Abstract
Angiostrongylus cantonensis is a neurotropic parasite which can cause injury to central nervous system and eosinophilic meningitis to human. Natural killer (NK) cells are specialized innate lymphocytes important in early defense against pathogens as in a variety of intracellular bacterial, viral, and protozoan infections. However, the number and function of NK cells in extracellular parasitic infection of A. cantonensis are unclear. In this study, on A. cantonensis infected mice which may mimic the human’s infection, we found that the percentage of splenic NK cells and the absolute number of peripheral blood NK cells were decreased at 21-day post infection compared with that of controls. When administrating with albendazole treatment at early stage of the infection, the changes of NK cells could be avoided. Further analysis confirmed that the reduction of NK cells was due to their apoptosis manifested as increased expressions of annexin V and activated caspase-3 after 16-day post infection. Moreover, both activated and inhibitory receptors such as CD16, CD69, NKG2D, and Ly49a on NK cells were down-regulated after 16-day post infection. Interestingly, NK cells isolated from mice of 21-day post infection showed enhanced IFN-γ production when stimulated with IL-12 for 24 h and cytotoxicity to YAC-1 cells, as well as elevated CD107a expression. It is evident that NK cell population and its function were changed in A. cantonensis infected mice, suggesting their involvement in pathogenesis of the infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiostrongylus cantonensis (A. cantonensis) was first discovered in the pulmonary arteries and hearts of domestic rats by Xintao Chen in 1935 (Wang et al. 2008). A. cantonensis is mainly causes human eosinophilic meningitis or meningoencephalitis in areas of South-East Asia and many Pacific Islands (Gelis et al. 2011; Maldonado et al. 2010; Wang et al. 2010). In recent years, several outbreaks of human angiostrongyliasis have been reported in China and in other countries, and it has been regarded as a public health problem (Lindo et al. 2002; Slom et al. 2002; Wang et al. 2010). Humans, as well as mice, are nonpermissive hosts for this parasite and become infected after eating intermediate or parasitic hosts or vegetables contaminating the third stage infective larvae of the worm. Once ingested, the infective larvae may invade intestinal tissues and migrate into the central nervous system in about 2 weeks. Unlike its life cycles in rats, the worms spontaneously die in human or mice meninges, brains, or eyes. Consequently, severe inflammation occurs in those affected organs (OuYang et al. 2012).
Previous study indicated that CD4+ T cells play a protective role in A. cantonensis infection since depletion of CD4+ T cells by monoclonal antibody impaired the clearance of the worm (Lee et al. 1996). The percentages of CD4+T lymphocytes of spleens in the mice infected with A. cantonensis markedly increased and polarized to Th2 phenotypes (Liu et al. 2013). Natural killer (NK) cells are cytotoxic lymphocytes that are able to kill virus-infected cells (De La Garza et al. 2005) and make critical contributions to control the infection of some intracellular bacteria and certain intracellular protozoan parasites (Lucas et al. 2007; Schleicher et al. 2007). NK cells also play important roles in extracellular parasitic infections. For example, binding of excreted and/or secreted products of Necator hookworms may cause the NK cells to become activated and secrete IFN-γ (Teixeira-Carvalho et al. 2008). In addition, NK cells expanded in the host defense against filariae, while in vivo depletion of NK cells strongly enhanced the worm load (Korten et al. 2002). However, the number and function of NK cells in A. cantonensis infections remain unclear. In view of this, the present study sought to observe the alteration of NK cells in mice infected with A. cantonensis.
Materials and methods
Animals and infection
Female BABL/c mice aged 6–8 weeks were purchased from the Comparative Medicine Center of Yangzhou University and maintained in the Animal Center of Nanjing Medical University according to the guidelines approved by the Nanjing Medical University Animal Experiment and Care Committee. Mice were orally infected with 20 third-stage larvae collected from infected snails.
Cell isolation
Mice were euthanized, and blood was collected into heparinized tubes. Single cell suspensions from spleens were prepared by forcing the tissues through a fine nylon mesh screen. The cells were washed with RPMI 1640, and the red blood cells were lysed by using red cell lysis buffer (8.3 g NH4Cl in 0.01 M Tris–HCl, pH = 7.4). DX5+ NK cells were isolated from splenocytes by using magnetic beads following the manufacturer’s instructions (MACS, Miltenyi Biotech, Germany).
Flow cytometry
Single cell suspensions (1 × 106 cells/sample) incubated with Fc-receptor block (eBioscience; except for panels examining CD16/CD32) for 15 min at 4 °C. Surface markers were stained with the specific antibodies: anti-CD49-PE, anti-CD3-APC, 7-AAD and anti-annexinV-FITC, anti-CD49-AlexaFluor® 647, anti-CD3-PE/Cy7, anti-CD16/32-FITC, anti-CD69-PE, anti-NKG2D-PE, anti-Ly-49A-FITC, and anti-CD107a-PE and anti-NKG2A-PE (Biolegend). Data were collected by FACS Calibur flow cytometer (BD Biosciences) and analyzed by FlowJo software (TreeStar, Ashland, OR).
For cell quantitation, each blood sample (100 μL) was stained with the specific antibodies (anti-CD49-FITC and anti-CD3-APC; eBioscience), and erythrocytes were then lysed with Cal-lyse Lysing Solution (Invitrogen). After thoroughly mixing with 100 μL of Caltag Counting Beads (Invitrogen), 10,000 beads were acquired in the FACS.
Western Blot
NK cells were washed in PBS. The proteins were extracted by using Protein Extraction Kit (Beyotime, China) and quantified by using BCA kit (Pierce, Rockford, IL). Lysates were separated on 12% SDS–polyacrylamide gel electrophoresis gels and transferred to PVDF(IPVH00010, Millipore, USA) followed by blocking in TBS/0.1% Tween 20 with 5% nonfat dry milk. Antibodies used as below: rabbit anti-mouse caspase-3 antibody (1:1,000); goat anti-rabbit IgG HRP-conjugated antibody (1:2,000); and mouse anti-β-actin antibody (1:1,000) (Cell Signaling Technology).
Cytokine assays and cytolytic activity of NK Cells
Following isolation, NK cells were cultured in 24-well tissue culture plates (Corning) in the presence or absence of 1 ng/mL IL-12 (R&D Systems) for 24 h. The levels of IFN-γ and TNF-α in culture supernatants were detected with ELISA method according to the instructions of the manufacturer (eBioscience). NK cell-mediated cytotoxic activity was determined in a colorimetric assay based on the measurement of LDH released from the cytosol of lysed YAC-1 target cells into the supernatant with the CytoTox 96R Non-Radioactive Cytotoxicity Assay (Promega, Madision, WI) according to the manufacturer's manual.
Albendazole treatment
Mice were infected with A. cantonensis, and then followed by intragastric administrations of albendazole (20 mg/kg/24 h) for seven consecutive days from 7-day post infection (dpi).
Statistical analysis
The data were analyzed by using two-tailed Student’s t test when compared between the two groups. A p value < 0.05 was considered statistically significant. All statistical analyses were operated by GraphPad Prism software 4.0 (GraphPad Software Inc., San Diego, CA).
Results
A.cantonensis infection induced decrease in NK cells
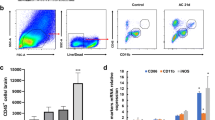
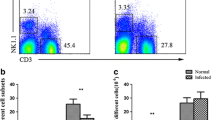
In this experiment, mice were infected with 20 third-stage larvae. The percentage of splenic NK cells was analyzed by flow cytometry. As shown in Fig. 1a, there was a significant decrease in the percentage of NK cells at 18 dpi. Subsequently, it was further reduced by more than 3-folds at 21 dpi. Likewise, the number of peripheral NK cells was significantly decreased at 21 dpi (Fig. 1b).
The decline in NK cells of mice following A. cantonensis infection. a Percentage of splenic NK cells in different time-points of infection. b The absolute number of total NK cells in peripheral blood. c Mice were infected with A. cantonensis first, followed by the albendazole treatment from 7 to 14 dpi. Percentages of NK cells in infected mice (14, 16, 18, and 21 dpi) with albendazole treatment. Uninfected mice were used as control. Values were shown as mean ± SEM. *Results differed from the control group; *P < 0.05; ***P < 0.001
It is noteworthy that this sharp decline of NK cells occurred after 2 weeks post infection when the larvae enriched in the brain. In order to determine whether the decrease of NK cells was related to brain injury resulted from the infection, we did the helminthicide experiments. Mice were infected with A. cantonensis, and then followed by intragastric administrations of albendazole (20 mg/kg/24 h) at 7 dpi for 7 days. We observed that NK cells were not decreased after treatment with albendazole (Fig. 1c). These data suggested that the decrease in NK cells was an event occurred after brain injury caused by A. cantonensis infection.
A. cantonensis infection induces apoptosis of splenic NK cells
To determine whether the reduction in NK cells was due to cell death, splenocytes were evaluated for the expression of annexin V. In comparison with control, NK cells showed an increase in apoptosis since 16 dpi (Fig. 2a). Further, we measured the levels of active caspase-3 in NK cells. Similarly, the levels of activated caspase-3 in NK cells from the infected mice were increased significantly since 16 dpi (Fig. 2b). These data were consistent with the above changes in NK cell number and revealed that the striking reduction in the NK cell number was partially because of apoptosis after A. cantonensis infection.
A. cantonensis induces apoptosis of splenic NK cells. a Splenocytes were collected from uninfected and infected mice. Cellular apoptosis was investigated by Annexin-V/7-AAD staining. b The expression of caspase 3 from the purified NK cells was detected by western blot. Values were shown as mean ± SEM. *Results differed from the control group; *P < 0.05; **P < 0.01; ***P < 0.001
Alteration in activated and inhibitory receptors on the NK cells following A. cantonensis infection
NK cell responses are mediated through cell surface receptors, including activated and inhibitory receptors (Pegram et al. 2011). To test whether the expressions of receptors on NK cells were affected in A. cantonensis infection, we performed flow cytometric analysis for NK cells. The results indicated that the expression of CD16 on NK cells in infected mice began to decline from 16 dpi and continued to 21 dpi (Fig. 3a). In addition, CD69 and NKG2D, the other activated receptors of on NK cells were also decreased in infected mice since 16 dpi compared to controls (Fig. 3b). NK inhibitory receptors have a potent regulatory function on the activation of NK cells (Karlhofer et al. 1992). Further, the common inhibitory receptors Ly49a and NKG2a were examined. As showed in Fig. 3c, the expression of Ly49a was decreased in the splenocytes of A. cantonensis infected mice compared to controls, but NKG2a was not. These results demonstrated that both activation receptors CD16, CD69, and NKG2D, and inhibiting receptor Ly49a on splenic NK cells were down-regulated after A. cantonensis infection.
The expression of activated and inhibitory receptors on the NK cells following A. cantonensis infection. Percentage of CD16 (a), activated receptor CD69 and NKG2D (b), and inhibitory receptor Ly49a and NKG2a (c) on splenic NK cells. Values were shown as mean ± SEM. *Results differed from the control group; *P < 0.05; **P < 0.01; ***P < 0.001
NK cell mediated cytotoxicity was enhanced after infection with A. cantonensis
NK cells are an important source of early cytokine production and typically produce IFN-γ and TNF-α (Lunemann et al. 2009). To assess the cytokines production of NK cell after A. cantonensis infection, we measured culture supernatants of NK cells expressing IFN-γ and TNF-α following exposure to IL-12 by ELISA. Compared with the naïve NK cells, the NK cells isolated from 21 dpi secreted higher level of IFN-γ after stimulation with IL-12 for 24 h (Fig. 4a). However, no significant difference was observed for the level of TNF-α (Fig. 4b).
NK cell-mediated cytotoxicity was enhanced after A. cantonensis infection. Splenic NK cells from 21 dpi and uninfected mice were isolated and incubated for 24 h with medium or IL-2. The concentration of IFN-γ (a) and TNF-α (b) in culture supernatants were determined by ELISA. c Splenic NK cells (effector) from 21 dpi and uninfected mice exposed to IL-12 or medium versus YAC-1 (target) cells to determine the NK cytotoxicity. d Percentage of CD107a on NK cells in the spleen. Values were shown as mean ± SEM. *Results differed from the control group; **P < 0.01; ***P < 0.001
We further evaluated whether infection of A. cantonensis modulates the cytolytic activity of NK cells. A LDH release assay was employed to determine functional NK cell alterations induced by A. cantonensis infection by their ability to kill YAC-1 cells. Our data showed that NK cell mediated cytolytic activity against target YAC-1 cells was increased in mice infected with A. cantonensis at 21 day (Fig. 4c). Knowing that CD107a is a marker to assess functionality of activated NK cells that have the capability to degranulate in the absence of cytokine secretion (Aktas et al. 2009), we tested the expression of CD107a on NK cells. The result showed that the levels of CD107a significantly increased at 16 dpi and maintained at a higher level at 18 and 21 dpi compared to the control group (Fig. 4d). Together, these studies demonstrated that NK cells of the infected mice presented enhanced cellular immune function.
Discussion
NK cells are innate immune effector cells and important component of the innate immunity to intracellular bacterium and protozoan parasite infections and tumors. They have the ability to provide an early source of inflammatory and immunoregulatory cytokines, and to lyse target cells (Yokoyama et al. 2004). Patients with onchocerciasis showed a higher number of natural killer cell phenotype (Brattig et al. 1987). Cytotoxic activity of NK cells in the lungs was substantially elevated in Trichinella spiralis infected mice (Bany et al. 1992). However, little is known about the alteration of NK cells in extracellular parasitic infection of A. cantonensis. In the current study of mouse model of A. cantonensis infection, we observed a significant reduction of NK cells both in spleen and peripheral blood at 21 dpi and cell apoptosis likely contributed to the quantitative changes of NK cells. In addition, both activated and inhibitory receptors such as CD16, CD69, NKG2D, and Ly49a on NK cells were down-regulated but the cytotoxicity and the expression of some cellular immunity-related molecules like IFN-γ and CD107a were up-regulated after A. cantonensis infection.
Non-permissive hosts including humans and mice appeared to have more serious neuropathological damages caused by A. cantonensis invading and developing in brain tissue (OuYang et al. 2012). Worms were first detected in cranial cavity at 10 dpi and the highest number of worms was found at 16 dpi. Histological examination revealed the damages including cavities and inflammation in the brain parenchyma (Guo et al. 2008). What is noteworthy in our study is that the decline of NK cells was observed after 2 weeks when larvae enrich in the central nervous system. The helminthicide experiments further supported that the decrease in NK cells occurred after brain injury resulted from the infection. Previous study reported that the absolute numbers of NK cells were decreased 2- to 3-fold in spleen after brain injury in cerebral ischemia experimental model (Prass et al. 2003). Significantly, less percentage of NK cells was found in infected patients with severe traumatic brain injury (Mrakovcic-Sutic et al. 2010). The underlying process that results in the sharp drop of NK cells after brain injury is not well understood. However, it has been proposed that brain injury induced immune depression through a mechanism of over-activation of the sympathetic nervous system or hypothalamo-pituitary-adrenal axis, leading to sustained lymphopenia (Prass et al. 2003; Tseng et al. 2005). Further experiment is needed to elucidate these possibilities in our A. cantonensis infection model. Another possibility of NK cells decline is apoptosis as other researchers described in cerebral ischemia (Prass et al. 2003).
NK cells express inhibitory and activated receptors recognizing self-ligands or microbial molecules on infected and tumor cells. Coordinated acquisition of the signals originating from inhibitory and activated receptors regulates NK cell effector functions (Grzywacz et al. 2006). Considering the decrease of NK cells after A. cantonensis infection, we further detected the expression of NK cell receptors, including CD16, CD69, NKG2D, LY49, and NKG2A. CD16 endows NK cells with the ability to detect cells coated with IgG and to eliminate them by antibody-dependent cellular cytotoxicity (ADCC) (Perussia 1998). CD69 is one of the stimulatory activation marker expressed after NK cells are activated. There is a strong relationship between CD16 and CD69 up-regulation, and both of them have the capacity to activate NK cytotoxic and anti-viral cytokine mechanisms (Marquez et al. 2010). In our study, the results showed that both CD16 and CD69 were decreased since 16 dpi, suggesting that activation receptors on NK cells were inhibited. Besides, NKG2D is a surface receptor of the NKG2 family (Cerwenka and Lanier 2001; Diefenbach and Raulet 2001) expressed by all NK cells which serves as a major recognition receptor for detection and elimination of transformed and infected cells (Elsner et al. 2007; Raulet et al. 2013). We found that the expressions of NKG2D in the infected mice were also decreased after 16 dpi compared with uninfected control. Similarly, the NK cells inhibitory marker Ly49 receptor was also decreased while NKG2A was not changed after A. cantonensis infection. The functional roles about down-regulation of activated and inhibitory receptors on NK cells following A. cantonensis infection also need further investigation.
Apart from the functional receptors, we further detected the cytotoxicity and cytokines production of NK cells in A. cantonensis infection. Interestingly, our data showed that NK cell cytotoxicity against YAC-1 cells was enhanced in A. cantonensis infected mice compared to that of the control. Consistently, the IL-12 stimulated splenic NK cells of 21 dpi produced higher level of IFN-γ, but not TNF-α compared to the control. Classically, to recognize and respond to inflamed or infected tissues, NK cells express a variety of activated and inhibitory receptors, as well as co-stimulatory receptors (Pegram et al. 2011). These receptors recognize cellular stress ligands as well as major histocompatibility complex class I and related molecules on target cells, which can lead to NK cell responses (Grzywacz et al. 2006). However, there is growing evidence showing that NK cells require signals from accessory cells to respond to pathogens (Newman and Riley 2007). Accessory cells including dendritic cells, macrophages, or monocytes serve as a source of soluble and contact-dependent signals to activate NK cells in response to murine CMV (Andoniou et al. 2005), Listeria monocytogenes (Hamerman et al. 2004), and Plasmodium falciparum (Cardillo et al. 1996; Newman et al. 2006). IFN-γ is a key contributor to antibacterial immune defense and is the predominant cytokine produced by activated NK cell. The protective role of IFN-γ co-administrating with TNF-α was shown in murine salmonellosis (Nakano et al. 1990). Results from the current experiments showed that the production of IFN-γ in response to IL-12 by splenic NK cells from mice 21 dpi was significantly increased. NK cells are able to exert their killing functions to tumor cells, virally infected cells, or other intracellular pathogens by several different mechanisms. One of these is the perforin/granzyme exocytosis granule system (Chowdhury and Lieberman 2008). After recognition of an infected cell, NK cells release both perforins and granzymes into the extracellular space act in synergy to activate endogenous caspases that trigger apoptotic pathways (Trapani et al. 1998). Lysosome associated membrane protein (LAMP) 1/CD107a is used as a marker for NK cell degranulation which capable of degranulation such as cytotoxic T cells and NK cells (Alter et al. 2004). Additionally, CD107a expression correlates with both IFN-γ secretion and NK cell-mediated lysis of target cells (Alter et al. 2004; Betts et al. 2003). Here, we showed that the expression of CD107a on splenic NK cells was significantly increased at 16 dpi, and then maintained at a higher expression level at 18 and 21 dpi compared to uninfected mice. Consistently, both IFN-γ secretion and NK cell-mediated lysis of YAC-1 were enhanced at 21 dpi. These data suggest that A. cantonensis infection increased degranulation of the splenic NK population and IFN-γ production of NK cells upon co-stimulation ex vivo may be the contributing factor of the enhanced cytotoxicity.
Taken together, our study demonstrated that the percentage of spleen NK cells, as well as activated and inhibitory receptors on the NK cells were down-regulated in A. cantonensis infection, but NK cell-mediated cytotoxicity was enhanced as increased IFN-γ production and CD107a marker. Our study described for the first time that NK cell population and function were changed after the occurrence of brain injury in mice infected with A. cantonensis and suggested that NK cells may play a role in A. cantonensis infection and its pathogenesis.
References
Aktas E, Kucuksezer UC, Bilgic S, Erten G, Deniz G (2009) Relationship between CD107a expression and cytotoxic activity. Cell Immunol 254(2):149–154
Alter G, Malenfant JM, Altfeld M (2004) CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods 294(1–2):15–22
Andoniou CE et al (2005) Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol 6(10):1011–1019
Bany J, Janiak MK, Budzynski W (1992) Activity of natural killer (NK) cells in the course of experimental trichinellosis in mice. Wiad Parazytol 38(3–4):117–126
Betts MR et al (2003) Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods 281(1–2):65–78
Brattig NW, Tischendorf FW, Albiez EJ, Buttner DW, Berger J (1987) Distribution pattern of peripheral lymphocyte subsets in localized and generalized form of onchocerciasis. Clin Immunol Immunopathol 44(2):149–159
Cardillo F, Voltarelli JC, Reed SG, Silva JS (1996) Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: Role of NK cells. Infect Immun 64(1):128–134
Cerwenka A, Lanier LL (2001) Ligands for natural killer cell receptors: Redundancy or specificity. Immunol Rev 181:158–169
Chowdhury D, Lieberman J (2008) Death by a thousand cuts: Granzyme pathways of programmed cell death. Annu Rev Immunol 26:389–420
De La Garza R, 2nd, Asnis GM, Fabrizio KR, Pedrosa E (2005) Acute diclofenac treatment attenuates lipopolysaccharide-induced alterations to basic reward behavior and HPA axis activation in rats. Psychopharmacology (Berl) 179(2):356–65
Diefenbach A, Raulet DH (2001) Strategies for target cell recognition by natural killer cells. Immunol Rev 181:170–184
Elsner L et al (2007) The heat shock protein HSP70 promotes mouse NK cell activity against tumors that express inducible NKG2D ligands. J Immunol 179(8):5523–5533
Gelis S, Spratt DM, Raidal SR (2011) Neuroangiostrongyliasis and other parasites in tawny frogmouths (Podargus strigoides) in south-eastern Queensland. Aust Vet J 89(1–2):47–50
Grzywacz B et al (2006) Coordinated acquisition of inhibitory and activating receptors and functional properties by developing human natural killer cells. Blood 108(12):3824–3833
Guo PJ, et al. (2008) [Pathological change in the brain of mice infected with Angiostrongylus cantonensis]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 26(5):353–5
Hamerman JA, Ogasawara K, Lanier LL (2004) Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J Immunol 172(4):2001–2005
Karlhofer FM, Ribaudo RK, Yokoyama WM (1992) The interaction of Ly-49 with H-2Dd globally inactivates natural killer cell cytolytic activity. Trans Assoc Am Physicians 105:72–85
Korten S et al (2002) Expansion of NK cells with reduction of their inhibitory Ly-49A, Ly-49C, and Ly-49G2 receptor-expressing subsets in a murine helminth infection: Contribution to parasite control. J Immunol 168(10):5199–5206
Lee JD, Wang JJ, Chang JH, Chung LY, Chen ER, Yen CM (1996) Role of T cell subpopulations in mice infected with Angiostrongylus cantonensis. J Helminthol 70(3):211–214
Lindo JF et al (2002) Enzootic Angiostrongylus cantonensis in rats and snails after an outbreak of human eosinophilic meningitis, Jamaica. Emerg Infect Dis 8(3):324–326
Liu H, Luo X, Shen E, Li H, Ding X, Chen D (2013) Alteration of T cell subtypes in spleen and antibodies of serum in mice infected with Angiostrongylus cantonensis. Parasitol Res 112(3):1255–1260
Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A (2007) Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity 26(4):503–517
Lunemann A, Lunemann JD, Munz C (2009) Regulatory NK-cell functions in inflammation and autoimmunity. Mol Med 15(9–10):352–358
Maldonado A Jr et al (2010) First report of Angiostrongylus cantonensis (Nematoda: Metastrongylidae) in Achatina fulica (Mollusca: Gastropoda) from Southeast and South Brazil. Mem Inst Oswaldo Cruz 105(7):938–941
Marquez ME et al (2010) CD16 cross-linking induces increased expression of CD56 and production of IL-12 in peripheral NK cells. Cell Immunol 264(1):86–92
Mrakovcic-Sutic I et al (2010) Early changes in frequency of peripheral blood lymphocyte subpopulations in severe traumatic brain-injured patients. Scand J Immunol 72(1):57–65
Nakano Y, Onozuka K, Terada Y, Shinomiya H, Nakano M (1990) Protective effect of recombinant tumor necrosis factor-alpha in murine salmonellosis. J Immunol 144(5):1935–1941
Newman KC, Riley EM (2007) Whatever turns you on: Accessory-cell-dependent activation of NK cells by pathogens. Nat Rev Immunol 7(4):279–291
Newman KC, Korbel DS, Hafalla JC, Riley EM (2006) Cross-talk with myeloid accessory cells regulates human natural killer cell interferon-gamma responses to malaria. PLoS Pathog 2(12):e118
OuYang L et al (2012) Differences of larval development and pathological changes in permissive and nonpermissive rodent hosts for Angiostrongylus cantonensis infection. Parasitol Res 111(4):1547–1557
Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH (2011) Activating and inhibitory receptors of natural killer cells. Immunol Cell Biol 89(2):216–224
Perussia B (1998) Fc receptors on natural killer cells. Curr Top Microbiol Immunol 230:63–88
Prass K et al (2003) Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med 198(5):725–736
Raulet DH, Gasser S, Gowen BG, Deng W, Jung H (2013) Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol 31:413–441
Schleicher U et al (2007) NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J Exp Med 204(4):893–906
Slom TJ et al (2002) An outbreak of eosinophilic meningitis caused by Angiostrongylus cantonensis in travelers returning from the Caribbean. N Engl J Med 346(9):668–675
Teixeira-Carvalho A et al (2008) Binding of excreted and/or secreted products of adult hookworms to human NK cells in Necator americanus-infected individuals from Brazil. Infect Immun 76(12):5810–5816
Trapani JA, Jans DA, Jans PJ, Smyth MJ, Browne KA, Sutton VR (1998) Efficient nuclear targeting of granzyme B and the nuclear consequences of apoptosis induced by granzyme B and perforin are caspase-dependent, but cell death is caspase-independent. J Biol Chem 273(43):27934–27938
Tseng RJ, Padgett DA, Dhabhar FS, Engler H, Sheridan JF (2005) Stress-induced modulation of NK activity during influenza viral infection: Role of glucocorticoids and opioids. Brain Behav Immun 19(2):153–164
Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR (2008) Human angiostrongyliasis. Lancet Infect Dis 8(10):621–630
Wang J et al (2010) An outbreak of angiostrongyliasis cantonensis in Beijing. J Parasitol 96(2):377–381
Yokoyama WM, Kim S, French AR (2004) The dynamic life of natural killer cells. Annu Rev Immunol 22:405–429
Acknowledgments
This work was supported by National Basic Research Program of China (973 Program) (No. 2010CB530004), the Postgraduate Student Research Training Program of Jiangsu Province (CXZZ13-0566), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors have declared that no competing interests exist.
We thank Prof. Zhong-Dao Wu, Sun Yat-sen University, Guangzhou, China, for providing us with A. cantonensis infected snails and for his helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Al., Qiu, Xy., Wang, W. et al. The quantitative and functional changes of NK cells in mice infected with Angiostrongylus cantonensis . Parasitol Res 113, 2087–2094 (2014). https://doi.org/10.1007/s00436-014-3858-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3858-0