Abstract
The poultry red mite, Dermanyssus gallinae, represents a key threat for the poultry industry worldwide. The control of D. gallinae is mainly achieved by continuous applications of acaricides. However, the fast-growing development of resistance, and the strict laws concerning chemicals admitted for treatments on food animals, highlighted the importance of alternative control tools. Here, we explored the potential of Artemisia sieberi essential oil against D. gallinae. In this study, the A. sieberi essential oil was analyzed using GC and GC-MS. The oil toxicity through contact and fumigant assays on adult mites was evaluated. The oil repellent activity was assessed on adult mites over different time intervals. Lastly, the residual toxicity of various doses of the oil was evaluated on D. gallinae until 14 days post treatment. GC and GC-MS showed that the oil was rich in α-thujone (31.5%), β-thujone (11.92%), camphor (12.3%), and 1,8-cineole (10.09%). Contact toxicity on adult mites showed 50% lethal concentration (LC50), LC90, and LC99 of 15.85, 26.63, and 35.42 μg/cm3, respectively. In fumigant assays, the oil was toxic on D. gallinae, and mortality was significantly higher in open containers over closed ones, underlining the key role of highly volatile constituents. Repellent assays showed that after 24 h from the treatment, all doses of the A. sieberi essential oil led to significant repellent activity over the control, except for 2 μg/cm3. After 48 h, A. sieberi essential oil tested at all doses led to significant repellent activity, if compared to the control. Residual toxicity assays showed that time exposure and concentration tested had a significant impact on mite mortality after 1, 2, 5, and 7 days from the treatment. Notably, mortality remained significantly higher over the control for 7 days after spraying with oil at 2%. Further field assays with selected molecules from the A. sieberi essential oil are ongoing, testing them in synergistic blends, as well as in microencapsulated formulations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dermanyssus gallinae (Acari, Dermanyssidae) (Fig. 1a), also known as the poultry red mite, represent a key threat for the poultry industry, with special reference to laying hen, in many parts of the world, including the USA, Europe, Japan, China, and Iran (Chauve 1998; Sparagano et al. 2009; Wang et al. 2010; Barimani et al. 2016). Studies conducted in Iran revealed that D. gallinae is the most prevalent blood-feeding mite in the breeder and caged layer flocks (Rahbari et al. 2009). The blood-feeding behavior of D. gallinae leads to stress, restlessness, irritation, anemia, and even death in heavy infestation cases, due to exsanguinations (Kirkwood 1968). Furthermore, poultry red mites are potential vectors of several pathogens, including Salmonella enterica (Hamidi et al. 2011), Erysipelothrix rhusiopathiae (Chirico et al. 2003), and Avipoxvirus (Chikuba et al. 2008). From an economical point of view, D. gallinae causes high production losses, including decreased meat (15%) and egg production (15–20%), and may even cause death of its hosts in a reduced amount of cases (6–7%) (Kilpinen et al. 2005). Particularly, the production in laying hens is affected through a decline in the growth rate and great decreases in egg production and egg quality (i.e., shell thinning and blood spotting on the shell surface) (Chauve 1998).
The control of poultry red mite populations is primarily achieved by continuous applications of acaricides, including organophosphates, pyrethroids, and carbamates. The fast-growing development of resistance to pesticides currently marketed (Naqqash et al. 2016), as well as the strict laws concerning the chemicals admitted for treatments on food animals, underlined the key importance to develop alternative methods for the control of D. gallinae (Kim et al. 2004, 2007; Nechita et al. 2015; Tabari et al. 2015). One alternative tool can be the formulation of novel acaricides using plant-derived compounds, including essential oils. However, botanical pesticide research has been mostly focused on mosquito (Benelli 2015; Pavela and Benelli 2016a, b) and tick control (Pavela et al. 2016), while studies on other arthropod pests and vectors of medical and veterinary importance still remain scarce (Benelli et al. 2017a). To the best of our knowledge, only few researches have focused on the potential employ of essential oil-based pesticides or repellents against the poultry red mite D. gallinae (Kim et al. 2004, 2007; George et al. 2009, 2010; Tabari et al. 2015; Masoumi et al. 2016).
The genus Artemisia represents a source of molecules and herbal preparations of high parasitological interest, as recently highlighted by the Nobel Prize to Y. Tu for the discovery of artemisinin (Benelli and Mehlhorn 2016). Besides antiplasmodial drugs extracted from Artemisia vulgaris, other Artemisia species, such as Artemisia absinthium, have been recently reported for their promising toxicity against arthropod vectors, with special reference to mosquitoes (Govindarajan and Benelli 2016). Artemisia sieberi Besser (Asteraceae) (Fig. 1b) is a dry land plant, mainly growing in Southwest and Central Asia. It is widely distributed in the semi-desert and desert areas of Iran (Mahboubi and Farzin 2009). This aromatic plant species has been studied for antimicrobial, antifungal, and anticoccidial activity (Arab et al. 2006; Khosravi et al. 2003; Mahboubi and Farzin 2009). In addition, it has been reported that the essential oil of A. sieberi showed a fumigant toxicity against several stored product pests of economical importance (Negahban et al. 2007). On this basis, here, we decided to explore the potential of A. sieberi essential oil against the poultry red mite, D. gallinae. In this study, the A. sieberi essential oil was analyzed using gas chromatography (GC) and GC-MS analyses. Furthermore, we evaluated the essential oil toxicity through contact and fumigant assays on adult mites. The repellent activity of A. sieberi essential oil on D. gallinae was assessed over different time intervals. Lastly, the residual toxicity of various doses of the oil was evaluated until 14 days post treatment.
Material and methods
Essential oil extraction and GC and GC-MS analyses
The aerial parts of A. sieberi at full-flowering stage were collected in Arak (34° 06′ 33.1″ N, 49° 47′ 35.5″ E) (Markazi Province, Iran) during September 2014. Voucher specimens of the plant were identified at species level at the Arak Agricultural Sciences University (Arak, Iran). Plant aerial parts were shade-dried at 25 °C and then hydro-distilled by using a Clevenger-type apparatus to extract the essential oil. Essential oil extraction was done according to Negahban et al. (2007). GC analysis was performed using a Shimadzu GC-9A with helium as a carrier gas on a DB-5 column (30 m × 0.25 mm i.d., film thickness 0.25 mm). GC-MS was carried out on a Varian 3400 GC-MS system equipped with a DB-5 column (30 m × 0.25 mm i.d., film thickness 0.25 mm), and oven temperature was 40–250 °C at a rate of 4 °C, with the following conditions: transfer line temperature, 260 °C; carrier gas, helium with a linear velocity of 31.5 cm/s; split ratio, 1:60; ionization energy, 70 eV; scan time, 1 s; and mass range, 40–300 amu. The identification of the A. sieberi essential oil compounds was based on the comparison of their retention indices and mass spectra with those in commercial libraries NIST 98.1 and MassFinder 3.1. The concentration of each essential oil component was calculated from the integration area of the chromatographer (Govindarajan and Benelli 2016).
Mites
D. gallinae adults used in experiments were collected from a commercial laying poultry farm in Gorgan (Iran). Mites were stored in a sealable, transparent glass container and stored at 25 ± 1 °C and 55 ± 5% relative humidity (R.H.) for 24 h before the testing phase.
Contact toxicity
Contact toxicity assay was done according to the method described by Tabari et al. (2015). Briefly, we prepared different dilutions of the A. sieberi essential oil in 50 μl ethanol, leading to 2, 5, 10, 20, and 40 μg/cm3. The A. sieberi essential oil was applied on Whatman No. 1 filter papers. After drying, treated filter papers were placed on the bottom of Petri dishes (4.8 cm diameter × 1.4 cm). Then, 150 adult mites were introduced into each Petri dish containing treated filter papers and then sealed with Parafilm. Control filter paper discs were treated using only 50 μl of ethanol. All assays were done in the same condition at 25 ± 1 °C and 55 ± 5% R.H. Three replicates were carried out concurrently for all tested groups of mites.
Fumigant toxicity
To determine whether the lethal activity of the essential oil on D. gallinae was related to contact or fumigant toxicity, the experimental apparatus described by Tabari et al. (2015) was used. It avoided direct contact of mites with filter papers. Vessels with mites in them were either sealed with a layer of Parafilm (method A, hereafter) or left uncovered (method B). The D. gallinae mortality rates were determined after 24 h under a loop by prodding mites with a pin. If no movement was observed, mites were considered as dead.
Repellent activity
The method used to assess the repellent activity of A. sieberi essential oil was described by Masoumi et al. (2016). Four conical flasks and a Y-tube olfactometer were assembled to develop a repellence measuring system. The air was pumped into the system at a rate of 1 l/min and filtered by passing through activated carbon; bubbling into distilled water humidified it. Then, using a Y-tube olfactometer, airflow was divided equally and each airflow was conducted to a 250-ml Erlenmeyer flask. One of these flasks contained the A. sieberi essential oil formulated on filter paper, while the other one contained the distilled water on filter paper. Air from these flasks was then directed to an olfactometer, where D. gallinae mites were released by using 0.2-mm mesh overall openings. In each assay, 50 D. gallinae adults were placed into the Y-tube olfactometer. The distribution of mites in the two opposing arms of the Y-tube olfactometer was recorded after 30 min. Then, the filter papers treated with four different concentrations of A. sieberi essential oil were added to the system and the number of mites in the untreated arm was recorded after 24 and 48 h. Three replications were used for each tested combination. Between successive runs of the experiment, all glassware exposed to test botanical products were washed with ethanol and finally rinsed in double-distilled water.
Residual toxicity assay
Three different concentrations of the A. sieberi essential oil (i.e., 0.5, 1, and 2%) evaluated over five different exposure times were tested in residual toxicity assay. The solutions containing A. sieberi essential oil were sprayed on aluminum foil surfaces, and then D. gallinae mites were exposed to the surfaces at 1, 2, 5, 7, and 14 days post spraying. The area of exposure on previously sprayed surfaces was limited using a converted Petri dish. Vaseline was applied to edges of Petri dishes to ensure mite’s contact on treated surfaces. For each solution and time exposure, three replicates were performed. All assays were done in the same condition at 25 ± 1 °C and 55 ± 5% humidity. Mite mortality (%) was determined after 48 h from the end of the exposure to the A. sieberi essential oil treatment.
Statistical analysis
To determine LD50, LD90, and LD99 values, mite mortality data were subjected to Probit analysis, and chi squares were not significant (Benelli 2017). Data of the fumigant toxicity, repellent activity, and residual toxicity assays were analyzed using ANOVA followed by Tukey’s HSD test. Values of P < 0.05 were considered significant. All the statistical analyses were carried out using SPSS, version 16.
Results and discussion
GC and GC-MS analyses
Our GC and GC-MS analyses showed that the major constituents of the A. sieberi essential oil were α-thujone (31.5%) (Fig. 1c), β-thujone (11.92%), camphor (12.3%), and 1,8-cineole (10.09%), as shown in Table 1. To the best of our knowledge, previous investigations shedding light on the chemical composition of A. sieberi essential oil showed several key quantitative and qualitative differences. For example, Weyerstahl et al. (1993) noted that major constituents of the A. sieberi essential oil are camphor (44%), 1,8-cineole (19%), camphene (5%), terpinen-4-ol (2.5%), and α-terpineol (2%). In addition, the main sesquiterpene component is dehydro-1,8-sesquicineole. Sefidkon et al. (2002) highlighted the high oil yield of A. sieberi (1.02%), reporting camphor (49.3%), 1,8-cineole (11.1%), and bornyl acetate (5.8%) as main molecules. Later on, Negahban et al. (2007) reported that the A. sieberi essential oil mainly contained camphor (54.7%), camphene (11.7%), 1,8-cineol (9.9%), β-thujone (5.6%), and α-pinene (2.5%). We hypothesize that these differences can be due to the different collection sites [i.e., Qom Province, Iran (Negahban et al. 2007); Arak, Markazi Province, Iran (our study)] and harvesting periods; indeed, we collected flowering aerial parts, while plant material at vegetative stage was probably collected by Negahban et al. (2007).
Toxic and repellent activity
Pest management strategies relying to conventional pesticides have been progressively hindered by the fast-growing development of arthropod resistance (Naqqash et al. 2016), increases in consumers demand for safe and residue-free foodstuffs, and a decrease in available products due to stricter legislation (George et al. 2009; Pavela and Benelli 2016b). This has lead to considerable market opportunities for alternative products (Khater et al. 2016), including botanical pesticides (Benelli 2015). Selected plant extracts, isolated metabolites, and essential oils meet the criteria of minimum risk pesticides, so many researches recently focused on them as potential starting materials to develop eco-friendly pesticides (Kim et al. 2004; Abdel-Ghaffar et al. 2008, 2009; Semmler et al. 2009; Schmahl et al. 2010; Benelli and Mehlhorn 2016).
Notably, research on eco-friendly management of vectors and parasites showed that a wide number of botanicals have promising toxic and repellent properties, also on several mite and tick species of high economical importance (Mehlhorn et al. 2005; Govindarajan et al. 2016a, b, c; Benelli et al. 2016; Pavela et al. 2016, Mehlhorn 2016). However, limited information is still available about the eco-friendly control of poultry red mites using botanical pesticides. Locher et al. (2010) described a neem-based product, Mite-Stop®, as an effective botanical acaricide for the control of the poultry red mite, while George et al. (2009) showed that essential oils of thyme, manuka, and pennyroyal tested on D. gallinae achieved 99% lethal concentration (LC99) values lower than 0.30 mg/cm3. Furthermore, based on a comparison of LD50 values, Kim et al. (2004) have reported that acaricidal activity of some plant extract preparations on D. gallinae adults was almost comparable to that of the synthetic pesticide profenofos. These authors screened 56 essential oils using both filter paper assays and fumigation toxicity tests. In contact bioassays, 100% mortality testing 0.07 mg/cm2 has been reported for bay, cade, cinnamon, clove bud, coriander, horseradish, lime, mustard, pennyroyal, pimento berry, spearmint, red thyme, and white thyme oils (Kim et al. 2004).
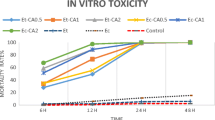
Mortality rates achieved testing the α-thujone-rich A. sieberi essential oil on D. gallinae in contact toxicity assays are presented in Table 2. The essential oil of A. sieberi showed significant toxicity against the poultry red mite with LC50, LC90, and LC99 values of 15.85, 26.63, and 35.42 μg/cm3, respectively. The toxic effects of A. sieberi essential oil in fumigant assays on D. gallinae are given in Fig. 2. The comparison of mortality rates in A. sieberi essential oil-treated groups and controls showed that A. sieberi essential oil tested using closed and open containers led to significant mortality in D. gallinae; mortality rate using the closed container method (A) was significantly higher over the open container method (B) (P < 0.05). In fumigant assays, the highest mortality rate was observed for the A. sieberi essential oil tested with the closed container method, which was statistically significant in comparison to all the other groups (P < 0.05). In agreement with our findings, fumigation tests carried out by Kim et al. (2004) at 0.28 mg/cm2 on poultry red mites showed that cade, clove bud, coriander, horseradish, and mustard oils were effective in closed containers than in open ones, pointing out that the effect of these essential oils can be largely due to the action in the vapor phase (Kim et al. 2004).
Fumigant toxicity of α-thujone-rich Artemisia sieberi essential oil against the poultry red mite, Dermanyssus gallinae: closed (A) and open (B) arena assays showing the LD50 value estimated on adult mites. T - bars represent standard errors. Above each column, different letters indicate significant differences among treatments (ANOVA, Tukey’s HSD, P < 0.05)
Furthermore, in repellent activity assays, before initiation of treatments, no significant differences were detected about the distribution of D. gallinae in the arms of the olfactometer (P > 0.05) (Table 3). After 24 h, all doses of the α-thujone-rich A. sieberi essential oil led to significant repellent activity in comparison to the control (P < 0.05), except for the dose of 2 μg/cm3. After 48 h, A. sieberi essential oil tested at all doses led to significant repellent activity, if compared to the control (P < 0.05). As it could be expected, the highest repellency was seen in the highest dose of essential oil after 48 h.
Data obtained from residual toxicity assays showed that time exposure and concentration tested had a significant impact on poultry red mite mortality at time intervals of 1, 2, 5, and 7 days post application (P < 0.05) (Table 4). Mean mortality rates of mites remained significantly higher over those of the control for 7 days after spraying for 2% concentration (P < 0.01). For 0.5% α-thujone-rich A. sieberi essential oil, significant mortality in comparison to the control was maintained for 5 days (P < 0.001). When testing 1% concentration, this time was significantly longer, reaching to 7 days (P < 0.05). Overall, several essential oils and plant-derived products have been tested for their acaricidal activity on D. gallinae. However, it has been also pointed out that the compounds currently proved effective against red poultry mites are highly volatile, and any acaricidal effects attributed to them might be temporary, lacking in long-term residual toxicity (George et al. 2008, 2009). From an environmental friendly viewpoint, such a lack of residual toxicity could be considered as beneficial. On the other hand, the effective toxicity and repellent action over time are a key feature for a botanical pesticide (Pavela and Benelli 2016b). In this framework, our results are promising, since the A. sieberi oil at 2% maintained its toxicity for at least 7 days after the treatment, at variance with a number of evidences reported by the earlier literature. For example, lavender essential oil did not showed prolonged toxicity against D. gallinae. When lavender-treated filter papers were used in toxicity assay immediately after impregnation, the mortality rates of D. gallinae were 66–90% but, if used 24 h after impregnation, the mortality rates of D. gallinae fell to 11% or less (George et al. 2008). Similar findings have been also reported for thyme essential oil (George et al. 2010).
Conclusions
Overall, this study firstly sheds light on the promising toxicity and repellent activity of α-thujone-rich A. sieberi essential oil on adults of the poultry red mite, D. gallinae. The residual toxicity tests also highlighted the prolonged toxicity of this essential oil, until 7 days from a single treatment. Further field assays with selected molecules (i.e., α-thujone, β-thujone, and camphor) from the A. sieberi essential oil are ongoing, testing them alone and in synergistic blends (see also Benelli et al. 2017b, c). Lastly, future research will focus on microencapsulation of the α-thujone-rich A. sieberi essential oil (Pavela 2016) as a suitable technique to maintain the oil toxicity and repellent effect in field conditions.
References
Abdel-Ghaffar F, Sobhy HM, Al-Quraishy S, Semmler M (2008) Field study on the efficacy of an extract of neem seed (Mite-Stop®) against the red mite Dermanyssus gallinae naturally infecting poultry in Egypt. Parasitol Res 103:481–485
Abdel-Ghaffar F, Semmler M, Al-Rasheid K, Mehlhorn H (2009) In vitro efficacy of ByeMite® and Mite-Stop® on developmental stages of the red chicken mite Dermanyssus gallinae. Parasitol Res 105:1469–1471
Arab HA, Rahbari S, Rassouli A, Moslemi MH, Khosravirad F (2006) Determination of artemisinin in Artemisia sieberi and anticoccidial effects of the plant extract in broiler chickens. Trop Anim Health Prod 38:497–503
Barimani A, Youssefi MR, Tabari MA (2016) Traps containing carvacrol, a biological approach for the control of Dermanyssus gallinae. Parasitol Res 115:3493–3498
Benelli G (2015) Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: a systematic review. Parasitol Res 114:3201–3212
Benelli G (2017) Commentary: data analysis in bionanoscience—issues to watch for. J Clust Sci 28:11–14. doi:10.1007/s10876-016-1143-3
Benelli G, Mehlhorn H (2016) Declining malaria, rising dengue and Zika virus: insights for mosquito vector control. Parasitol Res 115:1747–1754
Benelli G, Pavela R, Canale A, Mehlhorn H (2016) Tick repellents and acaricides of botanical origin: a green roadmap to control tick-borne diseases? Parasitol Res 115:2545–2560
Benelli G, Pavela R, Maggi F, Petrelli R, Nicoletti M (2017a) Commentary: making green pesticides greener? The potential of plant products for nanosynthesis and pest control. J Clust Sci 28:3–10. doi:10.1007/s10876-016-1131-7
Benelli G, Pavela R, Iannarelli R, Petrelli R, Cappellacci L, Cianfaglione K, Afshar FH, Nicoletti M, Canale A, Maggi F (2017b) Synergized mixtures of Apiaceae essential oils and related plant-borne compounds: larvicidal effectiveness on the filariasis vector Culex quinquefasciatus Say. Ind Crop Prod 96:186–195
Benelli G, Pavela R, Canale A, Cianfaglione K, Ciaschetti G, Conti F, Nicoletti M, Senthil-Nathan S, Mehlhorn H, Maggi F (2017c) Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: synergistic and antagonistic effects. Parasitol Int 66:166–171. doi:10.1016/j.parint.2017.01.012
Chauve C (1998) The poultry red mite Dermanyssus gallinae (De Geer, 1778): current situation and future prospects for control. Vet Parasitol 79:39–45
Chikuba T, Itou H, Sakakibara H, Inoue D (2008) Detection of fowl pox virus from red mite (Dermanyssus gallinae) at a layer farm occurring cutaneous fowlpox. J Jap Soc Poult Dis 44:113–117
Chirico J, Eriksson H, Fossum O, Jansson D (2003) The poultry red mite, Dermanyssus gallinae, a potential vector of Erysipelothrix rhusiopathiae causing erysipelas in hens. Med Vet Entomol 17:232–234
George DR, Callaghan K, Guy JH, Sparagano OAE (2008) Lack of prolonged activity of lavender essential oils as acaricides against the poultry red mite (Dermanyssus gallinae) under laboratory conditions. Res Vet Sci 85(3):540–542
George DR, Smith TJ, Shiel RS, Sparagano OAE, Guy JH (2009) Mode of action and variability in efficacy of plant essential oils showing toxicity against the poultry mred mite, Dermanyssus gallinae. Vet Parasitol 161:276–282
George DR, Olatunji G, Guy JH, Sparagano OAE (2010) Effect of plant essential oils as acaricides against the poultry red mite, Dermanyssus gallinae, with special focus on exposure time. Vet Parasitol 169:222–225
Govindarajan M, Benelli G (2016) Artemisia absinthium-borne compounds as novel larvicides: effectiveness against six mosquito vectors and acute toxicity on non-target aquatic organisms. Parasitol Res 115:4649–4661
Govindarajan M, Rajeswary M, Arivoli S, Tennyson S, Benelli G (2016a) Larvicidal and repellent potential of Zingiber nimmonii (J. Graham) Dalzell (Zingiberaceae) essential oil: an eco-friendly tool against malaria, dengue, and lymphatic filariasis mosquito vectors? Parasitol Res 115:1807–1816
Govindarajan M, Rajeswary M, Hoti SL, Bhattacharyya A, Benelli G (2016b) Eugenol, α-pinene and β-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol Res 115:807–815
Govindarajan M, Rajeswary M, Hoti SL, Benelli G (2016c) Larvicidal potential of carvacrol and terpinen-4-ol from the essential oil of Origanum vulgare (Lamiaceae) against Anopheles stephensi, Anopheles subpictus, Culex quinquefasciatus and Culex tritaeniorhynchus (Diptera: Culicidae). Res Vet Sci 104:77–82
Hamidi A, Sherifi K, Muji S, Behluli B, Latifi F, Robaj A, Postoli R, Hess C, Hess M, Sparagano OAE (2011) Dermanyssus gallinae in layer farms in Kosovo: a high risk for Salmonella prevalence. Parasit Vectors 4:136
Khater H, Hendawy N, Govindarajan M, Murugan K, Benelli G (2016) Photosensitizers in the fight against ticks: safranin as a novel photodynamic acaricide to control the camel tick Hyalomma dromedarii (Ixodidae). Parasitol Res 115:3747–3758
Khosravi A, Shirani D, Mahmoudi M (2003) Evaluation of the use of Artemisia sieberi essence on the treatment of cats and dogs with dermatophytosis. J Vet Res 58:293–295
Kilpinen O, Roepstorff A, Permin A, Norgaard-Nielsen G, Lawson LG, Simonsen HB (2005) Influence of Dermanyssus gallinae and Ascaridia galli infections on behaviour and health of laying hens (Gallus gallus domesticus). Br Poult Sci 46:26–34
Kim S, Yi J, Tak J, Ahn Y (2004) Acaricidal activity of plant essential oils against Dermanyssus gallinae (Acari: Dermanyssidae). Vet Parasitol 120:297–304
Kim S, Na YE, Yi JH, Kim BS, Ahn YJ (2007) Contact and fumigant toxicity of oriental medicinal plant extracts against Dermanyssus gallinae (Acari: Dermanyssidae). Vet Parasitol 145:377–382
Kirkwood AC (1968) Anemia in poultry infested with red mite Dermanyssus gallinae. Vet Rec 80:514–516
Locher N, Al-Rasheid KA, Abdel-Ghaffar F, Mehlhorn H (2010) In vitro and field studies on the contact and fumigant toxicity of a neem-product (Mite-Stop®) against the developmental stages of the poultry red mite Dermanyssus gallinae. Parasit Res 107:417–423
Mahboubi M, Farzin N (2009) Antimicrobial activity of Artemisia sieberi essential oil from central Iran. Iranian J Microbiol 1:43–48
Masoumi F, Youssefi MR, Tabari MA (2016) Combination of carvacrol and thymol against the poultry red mite (Dermanyssus gallinae). Parasitol Res 115(11):4239–4243
Mehlhorn H (2016). Available means to control bloodsucking ticks, mites and insects—an overview. In: Nanoparticles in the fight against parasites. Springer, Heidelberg, pp. 37–45
Mehlhorn H, Schmahl G, Schmidt J (2005) Extract of the seeds of the plant Vitex agnus castus proven to be highly efficacious as a repellent against ticks, fleas, mosquitoes and biting flies. Parasitol Res 95(5):363–365
Naqqash MN, Gökçe A, Bakhsh A, Salim M (2016) Insecticide resistance and its molecular basis in urban insect pests. Parasitol Res 115:1363–1373
Nechita IS, Poirel MT, Cozma V, Zenner L (2015) The repellent and persistent toxic effects of essential oils against the poultry red mite, Dermanyssus gallinae. Vet Parasitol 214(3):348–352
Negahban M, Moharramipour S, Sefidkon F (2007) Fumigant toxicity of essential oil from Artemisia sieberi Besser against three stored-product insects. J Stor Prod Res 43:123–128
Pavela R (2016) Encapsulation—a convenient way to extend the persistence of the effect of eco-friendly mosquito larvicides. Curr Org Chem 20:2674–2680
Pavela R, Benelli G (2016a) Ethnobotanical knowledge on botanical repellents employed in the African region against mosquito vectors—a review. Exp Parasitol 167C:103–108
Pavela R, Benelli G (2016b) Essential oils as eco-friendly biopesticides? Challenges and constraints. Tr Plant Sci 21(12):1000–1007
Pavela R, Canale A, Mehlhorn H, Benelli G (2016) Application of ethnobotanical repellents and acaricides in prevention, control and management of livestock ticks: a review. Res Vet Sci 109:1–9
Rahbari S, Nabian S, Ronaghi H (2009) Haematophagus mites in poultry farms of Iran. Iran J Arthropod Borne Dis 3:18–21
Schmahl G, Al-Rasheid KA, Abdel-Ghaffar F, Klimpel S, Mehlhorn H (2010) The efficacy of neem seed extracts (Tre-san®, MiteStop®) on a broad spectrum of pests and parasites. Parasit Res 107:261–269
Sefidkon F, Jalili A, Mirhaji T (2002) Essential oil composition of three Artemisia spp. from Iran. Flav Fragr 17:150–152
Semmler M, Abdel-Ghaffar F, Al-Rasheid K, Mehlhorn H (2009) Nature helps: from research to products against blood-sucking arthropods. Parasit Res 105:1483–1487
Sparagano O, Pavlicevic A, Murano T, Camarda A, Sahibi H, Kilpinen O, Mul M, van Emous R, le Bouquin S, Hoel K, Cafiero MA (2009) Prevalence and key figures for the poultry red mite Dermanyssus gallinae infections in poultry farm systems. Exp Appl Acarol 48:3–10
Tabari MA, Youssefi MR, Barimani A, Araghi A (2015) Carvacrol as a potent natural acaricide against Dermanyssus gallinae. Parasitol Res 114:3801–3806
Wang FF, Wang M, Xu FR, Liang DM, Pan BL (2010) Survey of prevalence and control of ectoparasites in caged poultry in China. Vet Rec 167:934–937
Weyerstahl P, Schneider S, Marschall H, Rustaiyan A (1993) The essential oil of Artemisia sieberi Bess. Flavor Fragr 8:139–145
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Tabari, M.A., Youssefi, M.R. & Benelli, G. Eco-friendly control of the poultry red mite, Dermanyssus gallinae (Dermanyssidae), using the α-thujone-rich essential oil of Artemisia sieberi (Asteraceae): toxic and repellent potential. Parasitol Res 116, 1545–1551 (2017). https://doi.org/10.1007/s00436-017-5431-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5431-0