Abstract
Species identification is generally assessed to be more difficult in larval stages than in adult forms. Especially closely related species such as Lucilia caesar and Lucilia illustris are difficult to identify. The aim of this study was to simplify species determination in Lucilia larvae for entomological and forensic purposes. Muscle attachment site (MAS) patterns were previously found to be a good tool for species determination in blowfly larvae. Here, distinctive MAS patterns are presented for European Lucilia ampullacea, L. caesar, L. illustris, L. richardsi, L. sericata, and L. silvarum. A joint pattern for the genus Lucilia is provided for a quick classification of a larva to the genus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Flies of the genus Lucilia Robineau-Desvoidy, 1830 in the family Calliphoridae are known as green bottle flies because of their brilliant metallic greenish colorations. In Europe, the genus is represented by 11 species (Schumann 1986). Many species of Lucilia present strong synanthropic tendencies and high abundance in anthropogenic ecosystems (Greenberg 1973; Rognes 1991). Ubiquity, abundant visiting, and active participation in decomposition of large carrion predestine at least five species of Lucilia as potential forensic indicators (Anton et al. 2011; Matuszewski et al. 2008; Matuszewski et al. 2010; Smith 1986): Lucilia ampullacea, Lucilia caesar, Lucilia illustris, Lucilia sericata, and Lucilia silvarum. The species differ in habitat preferences: They are present in a gradient of environments from dry/open to shadow/forest with a transition of many ecotones and overlapping occurrences of species (Draber-Mońko 1993; Draber-Mońko 1996; Draber-Mońko 2004; Fischer 2000; Fremdt and Amendt 2014; MacLeod and Donnelly 1957; Szpila 1999).
A crucial issue in the application of forensic insects is adequate species identification in the material collected (Amendt et al. 2011). This is generally assessed to be more difficult in larval stages than in adult forms (Byrd and Castner 2009; Smith 1986). Entomological evidence collected at crime scenes or autopsies, however, is usually composed of preimaginal stages (Byrd and Castner 2009; Smith 1986).
Taxonomy of necrophagous blowflies of the Palearctic ecozone was studied thoroughly due to their serious medical and veterinary importance. As a result of these scientific efforts, various keys for identification of European blowflies are available in several publications (e.g., Erzinçlioğlu 1985; Erzinçlioğlu 1996; Lehrer 1972; Rognes 1991; Schumann 1954; Schumann 1971; Szpila 2010; Szpila 2012). Among them are also descriptions, revisions, and keys useful for species identification of preimaginal stages. Articles with attempts to providing keys for identification of larval instars of European species of Lucilia were published by Schumann (1954, 1971), Szpila (2010), Szpila et al. (2013), and Velasquez et al. (2010). European species of Lucilia were also included in keys dedicated to other zoogeographical zones (Ishijima 1967; Knipling 1939; Liu and Greenberg 1989). Characters used in traditional keys are details of the cephaloskeleton, the distribution of spines on particular segments of the larval body, the position of papillae on the anal division, and details of posterior spiracles. Recently, the taxonomic value of some of these characters was critically revised especially in the context of identifying sister species like L. caesar and L. illustris (Szpila et al. 2013).
An innovative solution for further development of morphological methods in species identification of blowfly larvae is an implementation of muscle attachment site (MAS) patterns (Niederegger et al. 2013; Niederegger and Spiess 2012). MAS is located on the inside of the cuticle in blowfly larvae and presents themselves in a species-specific arrangement on every segment. Visibility of MAS is best obtained in larvae without protuberances of the cuticle such as are present in larvae of Chrysomya albiceps or Chrysomya rufifacies (Smith 1986). The MAS method can be applied for all larval stages, as the patterns are constant throughout the development of the larvae and change only in size during growth (Niederegger et al. 2013). The present study investigated MAS pattern characteristics for six species of the genus Lucilia: L. silvarum, L. illustris, L. richardsi, L. ampullacea, L. sericata, and L. caesar and introduces a basic pattern for the genus Lucilia.
The aim of this study was to simplify species determination in Lucilia larvae for entomological and forensic purposes. With the rise of genetic applications in insect determination, it was furthermore a desire to counteract negligence of morphological methods which still remain attractive for practitioners in the field of forensic entomology.
Materials and methods
Animals
Adult females were caught using baits with pig or chicken liver located in different habitats (stream banks, forests, rural habitats, and indoors) in Poland for oviposition. The keys of Rognes (1991) and Draber-Mońko (2004) were used for identification. All females were labeled and are available as voucher specimens in the insect collection of the Chair of Ecology and Biogeography, Faculty of Biology and Environmental Protection, Nicolaus Copernicus University in Toruń, Poland.
Resulting larvae were reared to the third instar, killed by dousing with boiling water, and stored in 70 % ethanol. For L. illustris and L. caesar, several larvae were bred to adult to obtain males for identification confirmation.
Preparation
The larvae were measured to the nearest 0.1 mm using a dissecting microscope (Zeiss Stemi 2000C) with digital camera (Zeiss AxioCam ICc1) and measuring software (AxioVision). All preparation and evaluation steps leading to the condensed patterns were performed as given in our previous publications (Niederegger et al. 2013; Niederegger and Spiess 2012).
Data evaluation
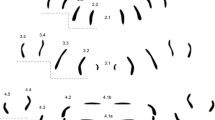
Due to the fact that more closely related species were analyzed than in our previous studies (Niederegger et al. 2013; Niederegger and Spiess 2012), all rows in segments 2–4 were documented, and labeling was altered. Starting at the center, rows were numbered according to the segment and the position within the segment (Fig. 1). What was previously known as row 2A now corresponds to 2.2, 3B = 3.1, 3A = 3.2, 4B = 4.1, and 4A = 4.2.
Genus pattern for Lucilia composed of 76 individual MAS patterns of Lucilia ampullacea, L. caesar, L. illustris, L. richardsi, L. sericata, and L. silvarum. Rows are labeled according to their location on and affiliation with a segment (e.g., 2.1 = central row in segment 2, 4.5 = most distal row in segment 4)
The patterns were evaluated using Inkscape (freeware) and Adobe Photoshop; means and standard deviations were calculated using Microsoft Excel 2010.
Results
The attachment sites of the transversal muscles form distinguishable, bilaterally symmetrical rows in each segment. The segments in all species we analyzed showed an average number of 36 (±8) MAS per hemisegment and an average total of 108 (±14) MAS for all three hemisegments (Table 1). The total number of MAS does not correlate with the size of the larvae (Table 2) as was already found in comparisons of less closely related blowfly species (Niederegger and Spiess 2012). Due to the similarities in MAS numbers and positions, we decided to pool all patterns to generate a basic pattern for the genus Lucilia (Fig. 1).
Genus pattern (n = 76) (Fig. 1)
The genus pattern shows a small row (2.1) in the lower center of segment 2 which is bordered by two almost straight vertical rows (2.2) with slight bends in their middles. More distally located are two vertical rows (2.3) with an angle of about 25° to the midline. The most distal rows 2.4 and 2.5 are about half as long as 2.2 and 2.3; 2.4 is located in the top part of the segment and has an angle of about 10° to the midline whereas 2.5 is lower and almost parallel to the midline.
The center of segment tree is characterized by two small curved rows (3.1) pointing their convex parts at each other and two longer curved rows (3.2) opposing the smaller ones and pointing their concave parts at each other. Rows 3.3–3.5 are slightly larger but very similar to rows 2.3–2.5.
The most conspicuous feature of segment 4 is the horizontal central row (4.1) in which, however, the MAS was often merged to a degree where no individual dots were definable. This row was therefore generally excluded from our analysis. The next rows (4.2) are bent into mirrored S-shapes. The 4.3 is an L-shape with a short row 4.4 on top of it. The most distal row 4.5 again is very similar to the most distal rows in segments 2 and 3.
For the comparison of the genus pattern to individual species patterns, we defined three grades of differences:
-
Grade 1: differences in the shape of the rows compared to the genus pattern.
-
Grade 2: disruptions and breaks in the pattern.
-
Grade 3: subtle differences (e.g., elongations) or differences in MAS numbers.
Lucilia silvarum (Meigen, 1826) (n = 10) (Fig. 2)
The pattern of L. silvarum is in accordance with the genus pattern (Fig. 1). All structures are present, and no shape differences (grade 1) or pattern disruptions (grade 2) are apparent. The average numbers of MAS for each row correspond to the average numbers for the genus (Table 1)
.
Condensed patterns of L. silvarum seem to be taking up much less room than indicated by the genus pattern. This is due to the high number of larvae from different species taken into account for the genus pattern and is valid for all of the following species patterns.
Lucilia illustris (Meigen, 1826) (n = 16) (Fig. 3)
The pattern of L. illustris also corresponds well with the basic pattern for the genus Lucilia (Fig. 1). All rows are present, and no shape differences can be detected. Indications for possible pattern disruptions can be found in segment 2 (2.3 in left hemisegment, dotted arrow), but they did not come through for all larvae examined. On the third segment, however, pattern disruptions were found in structures 3.3 (solid arrows) for all larvae and in both hemisegments. L. illustris has a comparable average number of MAS for each row (Table 1) as found in the genus pattern.
Lucilia richardsi Collin, 1926 (n = 7) (Fig. 4)
In L. richardsi pattern disruptions were found in rows 2.3 and 3.3 (arrows) for all larvae and in both hemisegments. The gaps in row 3.3 are wider than those in L. illustris (Fig. 3) and also very distinct on both sides in 2.3. These wider gaps, however, did not result in a change of MAS numbers for the rows compared to the genus pattern (Table 1). The very short patterns in rows 4.5 were not taken into account, as the row was not clearly visible in all examined larvae.
Lucilia ampullacea Villeneuve, 1922 (n = 10) (Fig. 5)
Pattern disruptions can be found in patterns of L. ampullacea in 2.3 and 3.3. (long arrows) and shape elongations (grade 3) in 2.2, 2.3, and 3.2 (short arrows). This is also reflected in the average numbers of MAS which is higher than that in the genus pattern (Table 1).
Lucilia sericata (Meigen, 1826) (n = 15) (Fig. 6)
Pattern disruptions were found in patterns of L. sericata in 2.3 and 3.3 (long arrows). Additionally, shape elongations were found in rows 4.3 (short arrow), although not in the same intensity for all larvae and not always on both sides of segment 4. MAS numbers of rows show no discrepancies compared to the genus pattern (Table 1).
Lucilia caesar (Linnaeus, 1758) (n = 18) (Fig. 7)
In L. caesar, we found neither pattern disruptions nor elongations, but grade 1 differences in the form of shape change in rows 4.3 (arrows). The typical L-shape could only be found in four larvae and only on the right hemisegment. All other larvae were lacking this feature and reduced the L-shape to a straight line with an angle of about 25° to the ventral midline. In five larvae, 4.5 showed a pattern similar to an L-shape opposing the expected shape of 4.3 as a possible compensatory measurement. This, however, did not manifest in the condensed patterns for L. caesar. MAS numbers of rows reflect both these differences, as 4.3 in average has fewer and 4.5 has more MAS than the basic pattern (Table 1).
L. caesar vs L. illustris (Fig. 8)
Determination is exceptionally difficult between L. caesar and L. illustris when using traditional morphologic and genetic methods (Rognes 1980; Rognes 1991; Schumann 1971; Sonet et al. 2012; Spence 1954; Szpila 2010). Using the MAS method, however, distinct differences can be found: In L. illustris (dotted), pattern disruptions were found in rows 3.3 (long arrows) and L-shaped elongations in 4.3 (short arrows) whereas in L. caesar (solid), neither pattern disruptions nor L-shapes could be detected. MAS numbers differ for 4.3 but not for 3.3 (Table 1).
Outlines of condensed MAS patterns for Lucilia caesar (solid lines) superimposed with outlines of the condensed muscle attachment site patterns for Lucilia illustris (dotted lines). Numbers indicate rows of transversal muscle patterns according to the location on and affiliation with a segment. Arrows indicate differences between the two species patterns
Discussion
In our previous work, we established the MAS method as promising tool for species determination in blowfly larvae. We challenged our method in this study, and it proved to deliver conclusive results for preimaginal stages of closely related and only recently diverged species (McDonagh and Stevens 2011) of the genus Lucilia. As expected, we had to broaden our field of analysis by including more MAS rows but were able to contain it to three segments in order to keep the method simple. The increased number of addressable rows required new labeling which can be continued if more segments should be needed in further investigations. Muscular patterns in Drosophila, however, were found to be unique only in the three thoracic and the first and last abdominal segments (Hooper 1986). Similar results were found in Calliphora vomitoria (Crossley 1965) and might be valid for all Calliphoridae species.
A joint pattern for Lucilia species could be found (Fig. 1) for a quick classification of the larvae to the genus. Species MAS patterns were then compared to the genus pattern. Mostly, grade 2 differences could be found, but also, grade 1 differences were present in at least one species. Grade 3 differences helped confirming results. A number of very small discrepancies to the genus pattern was present in each species. We concentrated on the most apparent differences, however, in order to provide an easy template for quick determinations of individual larvae. For L. illustris and L. caesar—where species determination of larvae was reported to be difficult (Smith 1986; Szpila 2010; Szpila et al. 2013) or impossible (Schumann 1971) even with genetic methods (Sonet et al. 2012)—we found distinctive features in their respective MAS patterns. Larvae originating from different habitats and different mothers displayed accordant MAS patterns.
Even though the MAS method is simple and quick, results can be improved by paying special attention to careful sampling, collection, and storage of larvae according to standards and guidelines of the European Association for Forensic Entomology (Amendt et al. 2007). The method can be applied to all larval stages. If the larvae are near to pupation, however, the cuticle forms a puparium which includes some chemical changes (Dennell 1958). This might result in fading or disappearance of MAS.
To further establish the method of MAS patterns, more dipteran species should be examined to find more genus and species patterns. Closely related species—such as L. illustris and L. caesar—from other continents should be analyzed and compared.
References
Amendt J, Campobasso CP, Gaudry E, Reiter C, LeBlanc HN, Hall MJR (2007) Best practice in forensic entomology—standards and guidelines. Int J Legal Med 121(2):90–104
Amendt J, Richards CS, Campobasso CP, Zehner R, Hall MJR (2011) Forensic entomology: applications and limitations. Forensic Sci Med Pat 7(4):379–392
Anton E, Niederegger S, Beutel RG (2011) Beetles and flies collected on pig carrion in an experimental setting in Thuringia and their forensic implications. Med Vet Entomol 25:353–364
Byrd JH, Castner JL (2009) Forensic entomology. The utility of arthropods in legal investigations, 2nd edn. Crc Pr Inc, Boca Raton
Crossley AC (1965) Transformations in Abdominal Muscles of Blue Blow-Fly Calliphora erythrocephala (Meig) during Metamorphosis. J Embryol Exp Morpholog 14:89
Dennell R (1958) The amino acid metabolism of a developing insect cuticle: the larval cuticle and puparium of Calliphora vomitoria. III. The formation of the puparium. Proc R Soc Lond B Biol Sci 149(935):176–183
Draber-Mońko A (1993) Calliphoridae i Rhinophoridae (Diptera, Calyptrata) Krainy Świętokrzyskiej. Fragmenta faunistica 36:235–273
Draber-Mońko A (1996) Muchówki z rodziny Calliphoridae (Diptera, Calyptrata) Roztocza. Fragmenta Faunistica 39:71–102
Draber-Mońko A (2004) Calliphoridae, Plujki (Insecta Diptera). Natura Optima Dux Foundation MalZ. PAS, Warsaw
Erzinçlioğlu YZ (1985) Immature stages of british Calliphora and Cynomya, with a re-evaluation of the taxonomic characters of larval Calliphoridae (Diptera). J Nat Hist 19(1):69–96
Erzinçlioğlu YZ (1996) Blowflies, vol 23. The Richmond Publishing Co, Ltd, Slough
Fischer OA (2000) Blowflies of the genera Calliphora, Lucilia and Protophormia (Diptera, Calliphoridae) in South Moravian urban and rural areas with respect to Lucilia bufonivora Moniez. Acta Vet Brno 69:225–231
Fremdt H, Amendt J (2014) Species composition of forensically important blow flies (Diptera: Calliphoridae) and flesh flies (Diptera: Sarcophagidae) through space and time. Forensic Sci Int 236:1–9
Greenberg B (1973) Flies and disease, vol. II. Biology and disease transmission. Princeton University Press, Princeton, New Jersey
Hooper JE (1986) Homeotic Gene-Function in the Muscles of Drosophila Larvae. EMBO J 5(9):2321–2329
Ishijima H (1967) Revision of the third stage larvae of synanthropic flies of Japan (Diptera: Anthomyiidae, Muscidae, Calliphoridae and Sarcophagidae). Jap J San Zool 18:47–100
Knipling EF (1939) A key for blowfly larvae concerned in wound and cutaneous myiasis. Ann Entomol Soc Am 32(2):376–383
Lehrer A (1972) Diptera Familia Calliphoridae. Fauna Republici Socialiste Romania 11(12):1–245
Liu DB, Greenberg B (1989) Immature Stages of Some Flies of Forensic Importance. Ann Entomol Soc Am 82(1):80–93
MacLeod J, Donnelly J (1957) Some ecological relationships of natural populations of calliphorine blowflies. J Anim Ecol 26(1):135–170
Matuszewski S, Bajerlein D, Konwerski S, Szpila K (2008) An initial study of insect succession and carrion decomposition in various forest habitats of Central Europe. Forensic Sci Int 180:61–69
Matuszewski S, Bajerlein D, Konwerski S, Szpila K (2010) Insect succession and carrion decomposition in selected forests of Central Europe. Part 2: Composition and residency patterns of carrion fauna. Forensic Sci Int 195:42–51
McDonagh LM, Stevens JR (2011) The molecular systematics of blowflies and screwworm flies (Diptera: Calliphoridae) using 28S rRNA, COX1 and EF-1 alpha: insights into the evolution of dipteran parasitism. Parasitology 138(13):1760–1777. doi:10.1017/S0031182011001089
Niederegger S, Miroschnikow A, Spiess R (2013) Marked for life: muscle attachment site patterns in blowfly larvae are constant throughout development. Parasitol Res 112(1):347–355
Niederegger S, Spiess R (2012) Cuticular muscle attachment sites as a tool for species determination in blowfly larvae. Parasitol Res 110(5):1903–1909
Rognes K (1980) The blow-fly genus Lucilia Robineau-Desvoidy (Diptera, Calliphoridae) in Norway. Fauna Norv Ser B 27:39–52
Rognes K (1991) Diptera: Calliphoridae Blowflies of Fennoscandia and Denmark, vol 24. Brill, E.J., Leiden
Schumann H (1954) Morphologisch-systematische Studien an Larven von hygienisch wichtigen mitteleuropäischen Dipteren der Famlien Calliphoridae-Muscidae. Wiss Z Univ Greifswald III(1953–54):245–274
Schumann H (1971) Die Gattung Lucilia (Goldfliegen). Merkbl Angew Parasitenkd Jena 18:1–20
Schumann H (1986) Family Calliphoridae. In: Soos A, Papp L (eds) Catalogue of Palaearctic Diptera, Calliphoridae-Sarcophagidae, vol 12. Akadémiai Kiadó, Budapest, Amsterdam, pp 11–58
Smith KGV (1986) A manual of forensic entomology, vol 1. The Trustees of the British Museum (Natural History), London
Sonet G, Jordaens K, Braet Y, Desmyter S (2012) Why is the molecular identification of the forensically important blowfly species Lucilia caesar and L. illustris (family Calliphoridae) so problematic? Forensic Sci Int 223(1–3):153–159
Spence T (1954) A taxonomic study of the females of the British Lucilia species (Diptera: Calliphoridae). Proc R ent Soc Lond 23:29–35
Szpila K (1999) Muchówki z rodziny Calliphoridae (Diptera) Puszczy Białowieskiej. Parki Narodowe i Rezerwaty Przyrody 18:43–51
Szpila K (2010) Key for the identification of third instars of European blowflies (Diptera: Calliphoridae) of forensic importance. In: Amendt J, Goff ML, Campobasso CP, Grassberger M (eds) Current Concepts in Forensic Entomology. Springer, Dordrecht Heidelberg London New York, pp 43–56
Szpila K (2012) Key for identification of European and Mediterranean blowflies (Diptera, Calliphoridae) of medical and veterinary importance – adult flies. In: Gennard DE (ed) Forensic Entomology, an introduction, 2nd edn. Willey-Blackwell, Chichester, pp 77–81
Szpila K, Hall MJR, Pape T, Grzywacz A (2013) Morphology and identification of first instars of the European and Mediterranean blowflies of forensic importance. Part II. Luciliinae. Med Vet Entomol 27(4):349–366
Velasquez Y, Magana C, Martinez-Sanchez A, Rojo S (2010) Diptera of forensic importance in the Iberian Peninsula: larval identification key. Med Vet Entomol 24(3):293–308
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Niederegger, S., Szpila, K. & Mall, G. Muscle attachment site (MAS) patterns for species determination in European species of Lucilia (Diptera: Calliphoridae). Parasitol Res 114, 851–859 (2015). https://doi.org/10.1007/s00436-014-4248-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4248-3