Abstract
Three formulations; bait, encapsulation, and emulsion of Beauveria bassiana were prepared and evaluated for their insecticidal activity in simulated field settings. Tea waste-based bait formulation of B. bassiana showed 100 % mortality (within 72 h) in lab assay against adult houseflies. In field assay using traps, 65 % relative entrapment and 100 % mortality (within 60 h) of entrapped flies was observed. Although the bait formulation was low cost and easy to prepare and transport, its storage ability was limited. Hence, more advanced formulations in form of encapsulation and emulsion was attempted. Encapsulated B. bassiana conidia (using skimmed milk powder, polyvinyl pyrrolidone K-90 and glucose as additives) showed 100 % conidial germination and retained 78 % conidial viability, even after storage for 12 months at 30 °C. Encapsulated product showed 54.8 % (freshly prepared) and 30.6 % (after 12-months storage) mortality of housefly larvae in a simulated field condition. Emulsion formulation was prepared by using Tween 20 as surfactant with seven vegetable oils: soybean, rapeseed, sunflower, olive, castor, til, and linseed. Emulsion with linseed oil showing maximum conidial germination (94 %) was evaluated for shelf life and pathogenecity against housefly larvae. Shelf life analysis of emulsion revealed 28 % conidial germination and 19.9 % housefly larval mortality after 12 months of storage as opposed to 94 % conidial germination and 51.7 % of larval mortality with fresh product. Significant increase in shelf × targeted application of formulation is expected to increase its mass applicability for housefly control. Also, the variability among products presents diverse opportunities for commercialization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Entomopathogenic fungi are usually applied in the form of conidia, which need a stabilizing agent to facilitate application, stability during storage, and enhancement of activity (Alves et al. 2002; Meikle et al. 2008). In this regard, development of a suitable formulation is a crucial step towards successful implementation and scale up of entomofungal bioactivity. A biocontrol formulation with pest control potential should possess several desirable characteristics such as ease of preparation and application, stability, low cost, and abundant viable propagule (Sadanandane et al. 2003; Naranjo et al. 2013). Most of the mycoinsecticidal formulations discussed in the literature are concerned with the control of agricultural pests (Bateman et al. 1993; Feng et al. 2004a, b; Polar et al. 2008), while very few of these studies evaluated potential of such formulations for control of housefly (Watson et al. 1995; Geden et al. 1995; Mishra et al. 2011). Housefly is a major pest of medical and veterinary significance, the life cycle of which could be divided into larvae, pupa, and adult. A critical review of the product formulations for housefly control suggests that only primitive bait and dust formulations has been attempted and that too are confined against adults (Watson et al. 1995; Geden et al. 1995). An important aspect of more advanced formulations (encapsulation and emulsion) targeting the breeding stage of larvae and pupa, as well as adults is left unexplored.
Encapsulation of fungal conidia protects them from adverse environmental conditions, increases shelf life and bioefficacy, while easing the formulation handling (Patel et al. 2011; Kanis et al. 2012). With regards to housefly control, encapsulated fungal conidia with their protective coating could be a viable option for field application against housefly larvae and pupae. Similarly, emulsion of entomopathogenic fungi with vegetable oil protects fungal conidia from UV light and increases their efficacy by promoting conidial adhesion on the insect’s cuticle surface (David-Henriet et al. 1998; Malsam et al. 2002). Oil-based formulation of entomopathogenic fungi are also shown to enhance their pathogenecity to the host insects (Bateman et al. 1993; Bandani and Esmailpour 2006; Vega-Aquino et al. 2010; Luz et al. 2011). Furthermore, the ease of application for emulsion formulation makes them lucrative choice for housefly control. However, no reports are available in the literature on encapsulation and emulsion formulation for housefly control. Keeping in view the above facts, three formulations (bait, encapsulate, and emulsion) of B. bassiana, catering to the control of different life stages of housefly, site of applicability and need of end user, were prepared and evaluated in the present study.
Material and methods
Fungal culture
Beauveria bassiana HQ917687 used in the present study was isolated in our laboratory from soil samples collected from Northern part of Uttar Pradesh, India (data unpublished). The efficacy of fungal isolate against housefly has been reported earlier by Mishra and Malik (2012). The fungal isolate was grown on Potato Dextrose Agar (PDA) slants and maintained at 4 °C.
For preparation of conidial suspensions, conidia were harvested from fresh slants (cultured for 5 days at 28 °C) by adding 10 ml of 0.1 % sterile Tween 80 to the culture slant. The slant was vortexed for 5 min and the resulting suspension was filtered through a sterilized 8-μm membrane filter disk. The filtered conidial suspension was enumerated for conidia concentration and viability using an Automatic Cell Counter (Cellometer® Vision HSL, Nexcelom Bioscience). For determination of conidial viability, conidia were stained with Trypan blue. The conidial suspension was used as seed culture for formulation preparations.
Bait formulation of B. bassiana
Preparation of bait formulation
Preparation of fungus bait formulation was done using oven dried tea waste (80 °C, 24 h). The dried tea waste (150 g) was taken in Erlenmeyer flasks (250 ml) and inoculated with fungal conidial suspension (∼109 conidia/ml) along with appropriate moisture. Fungus was allowed to grow for 15–20 days at 28 °C. After the incubation period, the tea waste with fungal growth was mixed with 2 % carboxymethyl cellulose and glucose to prepare baits (diameter 1 mm).
Laboratory bioassay against adult housefly
Prepared fungal baits were adhered to molasses spread on the filter paper to prepare molasses strips (Fig. 1a). For the lab assay against housefly adults, these molasses strips were pasted on the inside wall of cylindrical mini-chamber (30 × 60 cm) made of plastic (Fig. 1b). In each of these cylindrical mini-chambers, 30 adult houseflies were placed along with housefly diet (consisting of wheat bran and groundnut oil cake in the ratio of 3:1). The mini-chambers were then covered with muslin cloth and incubated at 28 ± 2 °C, 65 % RH. The assay incorporated three replicates. Control set of experiment has molasses strip with tea bait without fungal growth. The mortality of adult flies was observed daily.
Field bioassay against adult housefly
Field assay against adult houseflies was performed in a restaurant (washing area) during the month of May 2010. The experiment was performed using traps (Fig. 1c), which were placed at the experimental site form 8 AM to 6 PM. The experimental set of traps contained either molasses strip with fungal bait or molasses strip without fungal bait, whereas the controlled set of traps had no strips. The number of flies entrapped inside the traps during the experimental period was observed to calculate relative entrapment in the test traps. The relative entrapment was calculated using the following formula:
where T T and T 0 is the flies entrapped within the treated and control traps in a given period of time. Further, the flies collected in the traps were kept at the room temperature (30 ± 2 °C) and was observed for mortality.
Encapsulated formulation of B. bassiana
Preparation of fungal encapsulate
Encapsulation of selected fungal isolate was prepared according to the method described by Horaczek and Viernstein (2004). An aqueous solution containing 5 % (w/v) skimmed milk powder and 1.25 % (w/v) polyvinyl pyrrolidone K-90 was prepared. To this aqueous mixture, an additive (5 %, w/v), consisting of one of three sugars (sucrose, glucose, and mannitol) was added. A negative control containing no additive was also incorporated. To the resultant aqueous mixture, 10 % (v/v) conidial suspension (∼108 conidia/ml) was added. The mixture was kept at −80 °C for 12 h and then exposed to freeze drying for the next 20 h, resulting in formation of encapsulated conidia.
Germination characterization of encapsulated conidia
For germination study, 1 g of encapsulated formulation was dissolved in double distilled water to prepare a conidial suspension (∼105 conidia/ml). A 20 μl of inoculums from the prepared suspensions were placed on PDAY (2 ml) medium on glass slides (75 × 25 mm) and observed microscopically at ×40 at regular interval of 4, 8, 16, 24, and 48 h. A total of 100 conidia were scored for each treatment in each of the trials and were analyzed statistically. For each additive, three replications were incorporated. Another set of control comprising non-encapsulated fungal conidia was also included for germination study.
Shelf life analysis
The fungus encapsulates (containing different additive) showing superior results for conidial germination rate was selected for further shelf life analysis under storage condition (30 ± 2 °C). The stored encapsulated conidia were periodically (0, 2, 4, 6, 8, 10, and 12 months) analyzed for its conidial germination rate till 12 months. Each observation incorporated data from three replications.
Bioassay against housefly larvae
Bioassay experiments with selected encapsulated conidia were performed during the period of October 2010 to September 2011. Housefly larvicidal assay was done in plastic trays (75 × 60 cm) containing decaying waste matrix as described earlier (Mishra et al. 2011). Dried waste matrix (5 kg) was placed in different trays and saturated with 70 % moisture. After 12 h, 20 g (∼106 conidia/g) of encapsulated entomopathogenic fungi was added to the matrix and mixed thoroughly. After 24 h, 300 housefly larvae were added to each tray. Experiment was done in triplicate with a control treatment. Trays were kept at room temperature within a netted cage (80 × 65 × 60 cm) and were checked daily for formation of pupae and emergence of flies for next 10 days. Number of pupae formed or flies emerged were counted together and rest of the larvae, which could not be accounted for, were considered as dead.
Emulsion formulation of B. bassiana
Preparation of emulsion
Emulsion formulation of B. bassiana was prepared using Tween 20, which was selected on the basis of best compatibility with the fungal isolate (Mishra et al. 2013). Seven vegetable oils: soybean, rapeseed, sunflower, olive, castor, til, and linseed were used for preparation of oil–water emulsions with Tween 20 according to the method described by Luz and Batagin (2005). Oils (5 ml), surfactant (2.5 ml), and sterilized water (45 ml) were adjusted separately to a temperature of 80 °C (distilled water) and 60 °C (oils and surfactant) in a water bath. The water was submitted to agitation at 200 rpm on a magnetic stirrer with continuous addition of oil and surfactant. After 30 min of agitation, prepared mixture was kept in a 100-ml flask and inoculated with 1 ml of conidial suspension (3.2 × 108 conidia/ml). Flasks were incubated at 28 °C and 180 rpm. After 48 h of inoculation, conidial germination rate was examined. For each oil–water emulsion, conidial germination was studied with three replicates.
Shelf life analysis
The best emulsion selected on the basis of conidial germination was evaluated under storage condition (30 ± 2 °C) at regular interval according to the method described above. SEM image analysis of the emulsion sample from freshly prepared and 12-months stored emulsion was also carried out.
Bioassay against housefly larvae
The larvicidal assay with emulsion was performed during the period of May, 2010 to April, 2011 with 20 ml (∼106 conidia/ml) of emulsion. The assay was done in simulated field condition according to the method described above.
Results and discussion
Bait formulation of B. bassiana
A bait formulation is an active ingredient mixed with food or another attractive substance. The conidial concentration in the tea waste bait formulation prepared in the present study was enumerated at ∼108 conidia/g. Insecticidal activity of baits was evaluated in cylindrical mini-chambers, whereas the field assay was performed using commercial traps.
Laboratory bioassay against adult housefly
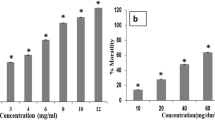
In a mini-chamber assay against adult houseflies, fungal bait was found to be very effective with 100 % mortality within 3 days (Fig. 2). Traps comprising molasses strips alone showed 10 % flies mortality. Most of the mortality in these traps was observed to be due to sticking of wings or probasis of adult houseflies to the wet surface of molasses. Previously, we evaluated mortality of adult housefly using B. bassiana conidial suspension at different concentrations in a similar mini-chamber bioassay (Mishra et al. 2011). The data from the study is included in Fig. 2 for comparison. In this study, absolute mortality of adult housefly with conidial suspension could be achieved only after 5 days, while the mortality data after 3 days was 47–56 %. Under similar condition in the present study, fungal baits provided 100 % adult mortality in 3 days. The above results reflecting increased mortality and decreased time of kill clearly indicate significant increase in B. bassiana efficacy when formulated as bait.
Mortality (mean ± S.D.) of adult house flies in mini-chamber bioassay [data represented by  indicates B. bassiana bait formulation;
indicates B. bassiana bait formulation;  indicates B. bassiana conidial suspension at 1 × 106 conidia/ml;
indicates B. bassiana conidial suspension at 1 × 106 conidia/ml;  indicates B. bassiana conidial suspension at 1 × 109 conidia/ml; number sign and asterisk are the data sets taken up from Mishra et al. (2011) for comparison]
indicates B. bassiana conidial suspension at 1 × 109 conidia/ml; number sign and asterisk are the data sets taken up from Mishra et al. (2011) for comparison]
Field bioassay against adult housefly
At the field level, due to apparent difficulty in monitoring of housefly population, data analysis for control experiments becomes difficult. The use of traps solves the problem to an extent, with ease in monitoring. Moreover, flies within traps, being representative population, gives an idea about population size and types. Hence, field assay with B. bassiana baits was performed within traps under three settings: (a) traps, (b) traps + molasses, and (c) traps + molasses + fungal baits. Traps with molasses showed a relative housefly entrapment of 62.5 %. The relative mortality of adult fly within this setting was 5 % (Fig. 3). The field setting containing traps with molasses and fungal baits showed a relative entrapment of 65 %. Such traps showed 100 % mortality (within 60 h) of entrapped flies. The results reflected molasses as good attractant for adult houseflies. However, no significant difference in the relative entrapment for traps containing molasses alone and molasses with fungal baits was obtained. Moreover, the attractant activity of molasses does not extend to its killing potentiality, reflected by low relative mortality (5 %) in traps containing only molasses. Thus, it is evident that the housefly mortality was solely due to the fungal baits.
Geden et al. (1995) reported the activity of sucrose bait formulation of two B. bassiana strains (P89 and L90) against housefly adults. In their study, the housefly mortality with baits of B. bassiana strains varied between 78 and 88 % (108 conidia/g) to 87–94 % (109 conidia/g) after 5 days of exposure. Renn et al. (1999) reported activity of bait formulation of M. anisopliae against housefly in a semi-field setting done with polythene cubicles. They observed housefly mortality to be dependent upon the time of exposure and the sex of the flies. In their study, females of housefly showed 20 % (fourth day) to 92.5 % (tenth day) mortality, while for male 9.3 % (fourth day) to 100 % (tenth day) mortality was achieved. Similarly, Sharififard et al. (2011) evaluated bait formulation (107 spore/g) of M. anisopliae (IRAN 437C) against housefly adult. In their study, fungal baits with sugar and powdered milk showed 72.4 % adult mortality after 9 days of observation. Further, it was observed that fungal baits along with sublethal doses of spinosad showed significant increase in Musca domestica adult mortality. They concluded that the sublethal spinosad exhibited synergistic and additive effect on insect mortality with the fungal dose of 105 and 107 spore/g, respectively. Naranjo et al. (2013) reported 92 % mortality of Aedes albopictus using boric acid sugar bait formulation in lab assay, while comparative mortality in semi-field condition was found to be 32.5 %, after an exposure period of 14 days.
In view of the above discussion, the present results with absolute mortality of adult houseflies within 72 h (mini-chamber assay) to 60 h (traps assay) were quite encouraging, and hold potentiality for B. bassiana bait formulations as housefly control agent. Further, the possibility of indirect transfer of infection by baits due to tendency of the houseflies to pick up lethal dose of conidia from dead flies, adds to their advantage. Moreover, tea waste being abundantly available, production of these baits would be economically viable and the technology could be easily replicated at the end user level. In fact, tea waste bait can easily be produced by small scale street food vendors for controlling the housefly menace at their eat-out. In spite of all the above advantages, the application and shelf life of bait formulations presents several disadvantages. Bait formulations of B. bassiana may kill the non-target beneficial organisms or may serve as a food supply to other pests after removal of fungal conidia. Further, during application of bait formulations, it is difficult to get an even distribution. However, the major problem is its short shelf life (2–3 months). The handicap bestowed due to the low shelf life, renders commercialization more difficult. Further, it also limits their area of use, which remains confined to local production and utilization.
Encapsulated formulation of B. bassiana
In order to produce more effective and stable formulation, encapsulation of B. bassiana was attempted. Present study endeavored encapsulation formulation based on skimmed milk powder and polyvinyl pyrrolidone K-90. Saraiva et al. (2010) pointed that hydrophobic fungal agents such as B. bassiana are typically soluble in organic solvents and are distributed in microparticles as molecular dispersions within the amorphous polymer matrix. The effect of various sugar additives on efficacy of B. bassiana encapsules was studied along with assessment of encapsulated B. bassiana for its shelf life and bioefficacy against housefly larvae.
Effect of additives
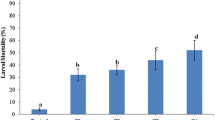
Figure 4 elucidates the effect of encapsulation on conidial germination in presence of various additives. Encapsulated B. bassiana conidia containing no sugar achieved 82 % conidial germination, while non-encapsulated control showed 100 % conidial germination within 48 h. The results indicated negative effect of encapsulation technique on conidial viability. The percentage conidial germination of encapsulated conidia varied significantly with different additives (F = 69.24; df = 2, 8; p < 0.001). Also, significant variation in conidial germination was observed for each additives for different observation time (F = 253.79; df = 4, 8; p < 0.001). Addition of glucose and sucrose showed a conidial germination of 100 % while addition of mannitol resulted in only 78 % conidial germination (Fig. 4). Hence, encapsulated conidia-containing additives (glucose and sucrose) showed comparatively higher conidial viability, suppressing detrimental effect of encapsulation process. The results suggested protective effect of these sugars during freeze drying.
The observation of germination kinetics showed an increasing trend for encapsulated conidia-containing glucose and sucrose; while regressing germination kinetics was observed with mannitol as additive (Fig. 4). Better germination kinetics observed with certain sugars further suggests their nutritive effect. Mannitol, however, could not perform optimally, showing negative effect on conidial growth and germination. Encapsulated B. bassiana conidia-containing glucose and sucrose depicted a healthy germinating conidium whereas with mannitol a negative impact on germinating conidia with visible shrinkage was observed.
To the best of author’s knowledge, no study dealing with encapsulation of B. bassiana exists in the literature. Hence, attempts were made to construe a meaningful comparison with similar studies on other entomopathogenic fungi, which too were scarce. A study concerning formation of wheat bran pellet with B. bassiana (Qu-B306) at different chitin combinations was reported by Gerding-Gonzalez et al. (2007). The improved conidial stability with the addition of stabilizers was reported by Burges and Jones (1998) while contradicting report by Burges (1998) indicated their toxic effect on conidial viability and production. In corroboration with the present study, Guijarro et al. (2007) recorded conidial germination of 100 and 96 % in Penicillium frequentans with addition of glucose and sucrose, respectively. Similarly, Jin and Custis (2011) reported higher colony-forming units in sucrose-mixed encapsulated conidia of Trichoderma harzianum than those containing no sugar. The positive effect of sugar (glucose and sucrose) on conidial growth and germination observed in the present study was possibly due to their nutritional effect, thereby providing fitness and endogenous reserves to B. bassiana conidia (Wraight et al. 2001).
Shelf life analysis
Encapsulated B. bassiana conidia-containing glucose was found to be the best for fungal growth and germination, and thus chosen for further shelf life studies under storage condition (30 ± 2 °C) for 12 months. Results revealed significant variation in conidial germination (F = 21.20; df = 4, 12; p < 0.001) for the encapsulated conidia at different storage period. The formulated conidia showed high conidial viability (78 %) even after a storage period of 12 months (Table 1), while the viability of non-encapsulated conidia decreased to 23 %. The encapsulation efficiency of microparticles is said to be dependent upon their particle size, and the partition coefficient of the active agent between polymer and release medium environment (Tafaghodi et al. 2011). Further, use of active agent as encapsulate not only improve their stability but also prolong their activity time (Kanis et al. 2012), as also reflected in the present study.
The available literature reports for various fungi indicated longer shelf life of encapsulated conidia (Liu and Liu 2009). Glucose as additive improved conidial viability and shelf life of the formulated P. frequentans conidia up to 365 days, when stored at 22 °C (Guijarro et al. 2007). Similarly, significant increase in survival percentage of encapsulated T. harzianum conidia with sucrose addition was observed by Jin and Custis (2011). Liu and Liu (2009) reported 80 % germination rate in the hydroxypropyl methyl cellulose-coated M. anisopliae conidia after 6 months of storage at 4 °C. Compared to the above studies, glucose-coated B. bassiana encapsule used in the present study showed better results with 92 % conidial germination after 6 months of storage at 30 °C.
Bioassay against housefly larvae
The evaluation of glucose-coated conidia for its pathogenecity against housefly larvae showed 54.8 % larval mortality which decreased to 30.6 % with the formulated product stored for 12 months (Table 1). During the different storage period, encapsulated conidia showed a positive correlation (0.99) between formulation efficacy (reflected in the conidial germinations) and larval mortality data. However, due to the absence of relevant literature reports concerning insecticidal activity of encapsulated fungal conidia, any meaningful comparison with the present study could not be attempted. Another study by our group (Mishra et al. 2011) reported 40 % mortality in housefly larvae under similar conditions using B. bassiana dust formulation with calcium carbonate. Hence, the present encapsulation method with higher insecticidal activity yields better product. In another study, significant mortality against larvae of Aedes aegypti and Anopheles stephensi was observed using silver nanoparticles of filamentous fungus, Cochliobolus lunatu (Salunkhe et al. 2011). The larval mortality rates in the above study were positively correlated with concentrations and exposure period of nanoparticles. Encapsulation of B. bassiana in the present study showed a high potentiality towards control of housefly in simulated field conditions. Moreover, its long shelf life obtained under storage condition was remarkable. Usage of sugar in encapsulation becomes highly relevant during field application, where sugars may protect the encapsulated conidia by creating a niche osmotic environment thus improving their viability (Jin and Custis 2011). However, dry formulations are restricted by the limitation in their range of application. In view of this, a liquid based formulation of B. bassiana (emulsion) was attempted.
Emulsion formulation of B. bassiana
Application of fungal conidia as emulsion frequently improves its environmental persistence and virulence against insects (Vega-Aquino et al. 2010). In our previous report, systematic study leading to selection of suitable surfactant was described. Among SDS, CABS-65, Tween 20, and Tween 80, Tween 20 was revealed to be the most compatible surfactant for B. bassiana (Mishra et al. 2013). In the present study, selection of suitable vegetable oil was done by preparing various vegetable oil-based emulsions using Tween 20. Subsequently, the best emulsion selected on the basis of conidial germination was evaluated for its shelf life and bioefficacy in semi-field conditions.
Evaluation of vegetable oils
The suitability of different vegetable oils was evaluated for emulsion preparation using Tween 20 as the surfactant. Growth and conidiogenesis of B. bassiana in different oil emulsions revealed significant variation in conidial germination (F = 41.01; df = 6, 24; p < 0.001). Also, conidial germination varied significantly between different observation time (F = 188.84; df = 4, 24; p < 0.001). Emulsion in linseed oil showed (Fig. 5) maximum conidial germination (94 %), followed by soyabean oil emulsion (87 %). The emulsions of sunflower, rapeseed, and castor oil were least effective in terms of conidial germination (51–52 %). The control treatment having conidia incubated in surfactant only showed 58 % conidial germination. Thus, the emulsions containing linseed and soyabean oil depicting better conidial germination kinetics are suitable for application.
Few studies attempting preservation of B. bassiana conidia in vegetable oil reported soybean and paraffinic oils (Moslim et al. 2004) or corn oil (Consolo et al. 2003; Luz and Batagin 2005) as best for B. bassiana conidial germination. However, contrary to the findings of the present study, Luz and Batagin (2005) reported poor conidial germination of B. bassiana with linseed (27 %) and soybean oil (19 %). Variation in conidial germination for different oils could be attributed to qualitative and quantitative composition of fatty acids, and their interaction with other oil components (Luz and Batagin 2005).
Shelf life analysis
Linseed oil emulsion supporting maximum conidial germination was taken up for further study concerning shelf life analysis and bioassay evaluation against housefly larvae. Conidial germination in linseed oil emulsion evaluated under storage condition (30 ± 2 °C) for 12 months, showed significant decrease in conidial viability with storage period (F = 24.21; df = 4, 16; p < 0.001). Fresh emulsion had maximum conidial germination of 94 % which decreased to only 28 % after 12 months of storage (Table 2). SEM micrograph of freshly prepared emulsion revealed B. bassiana with intact conidia and mycelium (Fig. 6a). After 12 months of storage, deterioration in mycelium was observed while fungal conidia could not be observed (Fig. 6b). Alves et al. (2002) obtained 56–72 % conidial viability in oil-formulated M. anisopliae conidia after 40 weeks of storage at 27 °C. They also reported significant decline in the conidial viability of formulations during storage period which became more prominent after 20 weeks of storage. The result was better than that obtained in the present study for B. bassiana conidia, where only 42 % conidial germination was seen after 9-months (36 weeks) of storage. However, further longevity of formulated conidia could be ensured by its storage at a lower temperature.
Bioassay against housefly larvae
Linseed oil emulsion was evaluated for its insecticidal efficacy against housefly larvae in a simulated field assay. The freshly prepared emulsion showed 51.7 % larval mortality which decreased significantly with increase in storage time for emulsion (Table 2). After 12 months of storage, larval mortality decreased to 19.9 %, revealing ≈2.6 time loss in bioefficacy. The larval mortality data obtained for emulsion with different storage period showed a high correlation (0.94) with conidial germination of emulsified conidia for the same period. Compared to the oil emulsion, fresh non-formulated conidia of B. bassiana resulted in 40 % mortality, which decreased drastically to 23 % mortality only after 3 months of storage. On the other hand, the emulsion caused >48 % mortality after 3 months of storage, indicating better preservation of the conidial efficacy.
Comparison of the conidial germination and larval mortality between encapsulated formulation (Table 1) and emulsion (Table 2) reveals that in spite of lower germination, larval mortality was higher when emulsion was used. For instance, after 6 months, conidial germination and larval mortality for emulsion were 60.5 and 43.3 %, respectively, while for encapsulation same were 92.1 and 44.8 %, respectively. Increase in virulence of fungus is supposed to be due to better adhesion between oil formulated conidia and lipid layer of insect cuticle through hydrophobic interaction (Bandani and Esmailpour 2006). Further, oils in the emulsion are reported to increase the conidial retention by preventing evaporation (Bateman et al. 1993).
The study against housefly using formulations of enotomopathogenic fungi has been limited to conidial dust, baits, or wetteable powder (Watson et al. 1995; Geden et al. 1995; Mwamburi et al. 2011), while no study concerning fungal emulsion against housefly larvae have been evaluated. Barson et al. (1994) studied potential of linseed and soya bean oil as carrier for M. anisopliae in aqueous suspension. A significant increase in fungal efficacy with complete mortality of housefly larvae in 3 days was reported. Similarly, oil formulated Nomuraea rileyi was more effective against larvae of Spodoptera spp. (Vega-Aquino et al. 2010). Synthetic oil (ShellSol T) formulation of M. anisopliae and B. bassiana conidia against Anopheles gambiae larvae resulted in significant reduction in pupae formation in lab (85.4 %) and field (39–50 %) conditions (Bukhari et al. 2011). In view of the above, ∼52 % housefly larval mortality obtained under the simulated field condition in present study is quite encouraging. The present study has limited itself to housefly larval mortality and the fate of survived larvae was not observed. Nevertheless, transmission of infection to next generation with sufficient reduction in conidial hydrophobicity as proposed by Gindin et al. (2006) might have increased the overall control efficacy. The study in this direction is warranted.
Comparative performance of different formulations
The results from this study indicated a workable approach regarding formulation of fungal conidia for various applications (Table 3). Baits formulation with immense applicability against adult houseflies in indoor as well as outdoor environments showed great potentiality for integration in housefly management program. Its simple preparation methodology, ease in transport and low cost production facilitates mass applicability and popularity in rural areas and slums infested with housefly menace. Encapsulation of B. bassiana with their protection of fungal conidia from adverse environmental condition not only increased their persistence, but also overall efficacy. Encapsulation also improved formulation handling and storage. However, significant increase in shelf life of B. bassiana conidia obtained with encapsulation was most remarkable. Emulsion of B. bassiana was superior spray carrier, and hence increased the probability of direct contact between fungal conidia and host insects. This property makes emulsion an excellent choice for management of housefly larvae and pupae inhabiting habitats difficult to penetrate with other formulations. Further, oil in emulsion increases conidial retention by preventing evaporation, while surfactants decrease conidial hydrophobicity. This results in increased persistence of fungal conidia during laboratory and field application as reflected by 51.7 % larval mortality in simulated field condition. Although, the shelf life of emulsions was inferior to encapsulated conidia, their properties of better sprayability render them more suitable for field application.
Conclusions
A previously isolated B. bassiana strain was formulated into three different formulations (bait, encapsulate, and emulsion). The B. bassiana bait showed higher and faster mortality of housefly adults over the bait formulations reported in literature. Encapsulate and emulsion of B. bassiana conidia depicted 54.7 and 51.7 % larvicidal activity, respectively, in the simulated field assay. This was the first report on encapsulate and emulsion that targeted against housefly larvae. Interestingly, the present work provided a basket of product options to choose as per the situation. While bait formulation was suited for local production and consumption cycle through cottage industries, advanced emulsion and encapsulated formulations could provide entrepreneurship models for organized sector.
References
Alves SB, Rossi LS, Lopes RB, Tamai MA, Pereira RM (2002) Beauveria bassiana yeast phase on agar medium and its pathogenicity against Diatraea saccharalis (Lepidoptera: Crambidae) and Tetranychus urticae (Acari: Tetranychidae). J Invertebr Pathol 81:70–77
Bandani AR, Esmailpour N (2006) Oil formulation of entomopathogenic fungus, Beauveria bassiana, against Sunn pest, Eurygaster integriceps Puton (Heteroptera: Scutelleridae). Commun Agric Appl Biol Sci 71:443–448
Barson G, Renn N, Bywater AF (1994) Laboratory evaluation of six species of entomopathogenic fungi for the control of house fly (Musca domestica L.), a pest of intensive animal units. J Invertebr Pathol 64:107–113
Bateman RP, Carey M, Moore D, Prior C (1993) The enhanced infectivity of Metarhizium flavoviride in oil formulations to desert locusts at low humidities. Ann Appl Biol 122:145–152
Bukhari T, Takken W, Koenraadt CJM (2011) Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasit Vectors 4:1–14
Burges HD (1998) Formulation of mycoinsecticides. In: Burges HD (ed) Formulation of microbial biopesticides: beneficial microorganisms, nematodes and seed treatments. Kluwer Academic, Dordrecht, pp 131–187
Burges HD, Jones KA (1998) Trends in formulation of microorganisms and future research requirements. In: Burges HD (ed) Formulation of microbial biopesticides. Kluwer Academic, Dordrecht, pp 311–332
Consolo VF, Salerno GL, Beron CM (2003) Pathogenicity, formulation and storage of insect pathogenic hyphomycetous fungi tested against Diabrotica speciosa. BioControl 48:705–712
David-Henriet AI, Pye BJ, Butt TM (1998) Formulation and application of the entomopathogenic fungus Metarhizium anisopliae for the control of crucifer pests in Europe, in Insect Pathogens and Insect Parasitic Nematodes (Smits, P.H., Ed.). IOBC WPRS Bull 21:89–90
Feng MG, Pu XY, Ying SH, Wang Y-G (2004a) Field trials of an oil-based emulsifiable formulation of Beauveria bassiana conidia and lowapplication rates of imidacloprid for control of false-eye leafhopper Empoasca vitis on tea in southern China. Crop Prot 23:489–496
Feng MG, Chen B, Ying SH (2004b) Trials of Beauveria bassiana, Paecilomyces fumosoroseus and imidacloprid for management of Trialeurodes vaporariorum (Homoptera: Aleyrodidae) on greenhouse grown lettuce. Biocontrol Sci Technol 14:531–544
Geden CJ, Rutz DA, Steinkraus DC (1995) Virulence of different isolates and formulation of Beauveria bessiana for house flies and the parasitoid Muscidifurax raptor. Biol Control 5:615–621
Gerding-Gonzalez M, France A, Sepúlveda ME, Campos J (2007) Use of chitin to improve a Beauveria bassiana alginate-chitin pellet formulation. Biocontrol Sci Technol 17:105–110
Gindin G, Levski S, Glazer I, Soroker V (2006) Evaluation of the Entomopathogenic Fungi Metarhizium anisopliae and Beauveria bassiana against the Red Palm Weevil Rhynchophorus ferrugineus. Phytoparasitica 34:370–379
Guijarro B, Melgarejo P, Cal AD (2007) Effect of stabilizers on the shelf-life of Penicillium frequentans conidia and their efficacy as a biological agent against peach brown rot. Int J Food Microbiol 113:117–124
Horaczek A, Viernstein H (2004) Comparison of three commonly used drying technologies with respect to activity and longevity of aerial conidia of Beauveria brongniartii and Metarhizium anisopliae. Biol Control 31:65–71
Jin X, Custis D (2011) Microencapsulating aerial conidia of Trichoderma harzianum through spray drying at elevated temperatures. Biol Control 56:202–208
Kanis LA, Prophiro JS, Vieira ES, Nascimento MP, Zepon KM, Kulkamp-Guerreiro IC, Silva OS (2012) Larvicidal activity of Copaifera sp. (Leguminosae) oleoresin microcapsules against Aedes aegypti (Diptera: Culicidae) larvae. Parasitol Res 110:1173–1178
Liu CP, Liu SD (2009) Formulation and characterization of the microencapsulated entomopathogenic fungus Metarhizium anisopliae MA126. J Microencapsul 26:377–384
Luz C, Batagin I (2005) Potential of oil-based formulations of Beauveria bassiana to control Triatoma infestans. Mycopathological 160:51–62
Luz C, Mnyone LL, Russell TL (2011) Survival of anopheline eggs and their susceptibility to infection with Metarhizium anisopliae and Beauveria bassiana under laboratory conditions. Parasitol Res 109:751–758
Malsam O, Kilian M, Oerke EC, Dehne HW (2002) Oils for increased efficacy of Metarhizium anisopliae to control whiteflies. Biocontrol Sci Technol 12:337–348
Meikle WG, Mercadier G, Holst N, Girod V (2008) Impact of two treatments of a formulation of Beauveria bassiana (Deuteromycota: Hyphomycetes) conidia on Varroa mites (Acari: Varroidae) and on honeybee (Hymenoptera: Apidae) colony health. Exp Appl Acarol 46:105–117
Mishra S, Malik A (2012) Comparative evaluation of five Beauveria isolates for housefly (Musca domestica L.) control and growth optimization of selected strain. Parasitol Res 111:1937–1945
Mishra S, Kumar P, Malik A, Satya S (2011) Comparative efficacy of Beauveria bassiana and Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) for the control of adult housefly, Musca domestica (Diptera: Muscidae). Parasitol Res 108:1483–1492
Mishra S, Kumar P, Malik A (2013) Evaluation of Beauveria bassiana spore compatibility with surfactants. World Acad Sci Eng Technol 73:109–113, ICABBBE 2013: International Conference on Agricultural, Biotechnology, Biological and Biosystems Engineering, Zurich, Switzerland, January 14–15, 2013
Moslim R, Wahid MB, Ali SRA, Kamarudin N (2004) The effects of oils on germination of Beauveria bassiana (Balsamo) Vuillemin and its infection against the oil palm bagworm, Metisa plana (Walker). J Oil Palmer Res 16:78–87
Mwamburi LA, Laing MD, Miller R (2011) Laboratory and field evaluation of formulated Bacillus thuringiensis var. israelensis as a feed additive and using topical applications for control of Musca domestica (Diptera: Muscidae) larvae in caged-poultry manure. Environ Entomol 40:52–58
Naranjo DP, Qualls WA, Müller GC, Samson DM, Roque D, Alimi T, Arheart K, Beier JC, Xue R-D (2013) Evaluation of boric acid sugar baits against Aedes albopictus (Diptera: Culicidae) in tropical environments. Parasitol Res 112:1583–1587
Patel AV, Jakobs-Schönwandt D, Rose T, Klaus-Dieter V (2011) Fermentation and microencapsulation of the nematophagous fungus Hirsutella rhossiliensis in a novel type of hollow beads. Appl Microbiol Biotechnol 89:1751–1760
Polar P, Moore D, Kairo MTK, Ramsubhag A (2008) Topically applied myco-acaricides for the control of cattle ticks: overcoming the challenges. Exp Appl Acarol 46:119–148
Renn N, Bywater AF, Barson GA (1999) Bait formulated with Metarhizium anisopliae for the control of Musca domestica L. (Dipt., Muscidae) assessed in large-scale laboratory enclosures. J Appl Entomol 123:309–314
Sadanandane C, Reddy CMR, Prabakaran G, Balaraman K (2003) Field evaluation of a formulation of Pseudomonas fluorescens against Culex quinquefasciatus larvae and pupae. Acta Trop 87:341–343
Salunkhe RB, Patil SV, Patil CD, Salunke BK (2011) Larvicidal potential of silver nanoparticles synthesized using fungus Cochliobolus lunatus against Aedes aegypti (Linnaeus, 1762) and Anopheles stephensi Liston (Diptera; Culicidae). Parasitol Res 109:823–831
Saraiva J, Lira AM, Esperandim VR, Ferreira DS, Ferraudo AS, Bastos JK, Silva MA, Gaitani CM, Albuquerque S, Marchetti JM (2010) (−)−Hinokinin-loaded poly(d,l-lactide-co-glycolide) microparticles for Chagas disease. Parasitol Res 106:703–708
Sharififard M, Mossadegh MS, Vazirianzade B, Zarei-Mahmoudabadi A (2011) Interactions between Entomopathogenic fungus, Metarhizium anisopliae and sublethal doses of spinosad for control of house fly, Musca domestica. Iran J Arthropod Borne Dis 5:28–36
Tafaghodi M, Khamesipour A, Jaafari MR (2011) Immunization against leishmaniasis by PLGA nanospheres encapsulated with autoclaved Leishmania major (ALM) and CpG-ODN. Parasitol Res 108:1265–1273
Vega-Aquino P, Sanchez-Peña S, Blanco CA (2010) Activity of oil-formulated conidia of the fungal entomopathogens Nomuraea rileyi and Isaria tenuipes against lepidopterous larvae. J Invertebr Pathol 103:145–149
Watson DW, Geden CJ, Long SJ, Rutz DA (1995) Efficacy of Beauveria bassiana for the controlling of the house fly and stable fly (Diptera: Muscadiae). Biol Control 5:405–411
Wraight SP, Jackson MA, de Kock SL (2001) Production, stabilization and formulation of fungal biocontrol agents. In: Butt TM, Jackson C, Magan N (eds) Fungi as biocontrol agents. Progress, problems and potential. CABI Publishing, Wallingford, pp 253–287
Acknowledgments
This work was supported by Indian Council of Medical Research (IRIS_ID no. 2010–07860), India. CSIR fellowship to one of the authors (SM) is gratefully acknowledged. The authors also acknowledge Mr. Satendar Singh (IIT Delhi, India) for his help in experimental work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishra, S., Kumar, P. & Malik, A. Preparation, characterization, and insecticidal activity evaluation of three different formulations of Beauveria bassiana against Musca domestica . Parasitol Res 112, 3485–3495 (2013). https://doi.org/10.1007/s00436-013-3529-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3529-6