Abstract
The spleen is one of the most important peripheral immune organs, which is frequently affected in infectious diseases. Infectious diseases can induce splenic alterations including splenic atrophy and functional alteration, while splenic atrophy may in turn interferes with recovery of infectious diseases. Angiostrongyliasis is an infectious disease by Angiostrongylus cantonensis (A. cantonensis), which invade non-permissive hosts, such as humans and mice, to cause severe damage to the central nervous system (CNS) and acute inflammatory response. A. cantonensis infection-induced CNS injury has been confirmed to be due to profound immunopathology derived from peripheral immune components. However, the mechanism of immunopathology remains largely unknown. Here, we found that A. cantonensis invaded non-permissive hosts such as mice in the brain, but not in the other peripheral organs. However, this infection induced severe spleen atrophy. We further recognized that this atrophy is associated with a decrease of total splenocyte number and disruption of splenic structure due to reduced proliferation and increased apoptotosis. These also resulted in deterioration of T cell profile in the periphery with a low CD4/CD8 ratio and B/T cell ratio, and increased ratio of CD4+CD25+Foxp3+ Treg, CD8+CD28− T, and CD38+T lymphocyte of spleen. Albendazole treatment can alleviate spleen atrophy and set T cell immune reconstitution in some extend. Our data showed that A. cantonensis infection can cause splenic atrophy. These results are suggested to put more emphasis to improve the function of immune system. Meanwhile, infection and treatment model will be useful to evaluate new therapeutic approaches which can prevent or reverse immunosuppression and infectious complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The spleen, an important peripheral immune organ and a common target organ in infectious diseases, acts primarily as a blood filter and regulates immune response through humoral and cell-mediated pathways playing important roles in host immune defense (Brendolan et al. 2007; Cesta 2006). The white pulp and the red pulp as two main compartments of the spleen carry out important functions (Balogh et al. 2004; Medina 2016; Nolte et al. 2002). Clearance of circulating apoptotic cells is an important function of spleen. Meanwhile, regulatory T cells are critical to maintenance of peripheral tolerance and modulation of the immune system (Brousse et al. 2014). During systemic infections, several chemokine and adhesion molecules regulate the B and T cells activation (Morelli et al. 2003). However, various infections induce splenic alterations including splenic atrophy, modification in the splenic structure, and alteration in the function of spleen. When the spleen atrophies, it becomes smaller in size and cannot perform its function properly (Awaad and Moustafa 2016; Dillon et al. 1982; Elmore 2006; Gomez-Perez et al. 2014). Given the spleen’s intimate links with the immune system, its involvement in the pathogenesis of infectious diseases needs to be re-evaluated.
Angiostrongylus cantonensis (A. cantonensis) is the pathogen of angiostrongyliasis. As non-permissive host, humans are infected by eating food contaminating the third stage infective larvae of the worm. The infective larvae ultimately arrive at the central nervous system (CNS) and induce eosinophilic meningitis or meningoencephalitis (Baheti et al. 2009; Barratt et al. 2016). Mouse is also a nopernissoveost host and as an animal of human angiostrongyliasis. Previous studies focus on the damage to the central of nervous system (CNS) caused by A. cantonensis, including eosinophilic meningitis, meningoencephalitis, or optic neuritis (Feng et al. 2014); however, increasing evidence show that the CNS injury has profound effects on immune function. While interaction between the immune system and the nervous systems is bidirectional and occur under both physiological and pathological conditions, increasing reports showed that CNS injury have influence on the immune system (Gadani et al. 2015; Giulia et al. 2016; Soares-Schanoski et al. 2012). On the other hand, some studies have showed that the immune responses play an important role in the host’s resistance to the infection and pathogenesis of the parasitic nematode in mice infected with A. cantonensis. CD4+ T cells and type 2 immunity showed a protective function in A. cantonensis infection (Lee et al. 1996). Meanwhile, infection increases CD4+T, CD8+T, and natural killer (NK) cell counts after A. cantonensis infection (Liu et al. 2013; Wang et al. 2013). Interleukin 33 mediates type 2 immunity and inflammation in the central nervous system of the infected mice (Peng et al. 2013). CD8+CD28− T display immunosuppressive functions in immune system in infectious disease. CD8+CD28− T cells are reported to suppress CD4+ T cells functions (Cortesini et al. 2001; Onyema et al. 2015). CD38 is reported to have functions in cell adhesion, signal transduction, and calcium signaling (Malavasi et al. 2011). The level of CD8+CD28− T and CD38 T cell can reflect the status of immune system. Nevertheless, the basis for the pathogenesis of A. cantonensis infection is not fully understood, and there are only a few published reports that focused on the impact of the parasitic infection on mice immune system, especially on the spleen.
To further understand the association between the immune response and CNS, we used mouse models of A. cantonensis infection and investigated systematically and comprehensively the function of spleen in the infected mice. Our study based not only on the histological analysis of the spleen but also on the balance between an efficient immune response of the host and the development of pathology. We detected the capacity of CD4+ T to produce IL-2 and performed IHC staining for Ki67 to evaluate the ability of cellular proliferation and status of activation of the immune response in spleen. Meanwhile, to detect the states of the level of immune system after infection, we detected the expression of CD8+CD28− T cells and CD38+ T cells in spleen by flow cytometry. Since few reports concerned the recovering of the immune system after albendazole treatment in angiostrongyliasis, we also evaluated the albendazole treatment on the progression of damage of the brain, infiltration of inflammatory cells, eosinophilic meningitis, and recovering the immune system.
Methods and materials
Preparation of experimental animal and parasite
BALB/c mice (Male, 8 weeks old) were purchased from the Center of Experimental Animals of Sun Yat-Sen University (GB 14922.2-2011). Each mouse was orally infected with 30 larvae of A. cantonensis (third-stage, L3). The infective larvae were obtained from the tissues of infected snail, Biomphalaria straminea according to the previous methods (Yu et al. 2015). Mice were euthanized respectively on 7, 14, and 21 days post infection. For the treatment group, mice were treated by intragastric administrations of albendazole (20 mg/kg/day) and dexamethasone (0.5 mg/kg/day) for seven consecutive days from 7-day post infection, and sacrificed at 21 days after infection (Tu and Lai 2006; Wang et al. 2013). Each group contained six mice, and we performed three independent experiments.
Monitoring of infection
After mice were sacrificed, we removed the brain and shredded them in phosphate-buffered saline (PBS) and the larvae were examined under dissecting microscope at ×20 magnification (OuYang et al. 2012). Brains were fixed in 4% paraformaldehyde and embedded in paraffin. Sections were stained with hematoxylin and eosin. Images were captured using an AxioImager Z1 microscope (CarlZeiss, Jena, Germany).
Histological examination, immunofluorescence, and immunohistochemistry analysis
Spleen were excised and fixed in 4% paraformaldehyde. Tissues were embedded in paraffin, and 5-μm paraffin sections were stained with hematoxylin and eosin for the assessment of general histopathology. After deparaffinization and hydration of the spleen section, antigens were retrieved using a microwave. The primary antibodies used were Ki67 (Abcam). After incubated overnight at 4 °C and three washes with PBST, the samples were incubated with AB composite liquid and then DAB. Lastly, counterstained with Mayer’s hematoxylin and mounted.
According to manufacturer’s guidelines, splenic apoptotic cells were stained by TUNEL using the In Situ Cell Death Detection Kit, POD (Roche Applied Science, Mannheim, Germany). The slides were lightly counterstained with hematoxylin, dehydrated, and mounted. For each group, at least three samples were evaluated. All of the slices were examined using confocal microscope (Olympus BX63).
Lymphocyte preparation and splenocyte subpopulations analysis by flow cytometry
Spleens of the mice were mechanically dissociated. After being filtered through a cell strainer (100 μm), lysed RBCs, washed with PBS, spleen single cell suspensions were obtained. Their viability was determined by Trypan blue exclusion assay. The number of total splenocytes was counted using blood count plate. The splenocytes were resuspended to a concentration of 1 × 106 cells per milliliter and stained with antibody (CD3ɛ-PE, CD3ɛ-PE-Cyanine5, CD4-PE-Cyanine5, CD4-FITC, CD8-FITC, CD19-FITC, CD28-PE, CD38-PE, and CD49b (Integrin alpha 2)-PE-Cyanine5 (all from e-Biosciences Inc., San Diego, CA, USA)) at 4 °C for 30 min. After washing with PBS, the data were acquired by FACS. For IL-2 intracellular cytokine staining, the cells were seeded into 24-well microplates, treated with 2 μg/ml anti-CD3 mAb, anti-CD28 mAb (e-Biosciences), and Golgi Stop (BD Biosciences) at 4 °C for 5 h;, after surface stained with CD3ɛ and CD4, the cells were permeabilized with Permeabilization Buffer and doubly stained for surface and intracellular IL-2-PE antibodies for 30 min at 4 °C. After washing with PBS, the data were acquired by FACS. Lymphocyte was surface stained (CD4-FITC, CD25-PE), fixed, and then permeabilized for intracellular staining (Foxp3-PE-Cyanine5) to analysis of CD4+ CD25+ Foxp3+ Treg. The protocol is carried out following manufacturer’s instruction of mouse regulatory T cell staining kit (e-Biosciences Inc., San Diego, CA, USA).
Cytokines mRNA level detection
Total RNA was extracted from spleen using Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions. After reversed transcribed, cDNA was stored at −20 °C. IFN-γ and IL-4 messenger RNA (mRNA) levels were detected by real-time PCR using primers shown in Table 1. Then, the RT-PCR was performed with SYBR Green Supermix (Bio-Rad). β-Actin gene was used as an endogenous control.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). The significance of the difference was determined by one-way analysis of variance (ANOVA) with Tukey test using SPSS (version 19.0). Differences between groups were considered statistically significant at P ≤ 0.05.
Results
The brain pathological changes after A. cantonensis infection
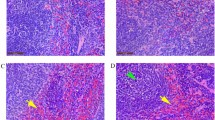
The brain pathological changes were observed by histological examination. Hemorrhages (black arrows) and infiltration of inflammatory cells (green arrows) were observed at 14 dpi and aggravated significantly at 21 dpi after infection. Albendazole treatment attenuated the progression of brain damage. Infiltration of inflammatory cells reduced in treatment group (Fig. 1). These pathological changes are similar to human angiostrongyliasis.
Pathological status of mice after A. cantonensis infection. Brain tissues were collected for pathology evaluated by H&E (hematoxylin and eosin) staining. Images are shown using an automatic upright microscope (AxioImager Z1) at ×20 magnification. Black arrows showed hemorrhages and green arrows for infiltration of inflammatory cells. The results are representative of at least three independent experiments. Each group contained 5–6 mice
A. cantonensis infection causes spleen atrophy
The size of the spleen of infected mice decreased, the texture of spleen became fragile with a more shallow color with the extension of time, and all of those changes gradually got pronounced with the progression of infection time, with maximum changes at 21 days post infection (dpi) (Fig. 2a). The spleen index of infected mice was significantly lower at 14 dpi, especially at 21 dpi. Though the spleen index showed a little decrease in 7 days after infection, there was no significant difference with control group (Fig. 2b). The number of splenocytes also remarkably decreased, leading to 11.6 and 71.2% reduction at 14 and 21 dpi, respectively (Fig. 2c). Albendazole treatment could significantly reverse the decrease of the spleen index and number of splenocytes. As shown in Fig. 2d, in control groups, the mouse spleens had normal splenic organization, with clearly identifiable regions of red and white pulp. However, spleens from infected mice exhibited marked histological disorganization. The most notable changes were the reduction of clearly defined splenic white pulp with expanded and disordered structure of red pulp. It showed significant disruption of the splenic architecture after infection with the extension of time, especially at 21 dpi. Albendazole treatment attenuated the progression of atrophy, and the spleen structure was restored in some extents compared with infected group.
A. cantonensis infection induces spleen atrophy. a Images of spleen of control, infected (21 dpi), and treated mice. b The spleen index of every groups. c The number of total splenocyte in spleens of different groups (n = 5). d Spleen tissues were collected for pathology evaluated by H&E staining. Images are shown using an automatic upright microscope (AxioImager Z1) at ×5 magnification. Data are mean ± SD of demyelination rates in control, 7 d, 14 d, and 21 d, respectively, after infection with A. cantonensis and represent three independent experiments. Asterisk indicates significant difference when compared with the control group. *P < 0.05; ***P < 0.001. Number sign indicates significant difference when compared with the straight line corresponded group. ##P < 0.01
Apoptosis is one of the reasons of spleen atrophy
To evaluate the effect of infection on apoptosis of spleen, apoptotic changes were assessed using TUNEL assay. It is important to determine whether A. cantonensis infection is directly associated with the apoptosis in spleen. We use TUNEL assay to evaluate the apoptotic changes in spleen. In control group, a small number of TUNEL-positive cells were observed within the main body of the spleen (Fig. 3). With the progression of infection, more and more TUNEL-positive cells were observed, especially at 21 dpi. The TUNEL-positive cells decreased after albendazole treatment.
CD4/CD8 ratio, B/T cell ratio, and the percentage of NK cell of splenocytes were significantly decreased in mice infected with A. cantonensis
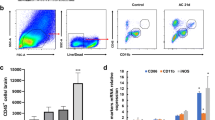
As mentioned above, A. cantonensis infections induce significantly spleen atrophy in the infected mice. Therefore, in order to analyze immune cell populations by a flow cytometer-based assay, we detected the proportions of T cell (CD3+ T cell), B cell (CD3−CD19+ T cell), and NK cell (CD3−CD49+ T cell) and subpopulations of T cell, CD4+T cell, and CD8+ T cell of spleen. As shown, there was a significant decrease in the ratio of CD4/CD8 T cells (Fig. 4a) and ratio of T/B cell at 21 dpi (Fig. 4b). We also observed significant decrease in the frequency of NK cell at 14 and 21 dpi (Fig. 4c) compared with the controls. Albendazole treatment restored the changes of CD4/CD8 ratio, B/T cell ratio, and the percentage of NK cell of splenocytes in some extents compared with 21 dpi group (Fig. 4).
A. cantonensis infection alters the CD4/CD8 ratio, B/T cell ratio, and percentage of NK cell of splenocytes. a–c Percentages of splenic T cells, B cells, and NK cells of splenocytes measured by FACS analysis. Statistical evaluation showed the CD4/CD8 ratio, B /T cell ratio, and percentage of NK cell of splenocytes. Data represent three independent experiments. Compared with the control group, asterisks indicate significant difference. ***P < 0.001. Compared with the straight line corresponded group, number sign indicates significant difference. ###P < 0.001
A. cantonensis infection can induce immunosuppression in spleen of the mice
To detect the function of peripheral T cells in spleen, we cultured the splenocytes in the presence of anti-CD3 and anti-CD28 for co-stimulation to induce IL-2 production. The capacity of CD4+ T to produce IL-2 by anti-CD3 and anti-CD28 co-stimulation dropped significantly at 14 and 21 dpi (Fig. 5a). Meanwhile, Ki67 staining showed there were very large numbers of positive cells throughout the spleen both in white pulp and red pulp in control group. The numbers of positive cells decreased at 21 dpi. The restoration of positive cells in spleen was more in treated group than in 21 dpi group (Fig. 5b). These results demonstrate that A. cantonensis infection can induce immunosuppression in spleen.
A. cantonensis infection reduces the activated T cells. a To analyze the changes of T cell function after infection, the expression levels of CD4+IL-2+ cells in the spleen were detected by FACS analysis. b Immunohistochemistry showing expression of Ki67 in the spleen. Magnification, ×10. Compared with the control group, asterisks indicate significant difference. *P < 0.05; ***P < 0.001. Compared with the straight line corresponded group, number sign indicates significant difference. ###P < 0.001
A. cantonensis infection induces dynamic expression of CD4+ CD25+ Foxp3+ Treg in spleen
The frequencies of Tregs were found to increase significantly during early infection, with a slight increase at 14 dpi, and obvious increase at 21 dpi. After albendazole treatment, the percentage of Treg showed a significant decrease (Fig. 6a). Histograms (mean ± SD) of the percentage of CD4+ CD25+ Foxp3+ Treg lymphocytes in total splenocytes were acquired (Fig. 6b). IFN-γ mRNA level of splenocytes showed no significant change after infection. The expression of IL-4 mRNA were increased at 21 dpi (Fig. 6c).
A. cantonensis infection effects dynamic expression of CD4+ CD25+ Foxp3+ Treg in spleen. a Splenic cells were gated on CD3+ T cells first, followed by analysis for T regulatory cells. b Histograms (mean ± SEA) of the percentage of CD4+ CD25+ Foxp3+ Treg lymphocytes in total splenocytes were acquired. c IFN-γ and IL-4 mRNA levels of splenocytes were measured. Each group of mice comprised five to six animals. Data presented represent one of three independent experiments. Compared with the control group, asterisks indicate significant difference. **P < 0.01; ***P < 0.001. Compared with the straight line corresponded group, number sign indicates significant difference. ###P < 0.001
A. cantonensis infection increased the expression of CD8+CD28− T and CD38+ T in spleen
We observed that the expression of CD8+CD28− T cell and CD38+ T cell using flow cytometry to detect the state of immune system. CD8+CD28− T cells showed began increase at 14 dpi. With the deterioration of brain inflammation, the percentage of CD8+CD28− T cells became apparent increase after infection especially at 21 dpi (Fig. 7a). Gated on CD3 positive splenocytes cells, CD38+ T cells increased after infection along with the progression of disease and reached a higher level at 21 dpi (Fig. 7b). Albendazole treatment could reduce the percentage of CD8+CD28− T cells and reversed the elevated expression of CD38 in spleen.
A. cantonensis infection effects dynamic expression of molecule CD28, CD38 on splenocytes. Lymphocytes were gated according to the forward scatter and side scatter characteristics for indicated dot plots. a–b Gated on CD3 positive splenocytes cells, we detected the percentage of CD8+CD28− T cells and CD38+ T cell. Data represent three independent experiments. C. Histograms (mean ± SD) showed the percentage of CD8+CD28− T lymphocytes and CD38+T lymphocytes in total lymphocytes in spleen. Asterisks indicate significant difference. **P < 0.01; ***P < 0.001. Compared with the straight line corresponded group, number sign indicates significant difference. ###P < 0.001
Discussion
A. cantonensis is a well-known food-borne causative agent of human eosinophiliomeningitis or meningoencephalitis in many areas. It is reported that the immune responses play an important role on responding properly to parasite antigen and exert anti-parasite effects. A. cantonensis infection induced Th2 immune response and CD4+ T cells played a key role (Feng et al. 2014; Chen et al. 2014; Spencer and Weller 2010). And it is reported that Th2 cytokines such as IL 4, IL5, IL10, and IL13 increase in the brain and periphery (Intapan et al. 2008; Diao et al. 2009). For a long time, studies on A. cantonensis largely focused on brain tissue damage and implied that peripheral immune disorders caused by infection are relatively limited. Considering this, we questioned whether the brain injury due to A. cantonensis infection would affect the peripheral immune system.
The results of our study showed a crucial change in the spleen structure after A. cantonensis infection. Spleen was gradually shrunken and the structure altered after A. cantonensis infection. It showed significant disruption of the splenic architecture, reduction of clearly defined splenic white pulp, and expansion and structural disorder of red pulp, which indicate immunosuppression. The number of splenocytes also remarkably decreased after infection, and this decrease in splenocytes number paralleled with the dramatic reduction in splenic index. It is important to determine whether A. cantonensis infection induced-splenic atrophy is directly associated with apoptosis in the spleen. Apoptotic changes were assessed using TUNEL assay. With lastingness of infection, more and more TUNEL-positive cells were observed. That is to say, apoptosis was one of the reasons of spleen atrophy.
To assess the balance between an efficient immune response of the host and the development of pathology, we investigated the characteristics of splenocyte subpopulations. A. cantonensis infection decreased CD4/CD8 ratio, B/T cell ratio, and the percentage of NK cell of splenocytes. In the general population, quantitative CD4/CD8 lymphocyte ratio can reflect the status of both CD4+ and CD8+ T cells in disease progression. A low CD4/CD8 and B/T cell ratio are considered a surrogate marker of immunosenescence, and has been associated with persistent infection (Smith et al. 2016; Walstra et al. 1985). As important innate lymphocytes, NK cells play an important role in early defense against pathogens as in a variety of pathogen infections. The decrease in percentage of NK cell of splenocytes after infection is in accordance with previous report (Chen et al. 2014).
Meanwhile, we detect the function of peripheral T cells in spleen. We cultured the splenocytes in the presence of anti-CD3 and anti-CD28 for co-stimulation to induce IL-2 production (Sojka et al. 2004). The capacity of CD4+ T to produce IL-2 by anti-CD3 and anti-CD28 co-stimulation dropped significantly after infection. Meanwhile, the result of IHC staining for Ki67, a cellular marker of proliferation, showed that the level of cellular proliferation and activation of the immune response were decreased after infection (Whitfield et al. 2006). The inhibition of proliferative activity of the immunocompetent cells suggests that infection can interfere with normal cell division. Spleen atrophy could also be due to the low proliferative activity except acceleration of apoptosis cells in spleen.
CD4+CD25+Foxp3+Treg cells play a key role in immune system (Plitas and Rudensky 2016). A. cantonensis infection induces the increase of the CD4+CD25+Foxp3+Treg cells in spleen. In addition, the Treg cell population might be relatively resistant to apoptosis or other mechanisms that reduce viable spleen cell numbers. Activated Th2 pathway of immune system was good for inflammatory response to against the parasites. However, the host’s own tissues also injured with too strong immune reaction. Thus, activated treg cells regulated the immune response when there is an excessively large or long immune response. While accumulated Treg cells also exert immunosuppressive roles in infectious disease. IL-2 is essential for Treg development and survival (Malek and Bayer 2004) and is also known to inhibit Th17 and to favor Treg differentiation (Oldenhove et al. 2009). It is reported that IL-2 played roles during acute toxoplasmosis (Salinas et al. 2014; Tenorio et al. 2011). The higher level of expression of CD4+CD25+Foxp3+Treg cells may therefore be correlated with low level of the IL-2 in the infected mice. Increased expression of IL-4 mRNA level and decrease of IFN-γ of splenocytes after A. cantonensis infection markedly confirmed the increasing and polarizing to Th2 phenotypes in A. cantonensis infection, which is consistent with other reports (Liu et al. 2013).
It has been reported that decrease expression of CD28 leads to decreased specificity in immune system. Loss of CD28 is also very common in chronic inflammation disease. High-expression CD38 is reported in many infective disease, and it could be reversed with effective treatment (Higashida et al. 2012). We found that mice infected with A. cantonensis showed a significant increase in the CD8+CD28− T cell and CD38+T cells population, which is parallel with inflammation in the brain. A. cantonensis infection inducing progressive loss of CD28 ultimately may suppress the antigen-presenting function and thus indirectly inhibit the T cells activation and cause immunosuppression. This model will be useful to evaluate new therapeutic approaches to prevent or reverse immunosuppression.
To study whether immunosuppression symptoms were directly caused by brain injury, we designed a therapeutic approach in A. cantonensis infection to kill worms and treat inflammation in the brain using albendazole early therapy. We found that albendazole treatment could alleviate spleen atrophy and setting of T cell immune reconstitution in some extend. This data suggests that the inhibition of immune responses was in part caused by parasitic migration-induced brain injury. This model will be useful to evaluate new therapeutic approaches to prevent or reverse immunosuppression and its infectious complications.
Conclusions
Our results indicate that A. cantonensis can induce acute and serious spleen atrophy and immunosuppression except in the brain. Further understanding of the mechanism of infection-induced immunosuppression will enable us to seek more effective therapies to modulation of the immune response. To improve the life quality of angiostrongyliasis patients, settings of T cell immune reconstitution and protecting immune system from damage should be considered besides killing helminths and decrease inflammation.
Abbreviations
- CNS:
-
Central nervous system
- Dpi:
-
Day post infection
References
Awaad A, Moustafa AY (2016) Immunotoxicity of skin acid secretion produced by the sea slug Berthellina citrina in mice spleen: histological and Immunohistochemical study. Acta Histochem 118:596–605
Baheti NN, Sreedharan M, Krishnamoorthy T, Nair MD, Radhakrishnan K (2009) Eosinophilic meningitis and an ocular worm in a patient from Kerala, south India. BMJ Case Rep 2009:bcr2007122093
Balogh P, Horvath G, Szakal AK (2004) Immunoarchitecture of distinct reticular fibroblastic domains in the white pulp of mouse spleen. J Histochem Cytochem 52:1287–1298
Barratt J et al (2016) Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen. Parasitology 143:1087–1118
Brendolan A, Rosado MM, Carsetti R, Selleri L, Dear TN (2007) Development and function of the mammalian spleen. Bioessays 29:166–177
Brousse V, Buffet P, Rees D (2014) The spleen and sickle cell disease: the sick(led) spleen. Br J Haematol 166:165–176
Cesta MF (2006) Normal structure, function, and histology of the spleen. Toxicol Pathol 34:455–465
Chen AL et al (2014) The quantitative and functional changes of NK cells in mice infected with Angiostrongylus cantonensis. Parasitol Res 113:2087–2094
Cortesini R, LeMaoult J, Ciubotariu R, Cortesini NS (2001) CD8+CD28− T suppressor cells and the induction of antigen-specific, antigen-presenting cell-mediated suppression of Th reactivity. Immunol Rev 182:201–206
Diao Z, Chen X, Yin C, Wang J, Qi H, Ji A (2009) Angiostrongylus cantonensis: effect of combination therapy with albendazole and dexamethasone on Th cytokine gene expression in PBMC from patients with eosinophilic meningitis. Exp Parasitol 123:1–5
Dillon AM, Stein HB, English RA (1982) Splenic atrophy in systemic lupus erythematosus. Ann Intern Med 96:40–43
Elmore SA (2006) Enhanced histopathology of the spleen. Toxicol Pathol 34:648–655
Feng Y et al (2014) The pathogenesis of optic neuritis caused by Angiostrongylus cantonensis in BALB/c mice. Parasites Vectors 7:339
Gadani SP, Walsh JT, Lukens JR, Kipnis J (2015) Dealing with danger in the CNS: the response of the immune system to injury. Neuron 87:47–62
Giulia P et al (2016) Brain atrophy, anti-smooth muscle antibody and cognitive impairment: an association study. Aging Dis 7:318–325
Gomez-Perez GP, van Bruggen R, Grobusch MP, Dobano C (2014) Plasmodium falciparum malaria and invasive bacterial co-infection in young African children: the dysfunctional spleen hypothesis. Malar J 13:335
Higashida H, Yokoyama S, Huang JJ, Liu L, Ma WJ, Akther S, Higashida C, Kikuchi M, Minabe Y, Munesue T (2012) Social memory, amnesia, and autism: brain oxytocin secretion is regulated by NAD+ metabolites and single nucleotide polymorphisms of CD38. Neurochem Int 61:828–838
Intapan PM, Kittimongkolma S, Niwattayakul K, Sawanyawisuth K, Maleewong W (2008) Cerebrospinal fluid cytokine responses in human eosinophilic meningitis associated with angiostrongyliasis. J Neurol Sci 267:17–21
Lee JD, Wang JJ, Chang JH, Chung LY, Chen ER, Yen CM (1996) Role of T cell subpopulations in mice infected with Angiostrongylus cantonensis. J Helminthol 70:211–214
Liu H, Luo X, Shen E, Li H, Ding X, Chen D (2013) Alteration of T cell subtypes in spleen and antibodies of serum in mice infected with Angiostrongylus cantonensis. Parasitol Res 112:1255–1260
Malavasi F, Deaglio S, Damle R, Cutrona G, Ferrarini M, Chiorazzi N (2011) CD38 and chronic lymphocytic leukemia: a decade later. Blood 118:3470–3478
Malek TR, Bayer AL (2004) Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol 4:665–674
Medina KL (2016) Overview of the immune system. Handb Clin Neurol 133:61–76
Morelli AE et al (2003) Internalization of circulating apoptotic cells by splenic marginal zone dendritic cells: dependence on complement receptors and effect on cytokine production. Blood 101:611–620
Nolte MA, Hamann A, Kraal G, Mebius RE (2002) The strict regulation of lymphocyte migration to splenic white pulp does not involve common homing receptors. Immunology 106:299–307
Oldenhove G et al (2009) Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31:772–786
Onyema OO, Njemini R, Forti LN, Bautmans I, Aerts JL, De Waele M, Mets T (2015) Aging-associated subpopulations of human CD8+ T-lymphocytes identified by their CD28 and CD57 phenotypes. Arch Gerontol Geriat 61:494–502
OuYang L et al (2012) Differences of larval development and pathological changes in permissive and nonpermissive rodent hosts for Angiostrongylus cantonensis infection. Parasitol Res 111:1547–1557
Peng H et al (2013) Interleukin 33 mediates type 2 immunity and inflammation in the central nervous system of mice infected with Angiostrongylus cantonensis. J Infect Dis 207:860–869
Plitas G, Rudensky AY (2016) Regulatory T cells: differentiation and function. Cancer Immunol Res 4:721–725
Salinas N, Olguin JE, Castellanos C, Saavedra R (2014) T cell suppression in vitro during Toxoplasma gondii infection is the result of IL-2 competition between Tregs and T cells leading to death of proliferating T cells. Scand J Immunol 79:1–11
Smith DM, Nakazawa M, Freeman ML, Anderson CM, Oliveira MF, Little SJ, Gianella S (2016) Asymptomatic CMV Replication during Early HIV-infection is Associated with Lower CD4/CD8 Ratio during HIV Treatment. Clin Infect Dis doi:10.1093/cid/ciw612
Soares-Schanoski A et al (2012) Impaired antigen presentation and potent phagocytic activity identifying tumor-tolerant human monocytes. Biochem Biophys Res Commun 423:331–337
Sojka DK, Bruniquel D, Schwartz RH, Singh NJ (2004) IL-2 secretion by CD4+ T cells in vivo is rapid, transient, and influenced by TCR-specific competition. J Immunol 172:6136–6143
Spencer LA, Weller PF (2010) Eosinophils and Th2 immunity: contemporary insights. Immunol Cell Biol 88:250–256
Tenorio EP, Fernandez J, Castellanos C, Olguin JE, Saavedra R (2011) CD4+ Foxp3+ regulatory T cells mediate Toxoplasma gondii-induced T-cell suppression through an IL-2-related mechanism but independently of IL-10. Eur J Immunol 41:3529–3541
Tu WC, Lai SC (2006) Angiostrongylus cantonensis: efficacy of albendazole-dexamethasone co-therapy against infection-induced plasminogen activators and eosinophilic meningitis. Exp Parasitol 113:8–15
Walstra K, Gratwohl A, Riederer I, Speck B (1985) B/T cell ratio of rabbit peripheral blood lymphocytes. Influence of separation technique on results. J Immunol Methods 79:143–147
Wang J et al (2013) Efficacy of tribendimidine against Angiostrongylus cantonensis infection in the mice. Parasitol Res 112:1039–1046
Whitfield ML, George LK, Grant GD, Perou CM (2006) Common markers of proliferation. Nat Rev Cancer 6:99–106
Yu L et al (2015) Preliminary expression profile of cytokines in brain tissue of BALB/c mice with Angiostrongylus cantonensis infection. Parasites Vectors 8:328
Acknowledgments
The manuscript was revised by Md Robiul Karim, who is a postdoctoral fellow in Sun Yat-sen University. This work was supported in part by the National Key Research and Development Plan (2016YFC1200500), the National High Technology Research and Development Program of China (No. 2015AA020934), and the National Natural Science Foundation of China (81271855, 81261160324, 81401688).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, Z., Wu, Y., Feng, Y. et al. Spleen atrophy related immune system changes attributed to infection of Angiostrongylus cantonensis in mouse model. Parasitol Res 116, 577–587 (2017). https://doi.org/10.1007/s00436-016-5322-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5322-9