Abstract
In this work, fermentation and formulation aspects of the nematophagous fungus Hirsutella rhossiliensis BBA were investigated. When incubated in 2% (w/w) glucose and 0.5% (w/w) yeast extract medium in a 1-L Erlenmeyer flask without baffles, heavy pellet formation was observed. Only 40% of the mycelium had a size less than 500 μm. When a flask with three baffles was used, the portion of mycelium <500 μm rose to 95%. In the next step, the influence of aeration rate and stirrer speed on production of finely dispersed mycelium in a stirred tank reactor was investigated. The best fermentation results were obtained at 0.4 vvm and 400 rpm stirrer speed with 90% mycelium <500 μm and 5 g/L biomass. Then, mycelium was microencapsulated in hollow beads based on sulfoethylcellulose (SEC). Experiments on the capsule nutrient reservoir showed that 15% (w/w) corn gluten and 0.5% (w/w) yeast extract could be replaced with 3% (w/w) autoclaved baker's yeast which was never used as capsule additive before. Radial growth of mycelium out of dried hollow beads containing 1% (w/w) biomass and 3% (w/w) baker's yeast was faster than for alginate beads containing equivalent amounts of biomass and yeast indicating a higher bio-control potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past, many microorganisms have been isolated and investigated for use as biological control agents or bio-fertilisers. Now, many promising strains are available for release into the environment and await further exploitation for large-scale application in agriculture. However, in practice, fermentation and formulation of the microorganisms are often limiting and deciding factors about the success of the bio-control measure (Burges 1998). This is particularly true for the use of nematophagous fungi to control plant-parasitic nematodes.
The endoparasitic nematophagous fungus Hirsutella rhossiliensis is an ubiquitous antagonist of many commercially important plant-parasitic nematodes (Sturhan and Schneider 1980; Velvis and Kamp 1995; Tedford et al. 1992; Cayrol et al. 1986).
However, no commercial product with this fungus is available yet, mainly because effective methods for fermentation, storage and delivery of fungal mycelium are missing. Conidia of the fungus cannot be used as they lose their infectivity when displaced from the mycelium (McInnis and Jaffee 1989).
A few reports on growth requirements and shake flask culture of Hirsutella indicated that H. rhossiliensis grows best on complex media (Lackey et al. 1992; MacLeod 1959a, b; Liu and Chen 2002). However, fermentation of the fungus has not been reported yet.
H. rhossiliensis is a weak saprophyte (Jaffee and Zehr 1985). To support fungal growth in soil, encapsulation of fungal mycelium in alginate beads was tested. Lackey et al. (1993) used alginate beads containing 25% (w/w) biomass but no nutrients. This high amount of fungal biomass is considered too costly for large-scale application. Therefore, a novel type of hollow beads based on sulfoethylcellulose (SEC) containing only 0.1% (w/w) biomass as well as corn gluten and yeast extract as a nutrient source was developed (Patel 1998; Rose et al. 2000; Patel et al. 2001). The hollow bead formation is analogous to that of hollow beads based on cellulosesulfate (Dautzenberg et al. 1985).
In first experiments it was shown that 1.5% (w/w) corn gluten and 0.05% (w/w) yeast extract resulted in up to 50% parasitism of Heterodera schachtii juveniles (Patel et al. 2002).
The objective of the present work was to suppress pellet formation of H. rhossiliensis in shake flask culture, optimise the fermentation process of fungal mycelium, and develop a novel capsule system with optimised nutrient content.
Materials and methods
All materials used were purchased from Merck, Darmstadt, if not mentioned otherwise. All concentrations are given as percent (w/w).
Strain
H. rhossiliensis isolate BBA was provided by the Julius Kühn-Institut (JKI), Institute for Epidemiology and Pathogen Diagnostics, Münster (formerly: Federal Biological Research Institute for Agriculture and Forestry, Institute for Nematology and Vertebrate Research). The strain was raised at 20 °C on GNA agar containing 2% glucose, 1.5% yeast extract, 1.5% agar-agar and 0.0025% penicillin-streptomycin at pH 6.0. Temperature optimum was found at 20 °C and pH optimum at 5–7 (data not shown).
Suppression of pellet formation in shake flask culture
Investigations of growth requirements were aimed at substitution of the yeast extract with other compounds and conducted according to MacLeod 1959a, b. As these experiments did not result in a change of medium composition (data not shown), the original shake flask medium was left unchanged and comprised 2% glucose, 0.5% yeast extract, 0.4% MES-NaOH buffer, 0.1% K2HPO4 and 0.0025% penicillin-streptomycin at pH 6.0 adjusted with 1 M HCl. The buffer was added to counter pH decrease down to pH 3.
As a starter inoculum mycelium stored at 5 ° C or −20 ° C or fresh biomass from GNA plates (see above) was used. For 20 shake flask cultures, 10 g biomass was suspended in 250 ml liquid medium and stirred for 30 min at 600 rpm. When fresh fungal mycelium from GNA plates was used, mycelium from two well-grown plates was scraped together and transferred into 250 ml liquid medium. Then either suspension was passed through a 500 μm sieve, respectively. In order to investigate the influence of baffles on pellet formation, 10 ml of the resulting filtrate were transferred into 1,000 ml shake flasks with three and without baffles containing 500 ml liquid medium.
The flasks were incubated at 20 °C and 80 rpm for 4 days and then at 120 rpm for another 4 days. Every day, 5 ml samples were taken to check for contamination under 1,000× magnification and to assay glucose concentration with HPLC or a modified blood sugar test. Fungal biomass for the encapsulation and bioreactor experiments was raised in shake flask culture with three baffles. After 8 days, fungal mycelium was harvested on a Buechner funnel, washed twice with sterile tap water and weighed. Then the particle size distribution was measured see below.
Optimisation of fermentation
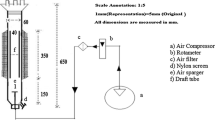
Batch fermentation was carried out in a 5 L Labfors stirred tank reactor (Infors, Bottmingen, Switzerland). For inoculation, the shake flask culture was used but without MES-NaOH buffer and antibiotics. In addition, glucose was lowered to 1% because the fermentation broth became highly viscous in later fermentation stages, which would have hindered complete consumption of 2% (w/w) glucose. Also, a few drops of the anti-foam agent Struktol J673 (Schill und Seilacher, Hamburg, Germany) were added before fermentation start. Fermentation was started by inoculating 500 ml of a 6–8-day old shake flask culture containing 4–5 g fungal dry biomass. Temperature was maintained at 20 °C and fermentation time was 50 h.
Analysis
During fermentation, glucose was routinely monitored with a rapid blood sugar test (Accutrend alpha, Boehringer, Mannheim, Germany). However, at selected sampling times the exact concentration was determined with a Shimadzu HPLC system consisting of a CTO-10A column oven at 80 ° C, a CHO-611 column (300 × 6.5 mm ID, sodium polystyrolvinylbenzene, a RID-10A refractive index detector and a SCL-10A system controller. As eluent a solution of 1 mM NaOH and 1 mM Na2-EDTA was used with a flow rate of 0.5 ml/min. 1.5 ml samples were centrifuged for 3 min at 20,800 ×g, diluted 1:10 with eluent, and centrifuged again. Glass vials containing 900 μl of the supernatant were placed in an autosampler directly or frozen at −20 °C to be used later.
For the determination of fungal wet and dry biomass paper filters (black band, Schleicher und Schuell, Düren, Germany) were pre-dried at 105 °C and stored over silica gel before weighing. Biomass was then placed on the filters and dried at 105 °C overnight and allowed to cool over silica gel before being re-weighed.
Particle size distribution analysis of fungal pellets was carried out with analytical sieves ranging from 1.000 to 100 μm. Suspensions with fungal pellets were diluted 1:5 with de-ionised water and poured over the stacked sieves. Then, all sieves containing fungal pellets were dried at 105 °C overnight before the dry weight of fungal pellets was determined.
Encapsulation
Preparation of microcapsules
The preparation of microcapsules (i.e. hollow beads) is based on a polyelectrolyte−polyelectrolyte complex where a semi-permeable membrane is formed by the polyanion sulfoethylcellulose (SEC, Wolff Walsrode, Bomlitz, Germany) and the polydiallyldimethylammoniumchloride (PDADMAC, Stockhausen GmbH & Co. KG, Krefeld, Germany).
In all experiments, SEC and PDADMAC were used in a final concentration of 2% (w/w). The pH of all solutions was adjusted to 6.0 before autoclaving. All other components were autoclaved separately and then added to the SEC solution to give the intended concentration in % (w/w). These components were: 15% corn gluten (Concentra 13883, Cerestar, Krefeld, Germany), 0.5% yeast extract (Ohly, Hamburg, Germany), and 1 or 3% baker's yeast (Deutsche Hefewerke GmbH&Co, Hamburg, Germany). After thorough mixing, fungal biomass harvested from shake flask culture was added so that the final encapsulation suspension contained either 1 or 15% fungal biomass.
Total mass of encapsulation suspension was 100 g which was pressed through a 500-μm sieve. Ten millilitres of the filtrate was then dripped with a syringe into 150 ml of a 2% PDADMAC solution. The resulting hollow beads had a diameter of ca. 3 mm and were left to stir in the PDADMAC solution for 20 min. As low-molecular-weight components diffuse through the semi-permeable capsule membrane, capsules were transferred into 200 ml washing solution containing the nutrients of the encapsulation liquid in the same concentration to ensure that the capsules really contained all substances in the concentrations given. Only hollow beads made from 1% biomass and 1% baker's yeast were washed either in 1% baker's yeast or in sterile tap water for 2 h, respectively.
Selected microcapsules were dried at 25 mbar over silica gel. In an accelerated storage test, dried microcapsules were stored at 50 °C over silica gel for 3 days.
To observe reswelling of the microcapsules, capsules were placed on water agar containing 1.7% agar adjusted at pH 6. Vitality of fungus was measured as radial growth of mycelium out of the capsules.
Preparation of calcium alginate beads
In order to compare the performance of the microcapsules with conventional alginate beads, a mix of 2% sodium alginate (Protanal LF20/60, Pronova Biopolymer, Dammen, Norway), 3% baker's yeast and 1% biomass was dripped into 150 ml 1% CaCl2 solution and cross-linked for 20 min. Beads were dried as mentioned above.
Results
Suppression of pellet formation in shake flask culture
In liquid cultures which were not shaken or shaken at low speed, pellets larger than 1.7 mm would turn hollow inside indicating massive cell death and lysis of the mycelium resulting in a fungal cell layer of 0.5 to 0.8 mm thickness (Fig. 1a, b).
It was observed that three baffles in the Erlenmeyer flask had a beneficial effect on pellet formation (Fig. 1c). No sporulation occurred in the liquid medium. After cultivation, particle size distribution was analysed. For flasks without baffles, less than 40% mycelium was retained on sieves below 500 μm, whereas for flasks with baffles, 95% of the mycelium was less than 500 μm. Total biomass yield in flasks with baffles was 4.1 g/l dry biomass (about 50 g/l wet biomass). Glucose concentration in flasks with baffles was approx. 5 g/l at the time when the mycelium was harvested (Fig. 2). The biomass yield in flasks without baffles was comparable but the fungus forms pellets during cultivation (data not shown).
Optimisation of fermentation
The filamentous fungus H. rhossiliensis isolate BBA was raised in a bioreactor. Two different fermentation strategies were investigated to produce high amounts of finely dispersed mycelium. In the first strategy the influence of six different aeration rates on the production of finely dispersed mycelium was tested while the stirrer speed was kept at 100 rpm (Fig. 3). The fermentation parameters and results are given in Table 1 and Fig. 4.
During 50 h fermentation time, 16 g/L wet biomass, respectively 3.2 g/L dry biomass, were produced. Max. specific growth rate μmax was 0.06 h−1. Only 15 h after reaching the max. specific growth rate the max. glucose consumption rate was reached. This is probably due to the consumption of compounds of the yeast extract before consumption of glucose started.
Average doubling time was 15.9 h. After 26 h, doubling time decreased to 11.5 h. The yield coefficient which was calculated by dividing dry biomass produced by glucose consumed was 0.44. Consumption of yeast extract could not be measured. Most important to note is that the aeration rate has a major impact on the production of finely dispersed mycelium as depicted in Fig. 3. Only about 10% of the mycelium was < 500 μm when the aeration rate was lower than 0.4 vvm. The best aeration rate to produce finely dispersed mycelium (e.g. 80%; <500 μm) was 1.6 vvm (Fig. 3). At an aeration rate of 2 vvm no mycelium was formed.
As a second strategy to increase the amount of finely dispersed mycelium the influence of stirrer speed was investigated at a conventional aeration rate of 0.4 vvm (Fig. 5). As a result the highest amount of finely dispersed mycelium was produced with a stirrer speed of 400 rpm. The fermentation parameters and results are given in Fig. 5 and Table 2.
The average doubling time at 100 rpm was 20.0 h, 3 h longer than at 400 rpm. Also, the min. doubling time in the exponential growth phase was 12.1 h, 1.6 h longer than at the higher stirrer speed. The slower growth resulted in a lower yield of 3.7 g/L. In contrast, 5.0 g/L dry biomass was obtained at 400 rpm (Fig. 6). As mentioned before, the yield coefficient in these fermentations is based on glucose only, as yeast extract compounds were not measured.
Optimisation of capsule compounds
In the first encapsulation experiments, 15% corn gluten and 0.5% yeast extract were found to serve as nutrients for H. rhossiliensis isolate BBA which would help to establish this weak saprophyte in the soil (Patel et al. 2002). However, nutrient components themselves or their metabolites showed some activity against infective juveniles of Heterodera schachtii (Gutberlet personal communication). In addition, low molecular weight components such as present in yeast extract are not suitable for encapsulation on a technical scale, as they are lost during the encapsulation process due to diffusion through the semi-permeable membrane of the microcapsules into the cross-linking solution. Therefore, whole yeast cells with a higher molecular weight were investigated for their effect on fungal growth. As shown in Fig. 7 there was no difference in the radial mycelium growth of H. rhossiliensis isolate BBA for the different combinations of fungal biomass, corn gluten, yeast extract and baker's yeast except for 15% biomass alone which resulted in the lowest fungal growth. However, it was observed that mycelium density was higher in formulations containing corn gluten and yeast extract. Even when washed in tap water for 2 h, microcapsules with baker's yeast showed fungal growth as good as capsules which were not watered (Fig. 5).
Drying
When microcapsules containing 1% biomass and 3% baker's yeast were dried down to residual moisture of 5% and then placed on water agar, a swelling of the microcapsules back to the original size could be observed within 2 days and growth of fungal mycelium could be observed as shown in Fig. 8. In comparison with calcium alginate beads (Fig. 9), the microcapsules showed better growth. Storage of the dried capsules (without drying additives) in an accelerated storage test at 50 °C for 3 days did not affect viability (data not shown).
Discussion
This study deals with the fermentation and formulation aspects of H. rhossiliensis and might prepare the way for further commercialisation of this nematophagous fungus.
-
➢ Suppression of pellet formation in shake flask culture
Previous experiments where the glucose-yeast extract medium or components thereof could be replaced by more economic media such as corn meal, corn steep liquor or potato meal led to a growth of mycelium but the yield could not be quantified because of the viscous and particulate nature of the cultivation broth (data not shown). Besides, particulate matter will clog the nozzles of the encapsulation equipment and must therefore be avoided. As a conclusion, a medium based on glucose and yeast extract shaken in a 1 L Erlenmeyer flask with three baffles is currently the most suitable method of producing finely dispersed mycelium for fermentation or encapsulation on lab scale, respectively. These results are in accordance with findings of others authors on influence of rheological conditions on fungal morphology (Paul et al. 1999; Riley et al. 2000).
The medium is also the most cost-effective medium studied so far for fermentation of the fungus when encapsulation is envisaged as a formulation method. Recently, more data on nutritional requirements of six H. rhossiliensis strains were published (Liu and Chen 2002). In that study, glycogen proved to be the best carbon source with regard to growth on agar but no data on biomass produced in liquid culture were given.
-
➢ Fermentation
Here, we reported a fermentation of H. rhossiliensis in liquid culture. As compounds of the yeast extract in the medium were not measured, the yield coefficients are based on the consumption of glucose only. The data indicate that yeast extract is consumed by the fungus and therefore, the yield coefficient should be lower.
The growth of the filamentous fungus generally follows a Monod kinetic as it is observed with bacteria. When fermentation data were used to calculate biomass produced it was found that theoretical biomass produced was 3.9 g/L while the “actual” yield was 3.2 g/L. This difference indicates the limitations of the simple Monod model for the filamentous fungus. The most important finding regarding fermentation is the suppression of pellet formation by using finely dispersed mycelium as inoculum.
To sum up the fermentation results of the two fermentation strategies, we recommend inoculating finely dispersed mycelium of H. rhossiliensis strain BBA and growing the mycelium at 400 rpm and 0.4 vvm to obtain 5.0 g/l dry biomass as finely dispersed mycelium after 50 h.
If during technology transfer and further scale up the problem of pellet formation rises again, other measures could be changing constructive characteristics of the bioreactor, such as geometry and stirrer type. Other measures could be an increased energy input, i.e., increased stirrer speed or aeration, or chemical additives (Qadeer Choudhary and Pirt 1965; Irvine 1990; Kleespies and Zimmermann 1998).
-
➢ Yeast cells as nutrient depot
The authors feel that the most important step towards commercialisation is the encapsulation of the weak saprophyte in a capsule with a nutrient reservoir. We argue that the key to successful bio-control of nematodes is the establishment of the fungus in the soil. Once it is established in the soil, it is probably going to kill nematode larvae as discussed in Jaffee (2000). It was shown in previous works that encapsulation can protect sensitive cells (Patel and Vorlop 1994; Patel et al. 2000), such as H. rhossiliensis. Furthermore, it was shown that the addition of 15% corn gluten and 0.5% yeast extract to the capsule allows fungal growth within the capsule and outside of the capsule (Patel et al. 2002).
It is often argued that encapsulation adds additional costs to the production process, thus rendering the whole process uneconomical. However, an appropriate formulation can also reduce overall costs (see also Burges 1998). The production of biomass is a major cost factor, and any approach reducing total biomass, such as a proper formulation, will finally also reduce overall costs. In our own experiments the fungal biomass within the capsules could be reduced from 15% to 0.1% without losing activity by the addition of nutrients (Patel et al. 2002). Further advantages of fungal encapsulation are the easy handling of the formulation, application with standard machinery, protection of the fungus from adverse environmental conditions such as temperature, drought and antagonists, controlled release into the environment (controlled by environmental conditions and material properties), extended shelf life and enhanced efficacy.
Although corn gluten and yeast extract did allow extensive fungal growth from the capsule, those additives showed adverse effects on plant growth (Patel et al. 2002) so that as an alternative a new capsule additive was tested: autoclaved baker's yeast. This additive supported fungal growth and resulted in a high efficacy in pathogenicity assays carried out at the Julius Kühn-Institute, Institute for Epidemiology and Pathogen Diagnostics at Muenster (Gutberlet 2000). Regarding sporulation, it was observed that H. rhossiliensis continuously forms phialides and spores. Attempts to quantify sporulation as a function of C/N ratio of nutrients did not result in significant differences as opposed to Liu and Chen (2002) who observed that certain nutrients would allow an increase in sporulation and/or germination with other H. rhossiliensis strains. The authors tend to agree with Jaffee's hypothesis “When it grows, it sporulates.” (Jaffee, personal communication). It is one of the fungus' advantages that it constitutively forms spores while other fungi need certain triggers to form traps or spores to kill nematodes and will otherwise not be harmful to nematodes (Sturhan and Schneider 1980; Hayes and Blackburn 1966a, b). We therefore recommend focusing on mycelium formation in soil (“the more mycelium, the more spores.”)
-
➢ Growth from dried hollow beads in comparison to alginate beads
It was previously known that encapsulated mycelium can survive drying and storage for 1 year at 5 °C without loss of viability and efficacy (Lackey et al. 1993). Therefore, the authors do not consider shelf life a major hindrance for commercialisation of the microcapsule formulation as is so often a problem with biological control agents (Burges 1998). When the formulation was stored in an accelerated storage test for 3 days at 50 °C, no loss of viability was observed (data not shown). A high thermostability usually indicates good storage ability of dried cells for longer periods (Milic and Otenhajmer 1974).
Interestingly, dried microcapsules exhibited a slightly better growth of mycelium than dried alginate beads, which is probably due to better water-retention properties of the polyelectrolyte-polyelectrolyte-complex making up the microcapsule membrane (Philipp et al. 1980). This may also be the reason why the hollow beads showed better bio-control ability in comparison with calcium alginate beads (Patel et al. 2002, Müller unpublished). The authors wish to point out that there is great need for research on alternatives to alginate as controlled release systems.
In conclusion, this report on fermentation and microencapsulation of the nematophagous fungus H. rhossiliensis in a novel type of hollow beads improved growth compared to alginate beads. Therefore, we consider microencapsulation of H. rhossiliensis a promising step towards commercialisation.
References
Burges HD (1998) Formulation of microbial pesticides. Kluwer, Dordrecht
Cayrol JC, Castet R, Samson RA (1986) Comparative activity of different Hirsutella species towards three plant parasitic nematodes. Revue Nématologique 12:331–336
Dautzenberg H, Loth F, Fechner K, Mehlis B, Pommerening K (1985) Preparation and performance of symplex capsules. Makromol Chem 9:203–210
Gutberlet V (2000) Dissertation Rheinische Friedrich-Wilhelms-Universität Bonn.
Hayes WA, Blackburn F (1966a) Studies on the nutrition of arthrobotrys oligospora Fes. and A. robusta Dudd. I. The saprophytic phase. Ann Appl Biol 58:43–50
Hayes WA, Blackburn F (1966b) Studies on the nutrition of arthrobotrys oligospora Fes. and A. robusta Dudd. II. The predaceous phase. Ann Appl Biol 58:51–60
Irvine TS (1990) Laboratory fermenters. In: McNeil B, Harvey LM (eds) Fermentation. Oxford University Press, Oxford
Jaffee BA (2000) Augmentation of soil with the nematophagous fungi Hirsutella rhossiliensis and Arthrobotrys haptotyla. Phytopathology 90:498–504
Jaffee BA, Zehr EI (1985) Parasitic and saprophytic abilities of the nematode-attacking fungus Hirsutella rhossiliensis. J Nematol 17:341–345
Kleespies RG, Zimmermann G (1998) Effect of additives on the production, viability and virulence of blastospores of Metarrhizium anisopliae. Biocontrol Sci Technol 8(2):207–214
Lackey BA, Jaffee BA, Muldoon AE (1992) Sporulation of the nematophagous fungus Hirsutella rhossiliensis from hyphae produced in vitro and added to soil. Phytopathology 82:1326–1330
Lackey BA, Muldoon AE, Jaffee BA (1993) Alginate pellet formulation of Hirsutella rhossiliensis for biological control of plant-parasitic nematodes. Biol Control 3:155–160
Liu XZ, Chen SY (2002) Nutritional requirements of the nematophagous fungus Hirsutella rhossiliensis. Biocontrol Sci Technol 12:381–393
MacLeod DM (1959a) Nutritional studies on the genus Hirsutella, I. Growth response in an enriched liquid medium. Can J Bot 37:695–714
MacLeod DM (1959b) Nutritional studies on the genus Hirsutella, II. Nitrogen utilization in a synthetic medium. Can J Bot 37:819–834
McInnis TM, Jaffee BA (1989) An assay for Hirsutella rhossiliensis spores and the importance of phialides for nematode inoculation. J Nematol 21:229–234
Milic S, Otenhajmer I (1974) Predicting the stability of freeze-dried suspensions of Lactobacillus acidophilus by the accelerated storage test. Cryobiology 11:116–120
Patel AV (1998) Verkapselungsverfahren für die biologische Schädlingsbekämpfung und zur Konstruktion von “vegetativen Samen”. Landbauforschung Völkenrode (Sonderheft) 188
Patel AV, Vorlop KD (1994) Entrapment of biological control agents applied to entomopathogenic nematodes. Biotechnol Tech 8(8):569–574
Patel AV, Pusch I, Mix-Wagner G, Vorlop KD (2000) A novel encapsulation technique for the construction of artificial seeds. Plant Cell Rep 19(9):868–874
Patel AV, Rose T, Vorlop KD (2001). Controlled release of Hirsutella rhossiliensis from hollow beads for biological control of phytopathogenic nematodes. In: Janssen FJJG (eds) COST 830—Microbial Inoculants for Agriculture and Environment Workgroup Meeting “Formulation of Microbial Inoculants” in Braunschweig, 05/02–06/02/1999, ISBN 92-894-0226-1, 50-52
Patel AV, Rose T, Vorlop KD (2002) Encapsulation of Hirsutella rhossiliensis in hollow beads based on sulfoethylcellulose to control plant-parasitic nematodes. Landbauforschung Völkenrode SH 241:145–150
Paul GC, Priede MA, Thomas CR (1999) Relationship between morphology and citric acid production in submerged Aspergillus niger fermentations. Biochem Eng J 3:121–129
Philipp B, Hong LT, Linow KJ, Dawydoff W, Arnold K (1980) Über Symplexe von Cellulosederivaten. 8. Mitt. Untersuchungen zur Wasserquellung unterschiedlicher Symplexfällungen. Acta Polym 31(10):654–658
Qadeer Choudhary A, Pirt J (1965) Metal-complexing agents as metal buffers in media for the growth of Aspergillus niger. J Gen Microbiol 41:99–107
Riley GL, Tucker KG, Paul GC, Thomas CR (2000) Effect of biomass concentration and mycelial morphology on fermentation broth rheology. Biotechnol Bioeng 68(2):160–172
Rose T, Neumann B, Thielking H, Koch W, Vorlop KD (2000) Hollow beads of sulfoethyl cellulose (SEC) on the basis of polyelectrolyte complexes. Chem Eng Technol 23(9):769–772
Sturhan D, Schneider R (1980) Hirsutella heteroderae, ein neuer nematodenparasitärer Pilz. Phytopathologische Zeitschrift 99:105–115
Tedford EC, Jaffee BA, Muldoon AE (1992) Effect of soil moisture on transmission of the nematophagous fungus Hirsutella rhossiliensis to cyst and root-knot nematodes. Phytopathology 82(10):1002–1007
Velvis H, Kamp P (1995) Infection of second stage juveniles of potato cyst nematodes by the nematophagous fungus Hirsutella rhossiliensis in Dutch potato fields. Nematologica 41:617–627
Acknowledgements
This research was funded by the Agency for Renewable Resources (FNR) of the German Ministry of Nutrition, Agriculture and Forestry (FKZ 98 NR 067) and by the “Gesellschaft der Freunde der FAL (GdF)” with a grant for junior scientists to Dr. A. Patel.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, A.V., Jakobs-Schönwandt, D., Rose, T. et al. Fermentation and microencapsulation of the nematophagous fungus Hirsutella rhossiliensis in a novel type of hollow beads. Appl Microbiol Biotechnol 89, 1751–1760 (2011). https://doi.org/10.1007/s00253-010-3046-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-3046-9