Abstract
Antioxidants are one of the key players in tumorigenesis, several natural and synthetic antioxidants were shown to have anticancer effects. The aim of the present study is to divulge the chemopreventive nature of carvacrol during diethylnitrosamine (DEN)-induced liver cancer in male wistar albino rats. Administration of DEN to rats resulted in increased relative liver weight and serum marker enzymes aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and gamma glutamyl transpeptidase (γGT). The levels of lipid peroxides elevated (in both serum and tissue) with subsequent decrease in the final body weight and tissue antioxidants like superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH), glutathione peroxidase (GPx), and glutathione reductase (GR). Carvacrol supplementation (15 mg/kg body weight) significantly attenuated these alterations, thereby showing potent anticancer effect in liver cancer. Histological observations and transmission electron microscopy studies were also carried out, which added supports to the chemopreventive action of the carvacrol against DEN-induction during liver cancer progression. These findings suggest that carvacrol prevents lipid peroxidation, hepatic cell damage, and protects the antioxidant system in DEN-induced hepatocellular carcinogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hapatocellular carcinoma (HCC) is the most frequent primary malignancy of the liver. It accounts for about 90% of all liver cancer and it represents more than 4% of all cancer cases worldwide and is the fourth most common cause of cancer mortality [1]. Most major well known risk factors of hepatocellular carcinoma includes hepatitis viral infection (HBV and HCV), food additives, alcohol, fungal toxins (aflatoxins), toxic industrial chemicals, and air and water pollutants [2]. Diethylnitrosamine (DEN, N-Nitrosodiethylamine) a potent hepatocarcinogen, is known to cause perturbations in the nuclear enzymes involved in DNA repair/replication [3]. N-nitroso compounds are considered to be a tragedic health hazards to man, and these compounds were present in tobacco products, cheddar cheese, cured and fried meals, occupational settings, cosmetics, agricultural chemicals, and pharmaceutical agents [4, 5]. It has been suggested that DEN, after its metabolic activation produces the pro-mutagenic adducts, O6-ethyl deoxy guanosine and 04 and O6-ethyl deoxy thymidine in liver that may cause carcinogenic effects [6]. It is also reported that, the oxidative stress plays a causative role during carcinogenesis [7]. Reactive oxygen species (ROS), are predominant stimulator for tissue injury, DNA damage, and mutagenesis associated with various stages of tumor formation process [8, 9]. Hence, the model of DEN-induced liver cancer is considered as one of the most accepted and widely used experimental models to study about the hepatocarcinogenesis [10]. One recent approach to control liver cancer is chemoprevention, by definition it is the means of cancer management in which the occurrence of the disease can be entirely prevented, slowed, or reversed substantially by the administration of one or more nontoxic naturally occurring and/or synthetic agent called as anticarcinogen [11]. These synthetic compounds have been identified as having some potential cancer chemopreventive value [12]. This is the pharmacological intervention aims to arrest/reverse the process of carcinogenesis. A number of macro, micronutrients, and nonnutrients have been reported as the chemopreventive agents for the carcinogenic effects [11]. A potential for inhibiting tumor development in both targeted high risk and general populations has increased significantly in recent years [13, 14]. Almost 30 classes of chemicals with cancer preventive effects that may have practical implications in reducing cancer incidence in human population have been described [15].
Carvacrol or cymophenol (2-methyl-5-isopropyl phenol), is a predominant monoterpenic phenol which occurs in many essential oil of the family Labiatae including Origanum, Satureja, Thymbra, Thymus, and Corydothymus species [16]. It has a characteristic pungent, warm odor of oregano and a pizza-like taste. The oil from origanum vulgare has the highest naturally occurring carvacrol content, at 80% and is thought to be the most biologically active compound (Fig. 1) [17]. It is well known that essential oils, which are rich in carvacrol, possess strong antioxidant properties equivalent to those of ascorbic acid, butyl hydroxyltoluene (BHT), and vitamin E [18, 19]. Since many antioxidant exerts antiplatelet [20] and anticarcinogenic effects [21, 22], it is possible that carvacrol functions in a similar way. Monoterpenoids manifest different remarkable biological effects like antioxidative, anti-inflammatory, and have preventive and therapeutic effects against many diseases [23, 24]. Moreover, monoterpenoids have a chemopreventive role in cancer through the induction of enzymes affecting carcinogen metabolism and inhibiting various activities of tumor promoters which are involved in the process of carcinogenesis [25]. Carvacrol was reported to have a broad range of therapeutic effects, including anti-inflammatory, anti-platelet, anti-proliferative, and anti-carcinogenic effects caused in cancers (lung, breast) which is strongly supported by in vitro studies [26]. Eventhough carvacrol has been used for centuries as a herbal medicine and its anticancer effects on various cancers are studied, its effect on hepatocarcinogenesis is not yet documented. In this investigation, we evaluated that efficacy of carvacrol on DEN-induced hepatocarcinogenesis in rats.
Materials and methods
Animals
Male, wistar strain albino rats weighing about 150–180 g were obtained from Tamilnadu Veterinary and Animal Sciences University (TANUVAS), Madhavaram, Chennai, India. The animals were housed in cages under proper environmental conditions and were fed with a commercial pelletted diet (M/S Hindustan Foods Ltd, Bangalore, India). The animals had free access to water. All the experiments were designed and conducted according to the ethical norms approved by Institutional Animal Ethics Committee guidelines (IAEC No: 02/018/2010).
Source of chemicals
DEN and carvacrol were purchased from Sigma Chemicals Co. (St. Louis, MO, USA). All other chemicals used were of analytical grade.
Experimental design
The experimental animals were divided into five groups, each groups comprising of six animals.
- Group 1:
-
Normal control rats fed with standard diet and pure drinking water
- Group 2:
-
Rats were induced with hepatocarcinogenesis by providing 0.01% DEN through drinking water for 16 weeks
- Group 3:
-
Rats treated with carvacrol alone by orally, alternative days in a week at a dose of 15 mg/kg body weight (based on effective dose fixation studies) for 16 weeks
- Group 4:
-
Rats pretreated with carvacrol (15 mg/kg body weight) one week before the administration of 0.01% DEN and continued till end of the experiment (i.e., 16 weeks)
- Group 5:
-
Rats post-treated with carvacrol (15 mg/kg body weight) for 6 weeks after the administration of DEN for 10 weeks (i.e., after administering DEN alone for 10 weeks the rats were treated with carvacrol along with DEN for another 6 weeks) and continued till end of the experiment
After the experimental period, the rats were fasted overnight, anaesthetized with diethyl ether, and then killed by cervical decapitation.
Evaluation of biochemical parameters
After cervical decapitation, the blood samples were collected from the experimental animals and liver tissue was removed and washed in ice-cold saline. The total protein in the liver was estimated by the method of Lowry et al. The activities of marker enzymes aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and γ-glutamyl transpeptidase (γGT) were assayed in the serum [27–29]. The activities of antioxidant enzymes—superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR) and glutathione (GSH)—were estimated in the liver tissue homogenate [30–33]. The levels of lipid peroxidation (LPO) products were estimated in serum and liver tissue homogenate [34].
Histopathological evaluation
Histological evaluation was performed on a lobe of the liver and a portion of the specimen was fixed in 10% formalin and embedded in paraffin wax, sectioned at 4 μm thickness and were stained with hemotoxylin–eosin. Light microscopy was used to evaluate pathological changes of liver.
Transmission electron microscopy
The liver samples were fixed in karnovsky’s fixative for 6–8 h at 4oC. These were post fixed in 1% osmium tetraoxide in 0.1 M phosphate buffer for 2 h at 4oC, dehydrated in ascending grades of acetone, infiltrated and embedded in araldite CY212, and polymerized at 60oC for 72 h. Thin (60–70 nm) sections were mounted on copper grids and stained with uranyl acetate and lead citrate and observed under a transmission electron microscope (TEM).
Statistical analysis
All the grouped data were significantly evaluated with SPSS/10 software. Hypothesis testing methods included one way analysis of variance (ANOVA) followed by least significant difference (LSD) test. P values of less than 0.05 were considered to indicate statistical significance. All these results were expressed as mean ± S.D for six animals in each group.
Results
General observations
The anticancer effects of carvacrol against DEN-induced hepatocellular cancer was elucidated in male wistar albino rats. Table 1 shows the total number of nodules and number of nodules per nodule-bearing liver and nodular sizes in millimeter in tumor-bearing animals. The carvacrol-treated groups 4 and 5 showed a significant decrease in the number of nodules when compared with group 2 animals. Figure 2 shows the body weight of control and experimental group of animals. In DEN-induced group 2 animals, there is a significant decrease in the final body weight when compared with group 1 control animals. The carvacrol-treated groups 4 and 5 showed a significant increase in the final body weight when compared with group 2 animals. During the course of the experiment, all rats showed the greater tolerance to treatment with carvacrol. Figure 3 shows the liver weight and relative liver weight of control and experimental group of animals. In group 2 animals, the relative liver weight is significantly increased when compared with group 1 animals and there is a significant decrease in the liver weight in carvacrol-treated groups 4 and 5 animals when compared with group 2 animals. No obvious changes were observed between the control and carvacrol alone treated group which is an indicative of nontoxic nature of carvacrol.
Liver weight and relative liver weight of control and experimental groups of rats. Results are expressed as mean ± S.D for six rats in each group. Statistical significance at P < 0.05 compared with with a group 1, b group 2, and c group 5. Liver weight is expressed in grams. Relative liver weight is the average of liver weight at final body weight multiplied by 100
Assessment of lipid peroxidation
Figure 4 shows the level of LPO in the serum and liver of control and experimental groups of animals which was analyzed for oxidative stress. In DEN-induced group 2 animals, there is a significant increase in the levels of lipid peroxides when compared with group 1 normal control animals. Which could be a tumor burden. Whereas in carvacrol-treated groups 4 and 5 animals, there is a significant decrease in the levels of lipid peroxides when compared with group 2 tumor-bearing animals. However, animals treated with carvacrol alone (group 3) did not show any significant changes when compared with group 1 control animals.
Effect of carvacrol on the levels of lipid peroxides in the serum and liver of control and experimental groups of rats. Results are expressed as mean ± S.D for six rats in each group. Statistical significance at P < 0.05 compared with with a group 1, b group 2, and c group 5. LPO levels are expressed as nmol of MDA formed/min/mg protein
Effect of carvacrol on the activities of marker enzymes
Table 2 portrays the effect of carvacrol on the activities of marker enzymes AST, ALT, ALP, LDH, and γGT in the serum of control and experimental group of rats. These marker enzymes are significantly (P < 0.05) increased in DEN-induced group 2 animals when compared with group 1 normal control animals. Carvacrol-treated groups 4 and 5 showed a significant decrease in the activities of these enzymes when compared with group 2 DEN-induced animals. This reveals that carvacrol has restoration potential of liver tissue.
Evaluation of antioxidant status in liver
Table 3 depicts the antioxidant status in the liver of control and experimental group of animals. DEN-induced group 2 animals exhibited a significant decrease in the activities of SOD and CAT when compared with group 1 normal control animals, carvacrol-treated groups 4 and 5 showed a significant increase in the activities of SOD and CAT when compared with group 2 DEN-induced animals. The activities of GPx, GR, and GSH also significantly decreased in DEN-induced group 2 tumor-bearing animals when compared with group 1 control animals. In carvacrol-treated groups 4 and 5 animals, there is a significant increase in the activities of GPx, GR, and GSH when compared with group 2 DEN-induced animals. No adverse effect was observed in group 3 animals.
Effect of carvacrol on macroscopic gross appearance of liver
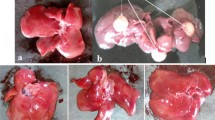
Macroscopic appearance of the liver cells of control group 1 animals shows normal morphology (Fig. 5a). DEN alone administered group 2 animals showing heavy enlargement and several grayish white nodules and foci on the peripheral surface of the liver (Fig. 5b, c). Liver cell shows normal morphology in carvacrol alone treated group 3 animals (Fig. 5d). Most of the foci and nodules disappeared in the liver from DEN + carvacrol-pretreated group (group 4) of rats showing the effect of chemoprevention (Fig. 5e). Many persistent but tiny nodules in a DEN + carvacrol post-treated group (group 5) of rats show the therapeutic effects (Fig. 5f).
Macroscopic gross appearance of livers at the end of experimental period in control and experimental group of rats. a Liver of a control animal showing normal morphology. b, c DEN alone administered group showing heavy enlargement and several grayish white nodules and foci on the peripheral surface of the liver as the features of HCC. d Liver cell showing normal morphology in carvacrol alone treated group of rats. e Most of the foci and nodules disappeared in the liver from DEN + carvacrol pretreated group of rats showing the effect of chemoprevention. f Many persistent but tiny nodules in an DEN + carvacrol post-treated group of rats
Effect of carvacrol on histological features of liver
Histological examination of liver sections from control group 1 animals revealed normal architecture and cells with granulated cytoplasm and small uniform nuclei (Fig. 6a). Group 2 animals revealed loss of architecture, marked a tendency to spread by intrahepatic veins, both hepatic and portal with significant tumor thrombi within portal vessels. Cytologically tumor cells are slightly larger, have more irregular nuclei and numerous mitotic figures (Fig. 6b, c). Group 3 animals showed normal architecture indicating the nontoxic nature of carvacrol (Fig. 6d). Whereas group 4 animals pretreated with carvacrol show few neoplastically transformed cells and hepatocytes maintaining near normal architecture (Fig. 6e), group 5 animals post-treated with carvacrol showed loss of architecture, comparatively less tendency to spread by intrahepatic veins, both hepatic and portal vessels (Fig. 6f).
Histopathological examination of liver tissue in control and experimental groups of rats. a Control showing a normal architecture (10×, HE). b, c DEN alone slides showing loss of architecture, a marked tendency to spread by intrahepatic veins, both hepatic and portal with significant tumor thrombi within portal vessels. Cytologically tumor cells are slightly larger, have more irregular nuclei and also numerous mitotic figures (20×, HE). d Drug control slides showing normal liver architecture (10×, HE). e Carvacrol pretreated showing few neoplastically transformed cells and hepatocytes maintaining near normal architecture (20×, HE). f Carvacrol post-treated slides showing loss of architecture, comparatively less tendency to spread by intrahepatic veins, both hepatic and portal vessels (20×, HE)
Effect of carvacrol on ultra-structural changes of liver
Ultra-structural studies of the liver cells of control group 1 animals show normal nucleus and cytoplasm (Fig. 7a). In DEN-induced group 2 animals, presence of multiple irregular nuclei close to each other with irregular cytoplasm demonstrates dysplasia (Fig. 7b, c). Carvacrol alone treated group 3 animals showed normal architecture (Fig. 7d). Morphological changes of apoptosis were seen in the liver cells of DEN + carvacrol-pretreated group 4 rats (Fig. 7e). Liver cell with shrunken nucleus and condensed chromatin undergoing apoptosis were seen in DEN + carvacrol post-treated group 4 rats (Fig. 7f).
Ultra-structural changes in liver cells viewed under TEM in control and experimental groups of rats. a Normal structure in the nuclei and cytoplasm of liver cells of control rats (10,000×). b, c Presence of multiple irregular shaped nuclei close to each other with irregular cytoplasm in the liver cells of DEN-induced rats (10,000×). d Liver cell showing normal architecture in carvacrol alone treated rats (15,000×). e Morphological changes of apoptosis in the liver cells of DEN + carvacrol-pretreated rats (10,000×). f Liver cell with little shrunken nucleus and condensed chromatin under going apoptosis in DEN + carvacrol post-treated rats (20,000×)
Discussion
Hepatic injury caused by DEN generally reflects instability of liver metabolism which leads to distinctive changes in the serum enzyme activities [35]. Intracellular enzymes, such as transaminases, ALP, LDH, and γGT are useful indicators for liver function; their increased levels are indicators of liver damage. Aminotransferases (AST and ALT) are a reliable marker enzymes of liver and they are the first enzymes to be used in diagnostic enzymology when liver damage has occurred [36]. Because of their intracellular location in the cytosol, toxicity affecting the liver with subsequent breakdown in membrane architecture of the cells leads to their spillage into serum, and their concentration rises in the latter. The discharge of LDH reflects a nonspecific alteration in the plasma membrane integrity and/or permeability. LDH is a familiar sensitive marker of solid neoplasm [37] and many studies revealed increased LDH activity in various types of tumor [38]. γGT is an enzyme embedded in the hepatocyte plasma membrane, mainly in the canalicular domain; again the liberation of this enzyme into serum indicates damage to the cell and thus injury to the liver. It is point outing that serum γGT activity is considered to be one of the best indicators of liver damage [35]. In the present study, carvacrol treatment significantly attenuated the increased activities of these enzymes. It is reported that carvacrol helps in parenchymal cell regeneration in liver, thus protecting membrane integrity and thereby minimizing enzyme leakage [39].
Oxidative stress is associated with damage to a wide range of macromolecular species including lipids, proteins, and nucleic acids thereby producing major interrelated derangements of cellular metabolism including peroxidation of lipids. Free radicals and nonradicals oxidizing species were produced in animals treated with carcinogens, and also in human tissues [40]. Reactive oxygen species (ROS) is formed from endogenous or exogenous sources are highly reactive, toxic, and mutagenic [41]. DEN has been shown to generate free radicals [42], an uncompromising free radical generation in the liver overwhelms the antioxidant status and ultimately proceeds to oxidative stress paving way to carcinogenesis [43]. Lipid peroxidation plays an important role in carcinogenesis [44], is the most studied biologically relevant free radical chain reaction. Induction of DEN has been reported to generate lipid peroxidation products like malondialdehyde and 4-hydroxy nonenal that may interact with various molecules leading to cause oxidative stress and carcinogenicity [45]. Increased level of LPO was recently reported during DEN-induced hepato carcinogenesis. This dynamic action may further lead to uncompromised production of free radicals overwhelming the cellular antioxidant defense [46]. It has been extensively reported that free radicals participated in DEN-induced hepatocarcinogenesis. LPO generation at the initiation stage can be prevented by free radicals scavengers and antioxidant action of carvacrol. Animals treated with carvacrol (both pre and post) exhibited significantly lowered the levels of LPO, both in liver and serum, when compared with animals induced with DEN. This shows the antilipid peroxidative role of carvacrol that is probably mediated by its ability to scavenge free radical generation [47, 48].
Antioxidants possess a variety of biological activities, including the induction of drug-metabolizing enzymes, inhibition of prostaglandin synthesis, inhibition of carcinogen-induced mutagenesis, and scavenging of free radicals [49]. Antioxidants may protect membrane from ROS toxicity by prevention of ROS formation by the interruption of ROS attack, by facilitating the repair caused by ROS and by providing cofactors for the effective functioning of other antioxidants [50]. Development of life threatening diseases like cancer is linked to the availability of these antioxidants [51]. Natural antioxidants are capable of inhibiting the ROS production and thereby reducing the associated intracellular oxidative stress [52].
SOD is the first line of defense in the antioxidant system against the oxidative damage mediated by superoxide radicals [53]. Superoxide dismutases catalyze the dismutation of superoxide radical to hydrogen peroxide and water [54]. Furthermore, CAT or GPx catalyze the transformation of H2O2 to harmless byproducts. Glutathione, a cysteine-containing tripeptide, is required to maintain the normal reduced state of cells and to counteract all the deleterious effects of oxidative stress. GSH is said to be involved in many cellular processes including the detoxification of endogenous and exogenous compounds. DEN, an electrophilic carcinogen may interact with the large nucleophilic pool of GSH thereby reducing the macromolecule and carcinogen interaction [55]. In carvacrol (both pre and post) treated animals, there was a significantly higher level of GSH in liver when compared to DEN-induced animals consistent with the idea of attenuation of DNA–carcinogen interaction and thereby averting a favorable environment for carcinogenesis.
Decreases in the activities of SOD, CAT, GPx, GR, and GSH are seen in tumor cells. The compounds that can scavenge excessive free radicals in the body are suggested to hinder the process of carcinogenesis [56]. Such studies support our findings as we had seen a significant decrease in the activities of antioxidant enzyme in both serum and liver of animals treated with carcinogen in comparison with normal animals. On the other hand, there is a significant increase in the activities of antioxidant enzymes in liver of the animals administered both carvacrol and carcinogen when compared with animals administered carcinogen alone.
Ultra-structural studies were performed to further confirm the occurrence of apoptotic morphological changes at the cellular level. The control animals showed normal nuclei and cytoplasm. The animals induced with DEN showed the presence of multiple irregular shaped nuclei close to each other with irregular cytoplasm were seen which might be due to the excessive free radicals generation during DEN administration [57, 58]. Carvacrol alone treated animals showed normal architecture, which shows that the carvacrol did not induce any intracellular morphology of liver cell, which ultimately shows its nontoxic nature at given dosage. Morphological changes of apoptosis were seen in the liver cells of DEN + carvacrol-pretreated rats, which reveal that the carvacrol may induce apoptosis. Liver cell with shrunken nucleus and condensed chromatin undergoing apoptosis were seen in DEN + carvacrol post-treated rats. Thus, the results of the ultrastructural studies undoubtedly confirmed that carvacrol has the ability to cause apoptosis in cancer cells.
In conclusion, the present study demonstrates that the carvacrol possesses potent free radical scavenging and antioxidant activities. From the results, it is evident that carvacrol is capable of modulating the levels of LPO and significantly increases the endogenous antioxidant defense mechanisms in DEN-induced hepatocellular carcinogenesis. Our results also show that the significant increase in the levels of serum markers was prevented by carvacrol treatment. Then, we suggest that carvacrol may be developed as an effective chemotherapeutic agent. Further studies are underway to elucidate the molecular mechanisms involved to prove carvacrol’s efficacy as an anti-cancer agent.
References
Harris CC, Sun T (1984) Multifactoral etiology of human liver cancer. Carcinogenesis 5:697–701. doi:10.1093/carcin/5.6.697
Farazi PA, Depinho RA (2006) Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer 6:674–687. doi:10.1038/nrc1934
Bhosale P, Motiwale L, Ignle AD, Gadre RV, Rao KVK (2002) Protective effect of Rhodotorula glutinis NCIM3353 on the development of hepatic preneoplastic lesions. Curr Sci 83:303–308
Sullivan BP, Meyer TJ, Stershic MT, Keefer LK (1991) Acceleration of N-nitrosation reactions electrophiles. IARC Sci Publ 105:370–374
Reh BD, Fajen JM (1996) Worker exposure to nitrosamines in a rubber vehicle sealing plant. Am Ind Hyg Assoc J 57:918–923
Verna L, Whysner J, Williams GM (1996) N-nitrodiethylamine mechanistic data and risk assessment: Bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther 71:57–81. doi:10.1016/0163-7258(96)00062-9
Kensler TW, Taffe BG, Egner PA, Trush MA (1989) Role of free radicals in tumor promotion and progression. Prog Clin Biol Res 298:233–248
Beckman KB, Ames BN (1997) Oxidative decay of DNA. J Biol Chem 272:19633–19636. doi:10.1074/jbc.272.32.19633
Parola M, Robino G (2001) Oxidative stress-related molecules and liver fibrosis. J Hepatol 35:297–306
Ha WS, Kim CK, Song SH, Kang CB (2001) Study on mechanism of multistep hepatotumorigenesis in rat: development of hepatotumorigenesis. J Vet Sci 2:53–58
Wattenberg LW (1992) Inhibition of carcinogenesis by minor dietary constituents. Cancer Res 52:2085s–2091s
Kellof GJ (2000) Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res 78:199–334
Morse MA, Stoner GD (1993) Cancer chemoprevention: principles and prospects. Carcinogenesis 14:1737–1746. doi:10.1093/carcin/14.9.1737
Hong WK, Sporn MB (1997) Recent advances in chemoprevention of cancer. Science 278:1073–1077. doi:10.1126/science.278.5340.1073
Wattenberg LW (1997) An overview of chemoprevention: current status and future prospects. Proc Soc Exp Biol Med 216:133–141
Krimer N, Baser KHC, Tumen G (1995) Carvacrol rich plants in Turkey. Chem Nat Compounds 31:37–42
Arcila Lozano CC (2004) Oregano: properties, composition and biological activity. Arch Latinoam Nut 54:100–111
Ruberto G, Baratta MT, Deans SG, Dorman HJ (2000) Antioxidant and antimicrobial activity of Foeniculum vulgare and Crithmum maritimum essential oils. Planta Med 66:687–693. doi:10.1055/s-2000-9773
Alma MH, Mavi A, Yildirim A, Digrak M, Hirata T (2003) Screening chemical composition and in vitro antioxidant and antimicrobial activities of the essential oils from Oreganum Synaceum L growing in Turkey. Biol Pharm Bul 26:1725–1729. doi:10.1248/bpb.26.1725
Karkabounas S, Sofis G, Evangelou A (1996) Implication of free radicals in platelet aggregation: antiplatelet effects of free radical scavengers ex vivo. Epith Klin Farmacol Farmakokin 10:84–91
Evangelou A, Kalpouzsos G, Karkabounas S, Liasko R, Nonni A, Stefanou D, Kallistratos G (1997) Dose-related preventive and therapeutic effects of antioxidants and anticarcinogens on experimentally induced malignant tumors in Wistar rats. Cancer Lett 115:105–111. doi:10.1016/S0304-3835(97)04712-5
Karkabounas S, Binolis J, Zelovitis J, Kotsis N, Charalabopoulos A, Avdikos A, Zouridakis A, Liasko R, Giannakopoulos X, Charalabopoulos K (2002) Inhibition and modification of benzo[a]pyrene-induced chemical carcinogenesis by ascorbic acid alone or in combination with α-tocopherol in Wistar rats. Exp Oncol 24:274–278
Kalemda D, Kunicka A (2003) Antibacterial and antifungal properties of essential oils. Curr Med Chem 10:813–829
Horvathova E, Sramkova M, Labaj J, Slamenova D (2006) Study of cytotoxic, genotoxic and DNA-protective effects of selected plant volatiles on human cells cultured in vitro. Neuro Endocrinol Lett 2:44–47
Cassady JM, Baird WM, Chang CJ (1990) Natural products as a source of potential cancer chemotherapeutic and chemopreventive agents. J Nat Prod 53:23–41
Zeytingolu M (1998) Inhibitory effect of carvacrol on DMBA induced pulmonary tumorigenesis in rats. Acta pharmaceuticaturcica 40:93–98
King J (1965) The transferase-alanine and aspartate transaminase. In: King J (ed) Practical clinical enzymology. D Van Nostrand Company Ltd, London, pp 121–138
King J (1965) The dehydrogenases or oxidoreductase-lactate dehydrogenase. In: King J (ed) Practical clinical enzymology. D Van Nostrand Company Ltd, London, pp 83–93
Rosalki SB, Rau D (1972) Serum gamma-glutamyl transpeptidase activity in alcoholism. Clin Chim Acta 39:41–47. doi:10.1016/0009-8981(72)90297-5
Misra HP, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WGO (1973) Selenium, biochemical role as a component of glutathione peroxidase purification and assay. Science 179:588–590. doi:10.1126/science.179.4073.588
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:380–395
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione- S-transferase activities in rat lung and liver. Biochem Biophys Acta 582:67–78. doi:10.1016/0304-4165(79)90289-7
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Bulle F, Mavier P, Zafrani ES, Preaux AM, Lescs MC, Siegrist S, Dhumeaux D, Guellaen G (1990) Mechanism of gamma-glutamyl transpeptidase release in serum during intrahepatic and extrahepatic cholestasis in the rat: a histochemical, biochemical and molecular approach. Hepatology 11:545–550
Whittby LG, Perey-Robb IW, Smith AT (1984) Enzymes tests in diagnosis, Lecture notes in clinical chemistry, 3rd edn. Black Well Scientific Publications, London, pp 138–169
Lippert M, Papadopoulos N, Javadpour NR (1981) Role of lactate dehydrogenase isoenzymes in testicular cancer. Urology 18:50–53
Ramakrishnan G, Augustine TA, Jagan S, Vinodkumar R, Devaki T (2007) Effect of silymarin on N-Nitrosodiethylamine induced hepatocarcinogenesis in rats. Exp Oncol 29:39–44
Canbek M (2008) Effects of carvacrol on defects of ischemia-reperfusion in the rat liver. Phytomedicine 15:447–452
Sun Y (1990) Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med 8:583–599
Halliwell B (1994) Free radicals, antioxidant and human disease: curiosity, cause or consequence? Lancet 344:721–724. doi:10.1016/S0140-6736(94)92211-X
Halliwell B, Gutteridge JMC (1989) Protection against oxidants in biological systems: the superoxide theory of oxygen toxicity. In: Cheeseman KH (ed) Free radicals in biology and medicine. Clarendon Press, Oxford, pp 144–147
Gey KF (1993) Prospects for the prevention of free radical disease, regarding cancer and cardiovascular disease. Br Med Bull 49:679–699
Banakar MC, Paramasivan SK, Chattopadhyay MB, Datta S, Chakraborty P, Chatterjee M, Kannan K, Thyagarajan E (2004) α1, 25-dihydroxyvitamin D3 prevents DNA damage and restores antioxidant enzymes in rat hepatocarcinogenesis induced by diethylnitrosamine and promoted by phenobarbital. World J Gasteroenterol 10:1268–1275
Hietanen E, Ahotupa M, Bartsch H (1987) Lipid peroxidation and chemically induced cancer in rats fed lipid rich diet. In: Lapis K (ed) In carcinogensis and tumor progression. Akademiaikiado Press, Budapest, pp 9–16
Klaunig JE, Kamendulis LM (2004) The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol 44:239–267. doi:10.1146/annurev.pharmtox.44.101802.121851
Aeschbach R (1994) Antioxidant actions of thymol, carvacrol, 6-gingerol, zingerone and hydroxytyrosol. Food Chem Toxicol 32:31–36
Yanishlieva NV (1999) Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem 64:59–66
Hirose M, Imaida K, Tamano S (1994) Cancer chemoprevention by antioxidants. In: Ho CT (ed) Food phytochemicals: teas, spices and herbs. American Chemical Society Press, Washington, pp 122–132
Sen CK (1995) Oxygen toxicity and antioxidants: state of the art. Int J Physiol Pharmacol 39:177–196
Gutteridge JM (1994) Antioxidants, nutritional supplement and life threatening diseases. Br J Biomed Sci 51:288–295
Feng Q, Kumagai T, Torii Y, Nakamura Y, Osawa T, Uchida K (2001) Anticarcinogenic antioxidants as inhibitors against intracellular oxidative stress. Free Radic Res 35:779–788
Oberley LW, Oberley TD (1986) Free radicals, cancer and aging. In: Johnson J (ed) Free radicals, aging and degenerative diseases. Alan R Liss Inc, New York, pp 325–371
Mccord JM, Fridovich I (1969) The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem 244:6056–6063
Chasseaud LF (1979) The role of glutathione and glutathione-s-transferase in the metabolism of chemical carcinogens and other electrophilic agents. Adv Cancer Res 29:175–274
Sumathi R, Baskaran G, Varalakshmi P (1996) Effect of dl-lipoic acid on tissue redox state in acute cadmium-challenged tissues. J Nutr Biochem 7:85–92
Bozzi A, Mavalli I, Finazzi A (1976) Agro, Enzyme defense against reactive oxygen derivatives. Part II: Erythrocyte and tumor cells. Mol Cell Biochem 10:11–16
Barretto OC, Zyngier SB (1984) Acquired disturbance of erythrocyte glutathione reductase in experimental tumors. J Cancer Res Clin Oncol 108:252–253
Acknowledgments
The authors wish to thank Dr. R. Sridharan, Pathologist, Department of Veterinary Pathology, Madras Veterinary College, Chennai, for his help in histopathological studies. The TEM work was carried out at EM facility, Department of Gastrointestinal Sciences, Christian Medical College, Vellore that is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jayakumar, S., Madankumar, A., Asokkumar, S. et al. Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol Cell Biochem 360, 51–60 (2012). https://doi.org/10.1007/s11010-011-1043-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-011-1043-7