Abstract
Resistance to conventional synthetic pesticides has been widely reported in Dermanyssus gallinae in poultry production systems. Introducing novel acaricides to poultry industry today is more urgent than ever. Research in this field recently focused on plants and plant-derived compounds as acaricides. In the present study, acaricidal activity of three plant bioactive components, carvacrol, thymol, and farnesol, was assessed against D. gallinae and compared with synthetic pesticide permethrin. Mode of acaricidal action was determined by contact toxicity and fumigant toxicity bioassays. Except farnesol which did not cause any mortality, carvacrol and thymol were found to be toxic to D. gallinae with LD50 values of 1 and 3.15 μg/cm3, respectively. Permethrin gave the LD50 value of 31.95 μg/cm3 which was less efficient than carvacrol and thymol. In fumigant toxicity bioassay, mortality rate in carvacrol- and thymol-treated groups in closed method was significantly higher than the open one. On the other hand, permethrin exhibited poor fumigant toxicity as there was no statistically significant difference between mortality rate in open and closed methods. These findings revealed that mechanism of acaricidal activity of carvacrol and thymol but not permethrin was mainly due to fumigant action. Results of the present study suggested that carvacrol and thymol, especially carvacrol, can be developed as a novel potent bioacaricide against D. gallinae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The poultry red mite, Dermanyssus gallinae (Acari, Mesostigmata, Dermanyssoidea, Dermanyssidae), poses the most economically deleterious threat to laying hen industry in many parts of the world, including the USA, Europe, Japan, China, and Iran (Chauve 1998; Sparagano et al. 2009a; Wang et al. 2010; Faghihzadeh Gorji et al. 2014). Prevalence of poultry red mite in laying hen houses varies from 20 to 90 %, based on the country and production system (Sparagano et al. 2009). Studies in Iran revealed that D. gallinae is the most prevalent blood feeder mite in the breeder and caged layer flocks (Rahbari et al. 2009). The blood feeding behavior of mite results in stress, restlessness, irritation, anemia, and even death in heavy infestations due to exsanguinations (Kirkwood 1968). Poultry red mites are potential vectors of several pathogens including Salmonella enterica (Hamidi et al. 2011), Erysipelothrix rhusiopathiae (Chirico et al. 2003), and Avipox virus (Chikuba et al. 2008). From the economical point of view, this blood-sucking pest causes production losses and decrease meat (15 %) and egg production (15–20 %) and may even cause death of its host (6–7 %) (Kilpinen et al. 2005). Production in laying hens is affected through decline in the growth rate and great decreases in egg production and egg quality (shell thinning and blood spotting on the shell surface) (Chauve 1998). Control of red mite population is primarily achieved by continued applications of various synthetic acaricides such as organophosphates, pyrethroids, and carbamates. Their repeated use has often resulted in the development of mite resistance (Kim et al. 2004; Fiddes et al. 2005; Kočišová and Plachʹy 2008). Also, in laying production system, residues in eggs may develop safety issues for human health (Kim et al. 2007). Therefore, it is becoming increasingly important to identify new sources for the control of D. gallinae in poultry production systems. Plants have been suggested as an alternative source for mite control because of low non-target organism toxicity, short environment persistence, biodegradation to non-toxic products, and organic food production (Isman 2008). Certain plant extracts and essential oils meet the criteria of minimum risk pesticides (US EPA 2004) so focus has been on them and their constituents as potential sources of acaricides. Recently, many research studies were done on studying the effects of plant extracts and essential oils on D. gallinae (Kim et al. 2004; Kim et al. 2007; Abdel-Ghaffar et al. 2008; Abdel-Ghaffar et al. 2009; George et al. 2009a, b, c). But, in applying essential oils as pesticides, some points should be considered; the most important is probable difference in chemical composition of essential oils from the same or taxonomically similar species of plants (Cimanga et al. 2002). These differences will affect the activity of plant essential oils, making it difficult to recommend any essential oil for its biocidal activity by taxonomy alone (Miresmailli et al. 2006). An approach to resolve this problem is isolation of bioactive components from the plant essential oils and applying them as acaricides. Sparagano et al. (2013) showed that three such components found in essential oils especially terpenes, as eugenol, geraniol, and citral, were effective against the poultry red mite. Carvacrol is a monoterpene phenol that occurs in many essential oils of the family Labiatae, including Origanum, Satureja, Thymbra, Thymus, and Coridothymus species (Jayakumar et al. 2012). Carvacrol was reported to have broad insecticidal and acaricidal activity against agricultural, stored-product, and medical arthropod pests (Ahn et al. 1998). Thymol is a monoterpene phenol which can be found in essential oils of thyme, Thymus vulgaris or Thymus zygis. Thymol constitutes up to 80 % of the major compounds of thyme essential oils (Archana et al. 2011). Farnesol is an acyclic sesquiterpene occuring in many herbs including chamomile that possesses antibacterial and antifungal properties(Horn et al. 2005). It was classified as an active ingredient in biochemical pesticides (Hollis 2009). Bearing in mind the above, the present study aimed to assess the acaricidal activity of carvacrol, thymol, and farnesol on D. gallinae.
Material and methods
D. gallinae used in experiments were collected from a commercial laying poultry farm in Gorgan, Iran. Mites were placed in a sealable, transparent glass container and stored at temperature of 25 (±1) °C and relative humidity of 55 % (±5) 1 day prior to use. Carvacrol, farnesol, and thymol were purchased from Sigma (Sigma-Aldrich, Germany) and stored in a sealed brown container until bioassays. Permethrin (95 % purity) was obtained from Fouman Chimie (Tehran, Iran). All other chemicals were of analytical grade and available commercially.
Contact toxicity bioassay
Contact toxicity bioassay was done according to the method described by Kim et al. (2007) and Locher et al. (2010) to evaluate the efficacy of plant-derived bioactive components (carvacrol, farnesol, and thymol) in comparison to synthetic insecticide (permethrin) against D. gallinae. For this purpose, out of Whatman filter papers (no. 2), circles with 4.25-cm diameters were made. Ten different dilutions of carvacrol, farnesol, and thymol (equal to 150, 70, 35, 20, 10, 5, 2.5, 1, 0.5, and 0.125 μg/ cm3) in 50 μl ethanol were applied to filter papers. Control filter papers received only 50 μl of ethanol. Permethrin served as standard reference and was prepared in dilutions similar to the test compounds. Treated filter papers were dried in a fume hood for 3 min; each paper was then placed on the bottom of a disposable petri dish (4.8 cm diameter × 1.4 cm). About 150 adult mites were introduced into the petri dishes containing treated filter papers on a piece of cotton impregnated with distilled water. Each petri dish was then sealed with another lid and wrapped with parafilm. Contact test for all of the groups of study was done in the same condition at 25 (±1) °C and 55 % (±5) humidity. Three replicates were run concurrently for all tested compounds as well as control.
Fumigant toxicity bioassay
LD50 values obtained from contact test were used in fumigant bioassay to determine whether the lethal activity of the test compounds against D. gallinae was related to contact or fumigant toxicity. Selected dilutions of test compounds, each in 50 μl of ethanol, were applied to 4.25-cm diameter Whatman no. 2 filter papers. Filter papers were dried and then placed in polyvinyl chloride (PVC) containers with screw caps (4 cm diameter × 7 cm). Groups of 50 adult mites were placed in 1.5-ml cylindrical containers and then both sides of the container were covered with 200-mesh screen to allow for entrance of vapors from the test compounds. Then, the cylindrical containers with mites in them were transferred to PVC vessels. Vessels were either sealed with a layer of parafilm and then screw caps were added (method A), or left uncovered (method B). This system avoided direct contact of mites with filter papers. All treatments were replicated three times under the same experimental conditions as contact test. In both contact and fumigant bioassays, after 24 h, mite mortality rates were determined under a loop by prodding mites with a pin. If no movement was observed, mites were considered as dead.
Data analysis
To determine LD50, LD90, and LD99 values, mortality data were subjected to probit analysis (SPSS, 2013). The LD50, LD90, and LD99 values of the treatments were considered to be significantly different from one another when 95 % confidence limits (CLs) failed to overlap. The Student-Newman-Keuls multiple comparisons test was used to test for significant differences between open and closed fumigant toxicity methods.
Results
Contact toxicity
In contact toxicity assay, comparison of LD50 of different treatments showed that carvacrol with the lowest LD50 (1.00 μg/ cm3) had the highest efficacy against the poultry red mite in comparison to the other compounds (Table 1). On the other hand, surprisingly, farnesol not only had no effect on D. gallinae even in its undiluted pure form but also made the mites more active in comparison to the control group. As farnesol did not cause any mortality in contact test, no LD value was obtained for this compound. In the comparison of LD90 and LD99 values in different treatments, there was a significant difference between carvacrol and thymol and also permethrin. Carvacrol had the lowest LD90 (2.97 μg/cm3) and LD99 (7.24 μg/cm3) values which were significantly lower than those of thymol and permethrin. All LD50, LD90, and LD99 values of thymol and carvacrol, natural plant-derived compounds, were significantly lower than those of the synthetic chemical permethrin. In Fig. 1, mites are showed before and after treatment with carvacrol.
Fumigant toxicity
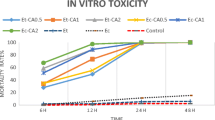
Toxic effects of carvacrol, thymol, and permethrin in vapor form on D. gallinae were evaluated by fumigant toxicity bioassay (Fig. 2). Comparison of mortality rate in permethrin-treated group and control group showed that permetrin in both closed and open methods did not cause any significant mortality in D. gallinae. Thymol resulted in higher mortality rate in comparison to the control and permethrin-treated groups, but it was statistically significant only in closed method (P < 0.001). The highest mortality rate observed in fumigant assay belonged to the carvacrol in closed method which was statistically significant in comparison to all other groups (P < 0.001). Only in carvacrol and thymol groups but not permethrin and control that mortality rate in closed method (A) was significantly higher than that in open method(B). As it could be expected, farnesol in fumigant toxicity test similar to contact test again did not cause any mortality in D. gallinae.
Mean mortality rate of D. gallinae in fumigant toxicity bioassay in open (a) and closed (b) methods at the pre-determined LD50 value for each compound. Means are presented with standard errors. Means not sharing a common letter are significantly different (Student-Newman-Keuls multiple comparisons test (P < 0.05)
Discussion
Resistance of D. gallinae to available synthetic acaricides has been widely reported from all over the world (Fiddes et al. 2005; Nordenfors et al. 2001; Beugnet et al. 1997). Due to increasing resistance of D. gallinae to synthetic acaricides and lack of newly developed effective acaricide, it is likely that D. gallinae will pose an increasing threat to the world poultry production. Therefore, new resource of acaricides for effective control of D. gallinae is urgently needed. Plant essential oils and plant-derived bioactive compounds have been suggested as alternatives for mite control. Several essential oils and extracts have been shown to pose acaricidal activity against D. gallinae (Kim et al. 2004; Abdel-Ghaffar et al. 2009; George et al. 2009a, b, c). Locher et al. (2010) described a neem product, MiteStop®, as an effective botanical acaricide for the control of the poultry red mite. Based on LD50 values, Kim et al. (2004) reported that acaricidal activity of some plant extract preparations was almost comparable to that of profenofos (a synthetic pesticide). George et al. (2009b) reported that essential oils of thyme, manuka, and pennyroyal gave for D. gallinae LC99 values less than 0.30 mg/cm3, confirming that these products are toxic to this pest. In the present study, carvacrol and thymol showed significant acaricidal activity against D. gallinae. As jugged from LD50 values, carvacrol was the most potent acaricides for D. gallinae. Jeong et al. (2008) reported acaricidal activity of thyme oil against Tyrophagus putrescentiae, a stored food mite. They purified biologically active constituents of thyme oil and revealed carvacrol and thymol as major acaricidal components. These researchers identified carvacrol as the most toxic component with LD50 of 4.5 μg/cm2 significantly different from thymol with 11.1 μg/cm2. Their findings were in line with the results of the present study which found carvacrol as the most efficient acaricide in comparison to other compounds. George et al. (2009b) reported LD50 values of 0.014 and 0.039 mg/ cm3 for thyme oil, while in the present study, carvacrol gave a LD50 value of 1 μg/cm3 and thymol 3.51 μg/cm3. Comparison of the obtained LD50 values with the results of George et al. (2009b) revealed that, probably, acaricidal activity of thyme oil primarily is for its thymol and carvacrol constituents. The lower LD50 value of carvacrol represented that it may be the main contributor of the toxicity of thyme oil against D. gallinae.
Kim et al. (2007) showed that toxic effects of some plant preparations against D. gallinae were mainly caused by action in the vapor phase. In the current study, fumigant toxicity of carvacrol and thymol to D. gallinae was noticeable. Mortality rate of D. gallinae for carvacrol and thymol in closed method was significantly higher than in the open one. These results indicated that the acaricidal action of carvacrol and thymol is mostly associated with fumigant toxicity. In common with these findings, George et al. (2009b) described fumigant toxicity of thyme, manuka, and pennyroyal oil against D. gallinae by fumigant toxicity. Acaricidal action against D. gallinae mainly by fumigant toxicity because of behavioral characteristics of this mite could be considered as a beneficial trait. The poultry red mite only feeds for short periods in dark hours every few days and other times hides in the cracks and crevices of the poultry cages. This may make it tough to eliminate D. gallinae by means of only contact toxic acaricide. Carvacrol and thymol due to significant fumigant acaricidal efficacy can be very useful as potential control agents against D. gallinae.
Permethrin exhibited less acaricidal activity than carvacrol and thymol in both contact and fumigant toxicity assays. Probably, population of mites used in the present study were partly resistant to permethrin. Meanwhile, mortality rate for permethrin in fumigant toxicity test in comparison of closed and open methods did not show any significant difference. This may be due to lower fumigant activity of permethrin than that of carvacrol and thymol. As previously mentioned, in farnesol-exposed mites, not only that no mortality was recorded but also that noticeable increase in mite activity was observed. This made the authors to explore more for understanding possible underlying mechanism of mite hyper-activation. Reviewing literature manifests that the identical chemical to farnesol was isolated from female mites, where the chemical serves as sex pheromones to attract male mites for mating (Regev and Cone 1976). This may explain why mites exposed to farnesol became more active and this compound per se could not serve as a pesticide against D. gallinae.
George et al. (2009a, 2009b) in two separate studies showed that in spite of using mites and essential oils from the same source, they obtained different LD50, LD90, and LD99 values for essential oils. To achieve more reliable and repeatable results and omitting inconsistence in findings, applying bioactive constituents of essential oils such as what was done in present study could be suggested. Variation in the efficacy of essential oils has been reported as one reason for the poor market penetration of plant-derived insecticides (Isman 2008). To compete synthetic acaricides and to obtain organic animal products with less concern about residues, potentially active constituents of plants, such as carvacrol and thymol, can be used as novel bioacaricide.
References
Abdel-Ghaffar F, Sobhy MH, Al-Quraishy S, Semmler M (2008) Field study on the efficacy of an extract of neem seed (Mite -Stop®) against the red mite Dermanyssus gallinae naturally infecting poultry in Egypt. Parasitol Res 103:481–485
Abdel-Ghaffar F, Semmler M, Al-Rasheid K, Mehlhorn H (2009) In vitro efficacy of ByeMite® and Mite-Stop® on developmental stages of the red chicken mite Dermanyssus gallinae. Parasitol Res 105:1469–1471
Ahn YJ, Lee SB, Lee HS, Kim GH (1998) Insecticidal and Acaricidal Activity of Carvacrol and β-Thujaplicine Derived from Thujopsis dolabrata var. hondai Sawdust. J Chem Ecol 24:81–90
Archana PR, Nageshwar Rao B, Satish Rao BS (2011) Modulation of gamma ray-induced genotoxic effect by thymol, a monoterpene phenol derivative of cymene. Integr Cancer Ther 10:374–383
Beugnet F, Chauve C, Gauthey M, Beert L (1997) Resistance of the red poultry mite to pyrethroids in France. Vet Rec 140:577–79
Chauve C (1998) The poultry red mite Dermanyssus gallinae (De Geer, 1778): current situation and future prospects for control. Vet Parasitol 79:39–45
Chikuba T, Itou H, Sakakibara H, Inoue D (2008) Detection of fowl pox virus from red mite (Dermanyssus gallinae) at a layer farm occurring cutaneous fowlpox. J Jpn Soc Poult Dis 44:113–117
Chirico J, Eriksson H, Fossum O, Jansson D (2003) The poultry red mite, Dermanyssus gallinae, a potential vector of Erysipelothrix rhusiopathiae causing erysipelas in hens. Med Vet Entomol 17:232–234
Cimanga K, Kambu K, Tona L, Apers S, De Bruyne T, Hermans N, Totté J, Pieters L, Vlietinck AJ (2002) Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J Ethnopharmacol 79:213–220
Faghihzadeh Gorji S, Faghihzadeh Gorji S, Rajabloo M (2014) The field efficacy of garlic extract against Dermanyssus gallinae in layer farms of Babol, Iran. Parasitol Res 113:1209–13
Fiddes MD, Le Gresley S, Parsons DG, Epe C, Coles GC, Stafford KA (2005) Prevalence of the poultry red mite (Dermanyssus gallinae) in England. Vet Rec 157:233–235
George DR, Sparagano OAE, Port G, Okello E, Shiel RS, Guy JH (2009a) Environmental interactions with the toxicity of plant essential oils to the poultry red mite Dermanyssus gallinae. Med Vet Entomol 24:1–8
George DR, Smith TJ, Shiel RS, Sparagano OAE, Guy JH (2009b) Mode of action and variability in efficacy of plant essential oils showing toxicity against the poultry mred mite, Dermanyssus gallinae. Vet Parasitol 161:276–282
George DR, Biron JM, Jolly G, Duvallet G, Sparagano OAE (2009c) Toxicity of geraniol solution in vitro to the poultry red mite, Dermanyssus gallinae. Parasit 16:319–321
Hamidi A, Sherifi K, Muji S, Behluli B, Latifi F, Robaj A, Postoli R, Hess C, Hess M, Sparagano OAE (2011) Dermanyssus gallinae in layer farms in Kosovo: a high risk for Salmonella prevalence. Parasit Vectors 4:136
Hollis J (2009) Biopesticides registration action document. Farnesol Nerolidol
Horn TL, Long L, Cwik MJ, Morrissey RL, Kapetanovic IM, McCormick DL (2005) Modulation of hepatic and renal drug metabolizing enzyme activities in rats by subchronic administration of farnesol. Chem Biol Interact 152:79–99
Isman MB (2008) Botanical insecticides: for richer, for poorer. Pest Manag Sci 64:8–11
Jayakumar S, Madankumar A, Asokkumar S, Raghunandhakumar S, Gokula Dhas K, Kamaraj S, Divya MG, Devaki T (2012) Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol Cell Biochem 360:51–60
Jeong EY, Lim JH, Kim HG, Lee HS (2008) Acaricidal activity of Thymus vulgaris oil and its main components against Tyrophagus putrescentiae, a stored food mite. J Food Prot 71:351–5
Kilpinen O, Roepstorff A, Permin A, Norgaard-Nielsen G, Lawson LG, Simonsen HB (2005) Influence of Dermanyssus gallinae and Ascaridia galli infections on behaviour and health of laying hens (Gallus gallus domesticus). Br Poult Sci 46:26–34
Kim S, Yi J, Tak J, Ahn Y (2004) Acaricidal activity of plant essential oils against Dermanyssus gallinae (Acari: Dermanyssidae). Vet Parasitol 120:297–304
Kim S, Na YE, Yi JH, Kim BS, Ahn YJ (2007) Contact and fumigant toxicity of oriental medicinal plant extracts against Dermanyssus gallinae (Acari: Dermanyssidae). Vet Parasitol 145:377–382
Kirkwood AC (1968) Anemia in poultry infested with red mite Dermanyssus gallinae. Vet Rec 80:514–516
Kočišová A, Plachʹy J (2008) Novel approach to controlling the poultry red mite (Acarina: Mesostigmata).In Proc. Int. Conf. Urban Pests, 6th, Budapest. OOK-Press Kft, Hungary, pp 349–54, July 13–16
Locher N, Al-Rasheid Kh AS, Abdel-Ghaffar F, Mehlhorn H (2010) In vitro and field studies on the contact and fumigant toxicity of a neem-product (Mite-Stop®) against the developmental stages of the poultry red mite Dermanyssus gallinae. Parasitol Res 107:417–423
Miresmailli S, Bradbury R, Isman MB (2006) Comparative toxicity of Rosmarinus officinalis L. essential oil and blends of its major constituents against Tetranychus urticae Koch (Acari: Tetranychidae) on two different host plants. Pest Manag Sci 62:366–371
Nordenfors H, Höglund J, Tauson R, Chirico J (2001) Effects of permethrin impregnated plastic strips on Dermanyssus gallinae in loose housing systems for laying hens. Vet Parasitol 102:121–31
Rahbari S, Nabian S, Ronaghi H (2009) Haematophagus Mites in Poultry Farms of Iran. Iran J Arthropod Borne Dis 3:18–21
Regev S, Cone WW (1976) Evidence of gonodotropic effect of farnesol in the twospotted spider mite, Tetranychus urticae. Environ Entomol 5:517–519
Sparagano O, Pavlicevic A, Murano T, Camarda A, Sahibi H, Kilpinen O, Mul M, van Emous R, le Bouquin S, Hoel K, Cafiero MA (2009) Prevalence and key figures for the poultry red mite Dermanyssus gallinae infections in poultry farm systems. Exp Appl Acarol 48:3–10
Sparagano O, Khallaayoune K, Duvallet G, Nayak S, George D (2013) Comparing Terpenes from Plant Essential Oils as Pesticides for the Poultry Red Mite (Dermanyssus gallinae). Transbound Emerg Dis 60:150–153
US EPA (2004) Biopesticides—25b Minimum Risk Pesticides. http://www.epa.gov/oppbppd1/biopesticides/regtools/25b_list.htm
Wang FF, Wang M, Xu FR, Liang DM, Pan BL (2010) Survey of prevalence and control of ectoparasites in caged poultry in China. Vet Rec 167:934–37
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tabari, M.A., Youssefi, M.R., Barimani, A. et al. Carvacrol as a potent natural acaricide against Dermanyssus gallinae . Parasitol Res 114, 3801–3806 (2015). https://doi.org/10.1007/s00436-015-4610-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4610-0