened larva with complete musculature. White triangle indicates ventral midline. d The longitudinal muscles were removed to reveal the underlying transversal muscle groups which produce the investigated attachment sites. e All muscles were removed from the transparent cuticle, and attachment sites coloured with brilliant blue. Patterns are laterally symmetrical. Investigated muscle groups labelled 2A, 3A, 3B and 4A, 4B, according to segments. f Patterns of ten larvae of the same species photographed, attachment sites marked with semitransparent dots. Resulting rows were stacked and areas with high degree of overlap are marked in black
The larvae were pinned, dorsal side up in a Petri dish coated with clear silicone (Wacker Silicones, Elastosil RT 601) using 0.1-mm pins made from shortened acupuncture needles. The preparation was covered with tap water. Under a dissecting microscope, an incision was made with fine scissors over the entire length of the larva along the dorsal midline. The first and last segments were cut off just anterior to the stigmata (Fig. 1b). The cuticle was then peeled off from the muscle layer and pinned down as flat as possible. Residual muscles were removed using a fine pair of forceps and a brush. The cleaned cuticle was covered in Coomassie brilliant blue solution (Sigma, 1% in tap water) for 1 min and subsequently washed. The procedure resulted in a light blue staining of the transparent cuticle while the muscle attachment sites appeared almost black (Fig. 1e). Preparations were photographed with a digital camera (BeyTec, Moticam 1000) mounted onto the dissecting microscope. The ventral midline was adjusted vertically. Only transversal muscle attachment sites (Fig. 1d) were investigated because (unlike longitudinal muscle attachment sites) they are of limited number and form an easily detectable pattern.
In one case, larvae previously preserved in 4% paraformaldehyde (PFA), resulting in a deep brown discolouration of the cuticle, were dissected (Fig. 2). In this case, the muscles did not detach from the cuticle and were therefore individually removed with fine forceps and a brush.
Discolouration of larvae. a Left normally coloured larvae killed with boiling water and stored in 70% ethanol. Right larva showing the typical dark discoloration caused by storage in 4% paraformaldehyde. Both larvae (C. vicina) were simultaneously photographed using identical illumination. b Cuticle of the dark larva. Patterns of the muscle attachment sites can be visualized and allow species determination. Inlet shows magnification of row 4A and 4B
Data evaluation
Where a muscle strand is attached to the cuticle, it forms a “dot” which becomes visible after the muscle is removed and the cuticle stained. A cluster of dots is called “row”. The term “pattern” refers to the shape (linear, curved) of a row.
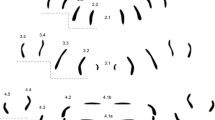
The clusters of muscle attachment sites, were labelled in accordance to the number of the segments: the longest longitudinal rows laterally to the ventral midline were labelled 2A, 3A and 4A. Starting from segment three, considerably shorter rows closer to the midline were present (3B and 4B).
Species-specific patterns were determined as follows: from ten larvae (for each species), photographs were taken with identical magnification from rows 2A, 3A, 4A, 3B and 4B. Using graphic software (CorelDRAW), each muscle dot of the five rows was covered with a semitransparent black circle. The resulting patterns were stacked, using the most anterior circle as reference point. The resulting areas with a high degree of overlap were marked and are referred to as “condensed patterns”.
Method validation
To validate the method, ten participants were requested to assign 40 individual patterns to the four species analysed in this study by use of the exact figures presented in this paper as determination keys. Five of the participants had previous experimental experience with fly larvae, albeit not with muscle attachment sites, and five participants had no previous experience with maggots at all. The results of the validation were statistically analysed using the momentum method.
N = 10 participants an (n = 1, …, N) correlated each element s m (m = 1, …, M) of a sample of M = 40 specimens to one of K = 4 prototypes t k (k = 1, …, 4). For each participant a n the number x n,k of correct classifications to prototype t k was registered (Table 1). The statistical approach was to compute an overall estimator for the probability p of correct classifications. This was done by the momentum method which led to the following equation:
Results
After removing the muscles and staining the cuticle, attachment sites of the longitudinal muscles forming circumferential rows of dots along each segment border became visible (Fig. 1e). These rows however did not provide enough characteristic features for species determination. The attachment sites of the transversal muscles on the other hand formed easily distinguishable, bilaterally symmetrical rows in each segment.
The differences in body length between Lucilia sp. (about 14.5 mm) and Calliphora sp. (about 17.5 mm) did not consistently correlate to the dot number of the rows. While in the larger Calliphora sp. larvae row 2A counted more dots (12) than in Lucilia (10), the opposite was true for row 3A. Here, the considerably smaller L. sericata (about 15 mm) had approximately the same number of dots as C. vomitoria (18 mm). C. vicina had the highest dot number (14) in row 3A (14), but was not the largest larva. In row 3B, the larger species had even fewer dots (about 4.5) than the smaller ones (six dots). The differences in dot numbers of row 3A were reflected in the pattern: in the Lucilia species, this row was clearly “C” shaped, while in Calliphora sp. it had a rather scattered, linear cluster.
A detailed description of the patterns for all four species is given below.
L. illustris
The larvae of this species had an average length of 14 mm (±0.9) and a diameter of 2.9 mm (±0.3; Fig. 3). Their patterns were the most conspicuous and easy to discriminate from the other three species. In row 2A, an average number of 10 (±0.8) dots formed a straight line in an angle of about 10° to the ventral midline. The pattern of row 3A (11 ± 1.2 dots) combined a straight section in an angle of 25° to the midline followed by a sharp bend toward the midline giving this row the shape of a boomerang. Row 3B was C-shaped and consisted of 5.9 ± 1.4 dots. Row 4A (11 ± 1.8 dots) showed the unique feature of a disrupted pattern: The anterior section formed a mirrored “S”. A second cluster of several dots was found closer to the midline, giving this row the shape of a crude question mark. In row 4B, 7 (±1.6) dots formed a straight line in a right angle to the midline.
Patterns of L. illustris. Row 2A forms a straight line. Row 3A shows a sharp bent giving it the shape of a boomerang, while row 4A is discontinuous and roughly resembles a question mark. Left photographs of the individual rows of one specimen. Middle stacked patterns from ten larvae. The average numbers of dots are given with standard deviations. Right condensed patterns. Vertical dashed lines are parallel to the ventral midline. The approximate angle of the rows in regard to the midline is indicated. Vertical scale bars 0.2 mm
Quick identification: the characteristic features of this species are the boomerang shape of row 3A and the disruption of row 4A.
L. sericata
These larvae had an average length of 14.9 mm (±1) and diameter of 2.7 mm (±0.2; Fig. 4). Row 2A consisted of 10 (±1.7) dots which formed a straight line in a 20° angle to the ventral midline. Row 3A (13 ± 1 dots) had a linear anterior portion aligned in a 20° angle. At about 2/3 the pattern curved toward the midline. Row 3B (6 ± 0.9 dots) was C-shaped as in L. illustris. Row 4A (13.5 ± 2 dots) also had a linear anterior section. At about half length the pattern first curved away from and then back to the midline. The 6 (±1.2) dots of row 4B formed a straight horizontal line.
Patterns of L. sericata. Anterior sections of rows 2A, 3A and 4A are linear. Small curves toward and away from the midline occur in the posterior section of rows 3A and 4A. The angles of the rows with regard to the midline are similar. Left photographs of the individual rows of one specimen. Middle stacked patterns from ten larvae. The average numbers of dots are given with standard deviations. Right condensed patterns. The vertical dashed lines are parallel to the ventral midline. The approximate angle of the rows with regard to the midline is indicated. Vertical scale bars 0.2 mm
Quick identification: compared to the other investigated species, the rows 2A, 3A and 4A are rather linear and aligned in a similar angle (about 20°) toward the midline.
C. vomitoria
The average larval body size was 17.9 mm (±1.5) in length and 3.1 mm (±0.4) in diameter. In this species, row 2A (12 ± 1.6 dots) was not linear over its entire length, but showed a characteristic curve toward the midline in its posterior section (Fig. 5). The anterior almost linear section was aligned in a 10° angle with reference to the midline. As a whole, row 3A (12.5 ± 1.6 dots) formed a quite even curve which was concave with respect to the midline. Unlike in the Lucilia species, row 3B (5 ± 1.2 dots) was not C-shaped but rather linear with a slight curve away from the midline in its anterior section. The anterior section of row 4A (14 ± 1.5) was linear. At about half length it showed a curve away from the midline followed by a linear section before the posterior end bent back to the midline again. The linear row 4B (6.5 ± 1.4 dots) was not aligned horizontally, but it rose toward the proximal end.
Patterns of C. vomitoria. Posterior section of row 2A curves toward the midline. Row 3A has almost no linear section but rather forms an arc which is concave with respect to the midline. Row 3B is quite linear. Row 4A has a linear anterior section followed by a slight outward and inward bent in the posterior half. Left photographs of the individual rows of one specimen. Middle stacked patterns from ten larvae. The average numbers of dots are given with standard deviations. Right condensed patterns. The vertical dashed lines are parallel to the ventral midline. The approximate angle of the rows with regard to the midline is indicated. Vertical scale bars 0.2 mm
Quick identification: the posterior end of row 2A curves toward the midline, and the entire length of row 3A forms an even curve.
C. vicina
Third instar larvae of this species had an average body length of 16.6 mm (±1) and a diameter of 3 mm (±1.3; Fig. 6). Row 2A (12.5 ± 1.5 dots) was linear with a 15° angle with respect to the midline. Row 3A (14 ± 2 dots) was also aligned in a 15° angle. It showed a concave bend framed by two linear sections, giving it the roughly shape of an inchworm. The dots of row 3B (4 ± 1) were rather scattered and covered a linear area parallel to the midline. The pattern of row 4A was unique: the 14 (±1.2) dots formed an “S” which is orientated in a −25° angle. Row 4B (6.5 ± 1 dots) formed a linear horizontal pattern.
Patterns of C. vicina. Row 2A is linear. The anterior and posterior sections of row 3A are quite linear and enclose a slight arc bending away from the midline. Row 3B is linear and often formed by some scattered dots. Row 4A shows a characteristic “S” shape bent away from the midline. Left photographs of the individual rows of one specimen. Middle stacked patterns from ten larvae. The average numbers of dots are given with standard deviations. Right condensed patterns. The vertical dashed lines are parallel to the ventral midline. The approximate angle of the rows with regard to the midline is indicated. Vertical scale bars 0.2 mm
Quick identification: row 3A is shaped like an inchworm, and row 4A forms an “S” oriented in a negative angle.
Method validation
The following results for x n were obtained by the momentum method:
Restriction to the subgroup of experienced examiners led to:
Whereas the subgroup of inexperienced examiners yielded (Table 1):
Discussion
A novel approach for fast and easy species determination in third instar blowfly larvae based on light microscopic visualization of morphological features is introduced: the species-specific arrangement of cuticular muscle attachment sites. The patterns of five clusters located in the second, third and fourth segments enable a reliable differentiation of L. sericata, L. illustris, C. vomitoria and C. vicina. These species are among the first flies to approach a corpse in Europe (Gennard 2007) and therefore play a vital role in PMI calculation based on larval growth of flies. As growth rates differ among species under identical environmental conditions (Niederegger et al. 2010), species determination is an important prerequisite for a correct PMI calculation.
Forensic entomologists have currently access to a variety of methods for species determination of fly larvae. Each method, however, has its own handicap: maintaining the larvae until they hatch is time-consuming; light microscopy fails when poor preservation causes fragmentation or discolouration and electron microscopy or DNA analysis is practicable only in specialized laboratories, take up to 3 days and are relatively pricy in the performance.
This new method only requires basic preparation skills and standard laboratory equipment such as a binocular microscope and dissection tools (small scissors and a pair of fine forceps). Traditional Coomassie brilliant blue is an easily obtained, nonpoisonous triphenyl methane dye dissolvable in tap water.
When the muscle layer has detached from the cuticle, it takes about 6 min to visualize the attachment sites: An experienced preparator requires about 5 min for the dissection and an additional minute for staining and rinsing of the preparation. If the muscles did not detach (e.g. when the larvae were fixed in PFA), the preparation becomes more time-consuming and delicate but is still manageable within 30 min.
In the present study, the condensed patterns were clearly species specific. Nevertheless, a certain degree of intraspecific variability was observed. Neither the number of attachment sites nor the pattern of a given row was exactly identical in the larvae of the same species. For this reason, the pattern of a single row (e.g. 2A) was not sufficient as a characteristic, but when all described rows were taken into account, the intraspecific variability was always smaller than the interspecific differences, allowing a reliable species determination.
The viability of the method was shown by the ten experienced and inexperienced probands which yielded an average of 83% of correct species identifications. Experienced probands achieved even 87%, the best of which scored 95% with 38 correct identifications out of 40 patterns (Table 1). With a complete description of all the patterns in the first three segments or even the whole cuticle, species determination will probably become even more reliable.
If the larvae are not handled carefully during preservation, the exposed CPS and the posterior spine bands are easily damaged, thus making species characteristics non-discernable. To jeopardize the muscle attachment sites however, the whole cuticle of the first three segments would have to be destroyed which is rather unlikely to happen.
Over time, even carefully handled larvae can develop severe discoloration if they are killed and stored in ethanol or PFA. Discolorations affect both cuticle and muscles and therefore hinder any clearance attempt to visualize the CPS for species determination. Staining and thus visualization of the patterns of the muscle attachment sites is still possible (Fig. 2).
Only five attachment site clusters in the meso-, metathoracic and first abdominal segments are featured in this study. Yet, there are seven more abdominal segments (Wallman 2001), each bearing at least three more clusters per hemisegment. In an ambitious project, a complete map could be drawn of all clusters in all accessible developmental stages for all forensically relevant larvae. The tempting prospect would be that even a fragment of a larva would be sufficient to determine its species and developmental state.
References
Amendt J, Krettek R, Niess C, Zehner R, Bratzke H (2000) Forensic entomology in Germany. Forensic Sci Int 113:309–314
Anderson GS (2000) Minimum and maximum development rates of some forensically important Calliphoridae (Diptera). J Forensic Sci 45:824–832
Boehme P, Amendt J, Disney RHL, Zehner R (2010) Molecular identification of carrion-breeding scuttle flies (Diptera: Phoridae) using COI barcodes. Int J Legal Med 124:577–581
Byrd JH, Castner JL (2001) Forensic entomology. The utility of arthropods in legal investigation, vol 1. CRC Press, Boca Raton
Cantrell BK (1981) The immature stages of some Australian Sarcophaginae (Diptera: Sarcophagidae). J Aust Entomol Soc 20:237–248
Carter DO, Yellowlees D, Tibbett M (2007) Cadaver decomposition in terrestrial ecosystems. Naturwissenschaften 94:12–24
Catts EP, Goff ML (1992) Forensic entomology in criminal investigations. Annu Rev Entomol 37:253–272
Donovan SE, Hall MJR, Turner B, Moncrieff CB (2006) Larval growth rates of the blowfly, Calliphora vicina, over a range of temperatures. Med Vet Entomol 20:106–114
Erzinclioglu YZ (1985) Immature stages of British Calliphora and Cynomya, with a re-evaluation of the taxonomic characters of larval Calliphoridae (Diptera). J Nat Hist 19(1):69–96
Gennard DE (2007) Forensic entomology. An introduction. Wiley, Chichester
Greenberg B, Kunich JC (2002) Entomology and the law. Flies as forensic indicators, vol 1. Paperback edn. Cambridge University Press, Cambridge
Kaneshrajah G, Turner B (2004) Calliphora vicina larvae grow at different rates on different body tissues. Int J Legal Med 118:242–244
Lane RP (1975) Investigation into blowfly (Diptera-Calliphoridae) succession on corpses. J Nat Hist 9:581–588
Niederegger S, Pastuschek J, Mall G (2010) Preliminary studies of the influence of fluctuating temperatures on the development of various forensically relevant flies. Forensic Sci Int 199:72–78
Niederegger S, Wartenberg N, Spiess R, Mall G (2011) Simple clearing technique as species determination tool in blowfly larvae. Forensic Sci Int 206:E96–E98
O’Flynn MA, Moorhouse DE (1980) Identification of early immature stages of some common Queensland carrion flies. J Aust Entomol Soc 19:53–61
Queiroz MMD, deMello RP, Lima MM (1997) Morphological aspects of the larval instars of Chrysomya albiceps (Diptera, Calliphoridae) reared in the laboratory. Mem Inst Oswaldo Cruz 92:187–196
Reiter C (1984) Zum Wachstumsverhalten der Maden der blauen Schmeißfliege Calliphora vicina. Z Rechtsmed 91:295–308
Reiter C, Wollenek G (1983) Zur Artbestimmung der Maden forensisch bedeutsamer Schmeißfliegen. Z Rechtsmed 90:309–316
Sukontason KL, Kanchai C, Piangjai S, Boonsriwong W, Bunchu N, Sripakdee D, Chaiwong T, Kuntalue B, Siriwattanarungsee S, Sukontason K (2006) Morphological observation of puparia of Chrysomya nigripes (Diptera: Calliphoridae) from human corpse. Forensic Sci Int 161:15–19
Szpila K, Pape T, Rusinek A (2008) Morphology of the first instar of Calliphora vicina, Phormia regina and Lucilia illustris (Diptera, Calliphoridae). Med Vet Entomol 22:16–25
Wallman JF (2001) Third-instar larvae of common carrion-breeding blowflies of the genus Calliphora (Diptera: Calliphoridae) in South Australia. Invertebr Taxon 15:37–51
Wells JD, Sperling FAH (2001) DNA-based identification of forensically important Chrysomyinae (Diptera: Calliphoridae). Forensic Sci Int 120:110–115
Wells JD, Pape T, Sperling FAH (2001) DNA-based identification and molecular systematics of forensically important Sarcophagidae (Diptera). J Forensic Sci 46:1098–1102
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niederegger, S., Spieß, R. Cuticular muscle attachment sites as a tool for species determination in blowfly larvae. Parasitol Res 110, 1903–1909 (2012). https://doi.org/10.1007/s00436-011-2716-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2716-6