Abstract
Various adjuvants and delivery systems have been evaluated for increasing the protective immune responses against leishmaniasis and mostly have been shown not to be effective enough. In this study, poly(d,l-lactide-co-glycolide) (PLGA) nanospheres as an antigen delivery system and CpG-ODN as an immunoadjuvant have been used for the first time to enhance the immune response against autoclaved Leishmania major (ALM). PLGA nanospheres were prepared by a double-emulsion (W/O/W) technique. Particulate characteristics were studied by scanning electron microscopy and particle size analysis. Mean diameter of ALM + CpG-ODN-loaded nanospheres was 300 ± 128 nm. BALB/c mice were immunized three times in 3-week intervals using ALM plus CpG-ODN-loaded nanospheres [(ALM + CpG-ODN)PLGA], ALM encapsulated PLGA nanospheres [(ALM)PLGA], (ALM)PLGA + CpG, ALM + CpG, ALM alone, or phosphate buffer solution (PBS). The intensity of infection induced by L. major challenge was assessed by measuring size of footpad swelling. The strongest protection, showed by significantly (P < 0.05) smaller footpad, was observed in mice immunized with (ALM + CpG-ODN)PLGA. The (ALM)PLGA, (ALM)PLGA + CpG, and ALM + CpG were also showed a significantly (P < 0.05) smaller footpad swelling compared to the groups received either PBS or ALM alone. The mice immunized with (ALM + CpG-ODN)PLGA, (ALM)PLGA + CpG, and ALM + CpG showed the highest IgG2a/IgG1 ratio, interferon-γ production, and lowest interleukin-4 production compared to the other groups. It is concluded that when both PLGA nanospheres and CpG-ODN adjuvants were used simultaneously, it induce stronger immune response and enhance protection rate against Leishmania infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Leishmaniasis with an annual incidence of about two million is a major health problem in some endemic countries (World Health Organization 2004 http://www.who.int/tdr/dw/leish2004.htm). The available drugs against leishmaniasis are expensive, difficult to administer, and with low efficacy, and emerging drug resistance is reported. Long lasting protection and strong immune response are induced upon recovery of cutaneous leishmaniasis lesion caused by either natural infection or leishmanization; it seems that development of an effective vaccine against leishmaniasis is achievable (Modabber 1995; Mauel 2002; Khalil et al. 2000; Khamesipour et al. 2005, 2006). Th1 type of immune response plays a major role in recovery and protection at least in animal model of leishmaniasis (Sacks and Noben-Trauth 2002; Reiner and Locksley 1995; Kedzierski et al. 2006). Recently, several immunoadjuvants like BCG, G-CSF (Follador et al. 2002; Satti et al. 2001), and CpG-ODN (Flynn et al. 2005; Iborra et al. 2005; Wu et al. 2006; Tewary et al. 2004; Jaafari et al. 2007) and various delivery systems like PLGA microspheres (Coelho et al. 2006) and liposomes (Shimizu et al. 2007; Bhowmick et al. 2007; Badiee et al. 2007; Jaafari et al. 2006) have been used to potentiate the immune response against Leishmania antigens in animal models.
Autoclaved Leishmania major (ALM) was used in humans in phase 3 trials with a limited efficacy (Noazin et al. 2008; Khalil et al. 2000; Armijos et al. 2004; Bahar et al. 1996; Khamesipour et al. 2006). Mycobacterium bovis Bacillus Calmette–Guérin (BCG) has been used as an immunoadjuvant in field efficacy trials of candidate vaccines against leishmaniasis (Armijos et al. 2004; De Luca et al. 1999; Mohebali et al. 2004; Bahar et al. 1996; Cabrera et al. 2000; Kamil et al. 2003; Misra et al. 2001). It has been shown in humans that the killed Leishmania vaccines mixed with BCG significantly increase the frequency and magnitude of LST response (Alimohammadian et al. 2002). The results of phase 3 clinical trials in humans showed that ALM mixed with BCG induce a Th1 type of immune response but not strong enough to protect against infection (Khamesipour et al. 2006; Bahar et al. 1996).
Biodegradable poly(d,l-lactic-co-glycolic acid) (PLGA) nanospheres and microspheres are promising delivery systems for protein, peptide, and DNA vaccines (Diwan et al. 2002; Johansen et al. 2000). Nano- and microparticles enhance the immune responses against encapsulated antigen by various mechanisms. They could impart particulate nature to soluble antigens and increase their interaction with antigen presenting cells (APCs) and macrophages (Diwan et al. 2002). They can deliver peptide antigens to APCs (Newman et al. 2002) to generate Th1 type immune response, even against poor immunogens (Newman et al. 1998; Venkataprasad et al. 1999). They have been used to co-encapsulate the antigen(s) and used an adjuvant to deliver the antigen and stimulatory adjuvant to the same APC and resulted in induction of stronger immune response compared to the free antigen and adjuvant (Diwan et al. 2002).
Oligodeoxynucleotides (ODN) containing unmethylated CpG motifs act as an immune adjuvant and induce strong humoral and cellular immune responses with a bias towards a Th1 response (McCluskie and Davis 1998; Gupta and Siber 1995; Krieg 1999). Leishmania antigens when used with CpG-ODN, as an adjuvant, induced a long-term protection in animal model (Mendez et al. 2003; Rhee et al. 2002; Verthelyi et al. 2002; Jaafari et al. 2007).
The immunogenicity of an antigen and the potency of an adjuvant have been substantially enhanced by co-delivery in biodegradable microspheres (Diwan et al. 2002). Therefore, in this study, the potential of PLGA nanospheres encapsulated with ALM and CpG-ODN adjuvant in the induction of immune response and protection rate against leishmaniasis have been studied.
Materials and methods
Materials, animals, parasite, and SLA
PLGA 50:50 co-polymer (MW 30000) was purchased from Boehringer Ingelheim (Germany). CpG oligodeoxynucleotide [number 1826, seq (5′–3′): tccatgacgttcctgacgtt] with a nuclease-resistant phosphorothioate backbone was purchased from Microsynth (Switzerland). ALM was produced at Razi Vaccine and Serum Research Institute, Hesarak, Iran and used in clinical trials (Alimohammadian et al. 2002; Bahar et al. 1996; Kamil et al. 2003; Khamesipour et al. 2006; Mohebali et al. 2004).
Female BALB/c mice 6–8 weeks old were purchased from Pasteur Institute (Tehran, Iran). The mice were maintained in animal house of Biotechnology Research Center and fed with tap water and standard laboratory diet (Khorassan Javane Co, Mashhad, Iran). Animals were housed in a colony room 12/12-h light/dark cycle at 21°C and had free access to water and food. Animal experiments were carried out according to Mashhad University of Medical Sciences, Ethical Committee Acts.
The L. major strain (MRHO/IR/75/ER) used in this experiment is the same strain which has been used for preparation of experimental Leishmania vaccine and leishmanization (Alimohammadian et al. 2002; Bahar et al. 1996; Kamil et al. 2003; Khamesipour et al. 2006; Mohebali et al. 2004; Noazin et al. 2008; Javadian et al. 1976).
Soluble Leishmania antigen (SLA) was prepared from promastigotes of L. major harvested at log phase (Scott et al. 1987); the protein concentration of SLA was determined using Lowry protein assay and stored in small aliquots at −70°C until use.
Preparation and characterization of PLGA nanosphere encapsulated with ALM and CpG-ODN
Nanospheres were prepared using a W/O/W emulsion and solvent evaporation technique (Tafaghodi et al. 2004). Briefly, CpG-ODN (40 μl, 10 μg/μl) and ALM (110 μl, 70 μg/μl) solutions were mixed and emulsified with PLGA solution (600 μl, 33% w/v) in dichloromethane for 40 s using a microtip probe sonicator (MSE, England) in amplitude of 18. Ice-water bath was used for prevention of temperature rise during sonication process. The W/O emulsion was combined with PVA (polyvinylalcohol) solution (8 ml, 7.5% w/v) and then sonicated (80 s) to form the W/O/W emulsion. The secondary emulsion was then added to PVA solution. The emulsion was further stirred for 2 h. Nanospheres were collected by centrifugation (20,000 g, 15 min, 4°C) and washed twice using distilled water, and then the preparations were lyophilized.
Scanning electron microscope (LEO, Germany) was used to check the morphology of nanospheres. Particle size and size distribution of the nanospheres were determined using a laser diffraction size analyzer (Shimadzu, Japan). The amount of encapsulated ALM was determined using Lowry protein assay method (Waterborg 2002), and amount of CpG-ODN was estimated based on absorbance at 260 nm (Barman et al. 2000). Because of interference of protein in 260 nm, PLGA nanospheres encapsulated only with CpG-ODN were prepared, and loading of CpG-ODN in these nanospheres were extrapolated to nanospheres both encapsulated with CpG-ODN and protein.
Immunization of BALB/c mice
Different groups of mice, ten mice per group, were subcutaneously (SC) immunized three times at 3-week intervals with one of the following formulations: (1) (ALM + CpG-ODN)PLGA (180 μg ALM + 10 μg CpG-ODN/10 mg nanosphere/100 μl phosphate buffer solution (PBS)/mouse), (2) (ALM)PLGA + CpG-ODN (180 μg ALM/10 mg nanosphere + 10 μg CpG-ODN/100 μl PBS/mouse), (3) (ALM)PLGA (180 μg ALM/10 mg nanosphere/100 μl PBS/mouse), (4) ALM + CpG-ODN (180 μg ALM + 10 μg CpG-ODN/100 μl PBS/mouse), (5) ALM (180 μg ALM/100 μl PBS/mouse), and (6) PBS (100 μl).
Challenge with L. major
The immunized mice (seven per group) were challenged SC into the left footpad with 1.5 × 106 L. major promastigotes harvested at stationary phase (in 50 μl volume), at 3 weeks after the last booster, and as a control, right footpads were injected with the same volume of PBS. Lesion development was recorded in each mouse by measurement of footpad thickness using a metric caliper (Mitutoyo Measuring Instruments, Japan). Grading of lesion size was carried out by subtracting the thickness of the uninfected contralateral footpad from that of the infected one (Jaafari et al. 2006, 2007).
Antibody isotype assay
Blood samples were collected from the mice before and at week 14 after challenge, and the sera were used to titrate anti-Leishmania IgG total, IgG1, and IgG2a antibodies using ELISA method (Zymed Laboratories Inc., San Francisco, CAS, USA) according to the manufacturer's instructions. Briefly, 96-well microtiter plates (Nunc) were coated with 50 μl of 10 μg/ml of SLA overnight at 4°C. Plates were washed and blocked with 1% bovine serum albumin in Tween 20 (PBS–Tween). Serum samples were diluted to 1:200 with PBS–Tween 20 and applied to the plates. Optical density was determined at 450 nm, using 630 nm as the reference wavelength (Badiee et al. 2007).
In vitro spleen cell response
Three mice from each group were sacrificed at week 3 after the last booster, at the same time as challenge experiment, the spleens were aseptically removed, and a single-cell suspension was prepared by homogenization of the tissue, and the erythrocytes were disrupted using ammonium chloride. The splenocytes were washed and resuspended in complete medium (RPMI 1640-FCS) and seeded at 2 × 106/ml in 96-well flat-bottom plates (Nunc). The spleen cells were stimulated in vitro with SLA (10 μg/ml) or Con A (2.5 μg/ml), or medium alone and incubated at 37°C in 5% CO2. Supernatants were collected at 72 h of culture, and the concentration of interleukin (IL)-4 and interferon (IFN)-γ was titrated using ELISA method according to the manufacturer's instructions (Badiee et al. 2007; Bender Med Systems GmbH, Vienna, Austria).
Statistical analysis
One-way ANOVA statistical test was used to assess the significance of the differences between various groups. In case of significant F value, multiple-comparison Tukey test was used to compare the means of different treatment groups; P < 0.05 was considered to be statistically significant.
Results
Characterization of PLGA nanospheres
As shown in Fig. 1, spherical nanospheres with smooth surfaces were prepared. Addition of encapsulates showed no effect on the morphology and surface roughness of nanospheres. The mean diameter of blank nanospheres or nanospheres encapsulated with ALM and CpG-ODN was 302 ± 129 and 300 ± 128, respectively. The mean encapsulation efficiencies of ALM and CpG-ODN in nanospheres were 71.6 ± 8.8% and 49.1 ± 2.4%, respectively.
The release profile of ALM and CpG-ODN from nanospheres was also evaluated (Fig. 2). The initial release of ALM was seen in the first 2 h of the study, in which 33.1 ± 0.7% of the ALM was released. This initial burst release was followed by a plateau with a mild slope. Finally, after 1 week, the cumulative percent of released ALM reached to 44.8 ± 0.8%. In the case of CpG-ODN, the same pattern of release was seen. The initial burst release in the first 2 h was 36.9 ± 2% and reached to 48.1 ± 0.1% after 1 week.
Lesion development after challenge
Lesion development was monitored by weekly measurement of footpad thickness (Fig. 3). The lesion size progressed at a more rapid rate in mice which received PBS or ALM alone than in mice immunized with ALM + CpG, (ALM)PLGA, (ALM)PLGA + CpG, and (ALM + CpG-ODN)PLGA (P < 0.05). At week 12 after challenge in mice immunized with (ALM + CpG)PLGA, lesion size was significantly (P < 0.05) smaller than all the other groups. The groups which received (ALM)PLGA, (ALM)PLGA + CpG, and ALM + CpG also showed a significantly (P < 0.05) smaller footpad swelling compared to the groups which received either PBS or ALM alone. The results also showed that there was no significant difference between mice immunized with (ALM)PLGA, (ALM)PLGA + CpG, and ALM + CpG.
Footpad swelling in BALB/c mice immunized SC, three times in 3-week intervals, with (ALM + CpG-ODN)PLGA, (ALM)PLGA + CpG-ODN, (ALM)PLGA, ALM + CpG-ODN, ALM, or PBS. At week 3 after the last booster, the immunized and control groups of mice were challenged in left footpad with 1.5 × 106 L. major promastigotes. The footpad thickness was measured on both footpads for 14 weeks. Each point represents the average increase in footpad thickness ± SEM (n = 7)
Antibody response
In order to determine the type of immune response generated in immunized mice, the anti-SLA specific IgG, IgG1, and IgG2a antibodies were titrated before (Fig. 4a) and at week 14 after L. major challenge (Fig. 4b). As shown in Fig. 4a, the significantly (P < 0.05) highest titers of IgG2a and IgG1 (before challenge) were seen in sera of mice immunized with (ALM + CpG)PLGA, followed by (ALM)PLGA + CpG group. The significantly (P < 0.001) lowest IgG1 and IgG2a titers were seen in mice immunized with ALM alone. The ratio of IgG2a/IgG1 in sera of mice immunized with ALM + CpG was significantly (P < 0.05) higher than all other groups (Fig. 4c). An intermediate IgG2a/IgG1 ratio was seen in group of mice which received (ALM)PLGA + CpG and (ALM + CpG)PLGA. Mice immunized with (ALM)PLGA showed the lowest IgG2a/IgG1 ratio.
Levels of anti-SLA-specific IgG, IgG2a, and IgG1 in sera of BALB/c mice immunized SC, three times in 3-week intervals, with (ALM + CpG-ODN)PLGA, (ALM)PLGA + CpG-ODN, (ALM)PLGA, ALM + CpG-ODN, ALM, or PBS. Blood samples were collected at week 3 after the last booster (a) and at week 14 after challenge (b). The SLA-specific IgG, IgG2a, and IgG1 levels were assessed using ELISA method. c The ratio of IgG2a/IgG1 based on absorbance. The assays were performed in triplicate at 200-fold dilution for each serum sample. Values are the mean ± SEM
L. major challenge induced elevation of IgG, IgG1, and IgG2a antibodies in all groups of mice compared with the antibody titers before challenge (Fig. 4b). The sera from mice immunized with ALM + CpG showed the significantly (P < 0.05) lowest level of IgG1 antibodies compared with the other groups. The groups immunized with nanospheric formulations showed nearly the same IgG1 titers. While before challenge the significantly (P < 0.05) lowest IgG2a titers were seen in ALM group and in (ALM)PLGA group, after challenge, these two groups showed the highest IgG1 Ab titers compared to the other groups. The significantly (P < 0.01) highest IgG2a/IgG1 ratio was seen in mice immunized with ALM alone compared to all other groups (except for ALM + CpG) followed by (ALM)PLGA (Fig. 4c).
In vitro cytokine production by splenocytes
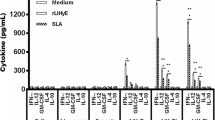
The supernatant of cultured splenocytes was analyzed to titrate the level of IFN-γ and IL-4 production. The levels of IFN-γ in the supernatant of SLA-stimulated splenocytes in groups of mice immunized with ALM + CpG, (ALM)PLGA + CpG, or (ALM + CpG)PLGA were significantly (P < 0.001) higher than the PBS, ALM alone, and (ALM)PLGA groups. The levels of IFN-γ production in these groups were as high as Con A-stimulated splenocytes. There was no significant difference in the levels of IFN-γ productin between mice immunized with ALM + CpG, (ALM)PLGA + CpG, or (ALM + CpG)PLGA.
The levels of IL-4 in the supernatant of SLA-stimulated splenocytes in groups of mice immunized with ALM + CpG, (ALM)PLGA + CpG, or (ALM + CpG)PLGA were significantly (P < 0.01) higher than the mice received either PBS or ALM alone or (ALM)PLGA. However, the levels of IL-4 production in these groups were significantly lower than Con A-stimulated splenocytes. There was no significant difference in the levels of IL-4 production between mice immunized with ALM + CpG, (ALM)PLGA + CpG, or (ALM + CpG)PLGA (Fig. 5).
Splenic T-cell response of BALB/c mice immunized SC, three times in 3-week intervals, with (ALM + CpG-ODN)PLGA, (ALM)PLGA + CpG-ODN, (ALM)PLGA, ALM + CpG-ODN, ALM, or PBS. At week 3 after the last booster, the spleens were removed, and the splenocytes were stimulated in vitro with SLA (10 μg/ml), concanavalin A (2.5 μg/ml), or with no stimulation. Production of IFN-γ (a) and IL-4 (b) was assessed using sandwich ELISA on supernatants removed at 72 h of in vitro culture. Cells from three mice per group were pooled. The bars represent the mean and SEM of triplicate wells
Discussion
Different Leishmania antigens showed to induce immune response and protection in animal models if used with IL-12 (Modabber 1995). Using first generation Leishmania vaccines in phase 3 trials showed little if any efficacy partially due to lack of an appropriate adjuvant. The efficacy of single and multiple doses of ALM mixed with BCG was checked in humans (Noazin et al. 2008; Khamesipour et al. 2006). ALM mixed with either BCG or Mycobacterium vaccae induced partial protection in C57Bl/6 and BALB/c mice (Keshavarz Valian et al. 2008).
To enhance the immunogenicity of ALM, PLGA nanospheres encapsulated with ALM alone or ALM plus CpG-ODN were used to immunize susceptible BALB/c mice. The protection rate, the extent, and the type of immune response generated were evaluated in immunized mice.
The mean diameter of particulate used for immunization is an important factor in generation of an efficient immune response. Particles smaller than 10 μm in diameter are directly taken up by macrophages and or dendritic cells through phagocytosis, whereas larger microspheres (greater than 10 μm) need to undergo biodegradation before phagocytosis occur (Sinha and Trehan 2003). Degradation, antigen release, location, and antigen presentation of particles smaller than 10 μm are different from larger ones (Sinha and Trehan 2003). At the present study, nanospheres with the mean diameter of about 300 nm were prepared. This range of particles' size is readily phagocytosed by macrophages. In vitro release studies showed that most of the encapsulated antigens and adjuvants remain entrapped in the nanospheres and are not easily released from the particles. Therefore, most of the injected antigens and adjuvants are in particulate form and, as such, stronger immune stimulation is induced, which is due to the potential of encapsulated antigens.
Resistance or susceptibility to L. major infection depends upon generation of Th1 or Th2 response, respectively. The key cytokine and antibody subtype indicative for Th1 response are IFN-γ and IgG2a, whereas IL-4 and IgG1 are key indicators for Th2 response (Sacks and Noben-Trauth 2002; Shimizu et al. 2003; Diwan et al. 2002).
Particulate antigens facilitate interaction with APCs and induce stronger immune response compared to soluble antigens (Rebelatto et al. 2001; Gupta and Siber 1995). Therefore, in this study, PLGA nanospheres were used to encapsulate soluble antigens to particulate ones. Furthermore, the PLGA microspheres and nanospheres induce a Th1 type of response (Diwan et al. 2002; Newman et al. 1998),
Progress of lesion development induced by L. major infection from week 4 after challenge was significantly (P < 0.05) slower in group of mice immunized with (ALM)PLGA than group of mice which received ALM alone, which is an indication of protection induced by nanospheric formulation. Encapsulated ALM induced higher IgG1 and lower IgG2a titers and lower IgG2a/IgG1 ratio compared with ALM alone, which is an indication that encapsulation of ALM in PLGA nanospheres skews the immune response towards Th2 response. However, the IFN-γ concentration in group of mice which received (ALM)PLGA was significantly (P < 0.001) higher than group of mice which received ALM alone, while IL-4 concentration was not significantly different in the two groups.
Previous studies on mice using PLGA nanospheres encapsulated with tetanus toxoid (TT) nanospheres efficiently induced stronger immune responses, which was even stronger than the conventional vaccine, alum absorbed TT. The IFN-γ titers induced with (TT)PLGA were eight times more than the TT solution and about two times more than the alum-TT group. However, similar to the present study, the IgG2b/IgG1 ratio in nanospheric group was lower than the TT solution (Diwan et al. 2002).
CpG-ODN as an immunomodulator adjuvant also affects the immune responses. Before challenge, ALM + CpG group induced a higher IgG1, IgG2a titers, and significantly (P < 0.0001) more IgG2a/IgG1 ratio compared to the group which received ALM alone. The antibody titers indicate that CpG-ODN skews the immune response towards Th1 type of response, but after challenge, the reverse order was observed. ALM + CpG induced far higher IFN-γ (ten times higher) and IL-4 (four times higher) than ALM group (P < 0.0001). This is an indication that immunization of mice with ALM + CpG induced more Th1 response.
In another study, immunization with ALM plus CpG-ODN markedly reduced lesion development in both susceptible BALB/c and resistant C57BL/6 mice, up to 12 weeks after immunization, and protection lasted for at least 6 months in immunized C57BL/6 mice (Rhee et al. 2002). BALB/c mice immunized with L. major ribosomal proteins (LRP) mixed with CpG-ODN were protected against challenge with L. major, and no dermal lesion was developed. The immunized group showed significantly lower parasite load; the protection induced in immunized mice was accompanied with induction of an IL-12-dependent IFN-γ, marked lower IgG1 antibody titer, and impaired IL-4 and IL-10 cytokine production (Iborra et al. 2008). It was previously shown that when CpG-ODN was co-administered with two other Leishmania antigens, recombinant major surface glycoprotein of Leishmania (rgp63) and recombinant L. major stress-inducible protein 1 (rLmSTI1), in liposomes, it protected the BALB/c mice against experimental leishmaniasis (Jaafari et al. 2007; Badiee et al. 2007).
CpG-ODN when co-administered with TT solution induced a higher IFN-γ titer (five times) and a higher IgG2b/IgG1 ratio in the group of mice which received TT + CpG than the group which received TT solution alone (Diwan et al. 2002). So, comparable with the present study, CpG-ODN induced the immune response more toward a Th1 type than Th2.
To improve the adjuvantcity, CpG-ODN was either linked directly to the antigen or CpG-ODN was co-encapsulated with antigens in PLGA nanospheres. These findings suggest that optimal immune stimulation occurs when antigen and adjuvant are presented to the immune system in a close spatial and temporal proximity (Jaafari et al. 2007). Moreover, improvement of adjuvant effect of CpG-ODN, which was seen when CpG-ODN was encapsulated in nanospheres, might be due to the fact that TLR9, the receptor for CpG motifs (Hemmi et al. 2000), localizes to endosomal/vacuolar/vesicular compartments but not to the cell surface (Krieg 2002). Internalization of CpG-ODN is a prerequisite to activate TLR9 for initiation of signaling (Ahmad-Nejad et al. 2002).
The superiority of co-encapsulation of CpG-ODN and antigen (TT) in PLGA nanospheres have also been reported (Diwan et al. 2002). Co-delivery of adjuvant and antigen by the same delivery system increase IFN-γ production (three times higher than TT + CpG solution), but the IgG2b/IgG1 ratio was lower (P < 0.05). (TT + CpG)PLGA enhanced immune response showed by a higher titer of IFN-γ, IgG1, and IgG2b compared with (TT)PLGA + CpG.
In summary, the results of this study showed that either PLGA nanospheres or CpG-ODN adjuvants improve the immune response against ALM antigen, but when both adjuvants were used simultaneously, a much stronger immune response was induced in susceptible BALB/c mice, and a higher protection rate was seen upon challenge with L. major. Therefore, co-encapsulation of ALM and CpG-ODN in PLGA nanospheres might be an appropriate strategy to induce a potent Th1 immune response and improve protection against leishmaniasis.
References
Ahmad-Nejad P, Hacker H, Rutz M, Bauer S, Vabulas RM, Wagner H (2002) Bacterial CPG-DNA and lipopolysaccharides activate toll-like receptors at distinct cellular compartments. Eur J Immunol 32(7):1958–1968
Alimohammadian MH, Khamesipour A, Darabi H, Firooz A, Malekzadeh S, Bahonar A, Dowlati Y, Modabber F (2002) The role of BCG in human immune responses induced by multiple injections of autoclaved Leishmania major as a candidate vaccine against leishmaniasis. Vaccine 21(3–4):174–180
Armijos RX, Weigel MM, Calvopina M, Hidalgo A, Cevallos W, Correa J (2004) Safety, immunogenecity, and efficacy of an autoclaved Leishmania amazonensis vaccine plus BCG adjuvant against new world cutaneous leishmaniasis. Vaccine 22(9–10):1320–1326
Badiee A, Jaafari MR, Khamesipour A (2007) Leishmania major: immune response in BALB/c mice immunized with stress-inducible protein 1 encapsulated in liposomes. Exp Parasitol 115(2):127–134
Bahar K, Dowlati Y, Shidani B, Alimohammadian MH, Khamesipour A, Ehsasi S, Hashemi-Fesharki R, Ale-Agha S, Modabber F (1996) Comparative safety and immunogenicity trial of two killed Leishmania major vaccines with or without BCG in human volunteers. Clin Dermatol 14(5):489–495
Barman SP, Lunsford L, Chambers P, Hedley ML (2000) Two methods for quantifying DNA extracted from poly(lactide-co-glycolide) microspheres. J Control Release 69(3):337–344
Bhowmick S, Ravindran R, Ali N (2007) Leishmanial antigens in liposomes promote protective immunity and provide immunotherapy against visceral leishmaniasis via polarized th1 response. Vaccine 25(35):6544–6556
Cabrera M, Blackwell JM, Castes M, Trujillo D, Convit J, Shaw MA (2000) Immunotherapy with live BCG plus heat killed Leishmania induces a T helper 1-like response in American cutaneous leishmaniasis patients. Parasite Immunol 22(2):73–79
Coelho EA, Tavares CA, Lima Kde M, Silva CL, Rodrigues JM Jr, Fernandes AP (2006) Mycobacterium HSP65 DNA entrapped into TDM-loaded PLGA microspheres induces protection in mice against Leishmania (leishmania) major infection. Parasitol Res 98(6):568–575
De Luca PM, Mayrink W, Alves CR, Coutinho SG, Oliveira MP, Bertho AL, Toledo VP, Costa CA, Genaro O, Mendonca SCF (1999) Evaluation of the stability and immunogenicity of autoclaved and nonautoclaved preparations of a vaccine against American tegumentary leishmaniasis. Vaccine 17(9–10):1179–1185
Diwan M, Tafaghodi M, Samuel J (2002) Enhancement of immune responses by co-delivery of a CPG oligodeoxynucleotide and tetanus toxoid in biodegradable nanospheres. J Control Release 85(1–3):247–262
Flynn B, Wang V, Sacks DL, Seder RA, Verthelyi D (2005) Prevention and treatment of cutaneous leishmaniasis in primates by using synthetic type D/A oligodeoxynucleotides expressing CPG motifs. Infect Immun 73(8):4948–4954
Follador I, Araujo C, Orge G, Cheng LH, de Carvalho LP, Bacellar O, Almeida RP, Carvalho EM (2002) Immune responses to an inactive vaccine against American cutaneous leishmaniasis together with granulocyte-macrophage colony-stimulating factor. Vaccine 20(9–10):1365–1368
Gupta RK, Siber GR (1995) Adjuvants for human vaccines—current status, problems and future prospects. Vaccine 13(14):1263–1276
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K et al (2000) A toll-like receptor recognizes bacterial DNA. Nature 408(6813):740–745
Iborra S, Carrion J, Anderson C, Alonso C, Sacks D, Soto M (2005) Vaccination with the Leishmania infantum acidic ribosomal p0 protein plus CPG oligodeoxynucleotides induces protection against cutaneous leishmaniasis in c57bl/6 mice but does not prevent progressive disease in BALB/c mice. Infect Immun 73(9):5842–5852
Iborra S, Parody N, Abánades DR, Bonay P, Prates D, Novais FO, Barral-Netto M, Alonso C, Soto M (2008) Vaccination with the Leishmania major ribosomal proteins plus CPG oligodeoxynucleotides induces protection against experimental cutaneous leishmaniasis in mice. Microbes Infect 10:1133–1141
Jaafari MR, Ghafarian A, Farrokh-Gisour A, Samiei A, Kheiri MT, Mahboudi F, Barkhordari F, Khamesipour A, McMaster WR (2006) Immune response and protection assay of recombinant major surface glycoprotein of Leishmania (rgp63) reconstituted with liposomes in BALB/c mice. Vaccine 24(29–30):5708–5717
Jaafari MR, Badiee A, Khamesipour A, Samiei A, Soroush D, Kheiri MT, Barkhordari F, McMaster WR, Mahboudi F (2007) The role of CpG ODN in enhancement of immune response and protection in BALB/c mice immunized with recombinant major surface glycoprotein of Leishmania (rgp63) encapsulated in cationic liposome. Vaccine 25(32):6107–6117
Javadian E, Nadim A, Tahvildare-Bidruni G, Assefi V (1976) Epidemiology of cutaneous leishmaniasis in Iran: B. Khorassan part V: report on a focus of zoonotic cutaneous leishmaniasis in Esferayen. Bull Soc Pathol Exot Fil 69(2):140–143
Johansen P, Men Y, Merkle HP, Gander B (2000) Revisiting PLA/PLGA microspheres: an analysis of their potential in parenteral vaccination. Eur J Pharm Biopharm 50(1):129–146
Kamil AA, Khalil EA, Musa AM, Modabber F, Mukhtar MM, Ibrahim ME, Zijlstra EE, Sacks D, Smith PG, Zicker F, El-Hassan AM (2003) Alum-precipitated autoclaved Leishmania major plus Bacille Calmette-Guerrin, a candidate vaccine for visceral leishmaniasis: safety, skin-delayed type hypersensitivity response and dose finding in healthy volunteers. Trans R Soc Trop Med Hyg 97(3):365–368
Kedzierski L, Zhu Y, Handman E (2006) Leishmania vaccines: progress and problems. Parasitology 133(Suppl):S87–S112
Keshavarz Valian H, Khoshabe Abdollah Kenedy L, Nateghi Rostami M, Miramin Mohammadi A, Khamesipour A (2008) Role of Mycobacterium vaccae in the protection induced by first generation Leishmania vaccine against murine model of leishmaniasis. Parasitol Res 103:21–28
Khalil EA, El Hassan AM, Zijlstra EE, Mukhtar MM, Ghalib HW, Musa B, Ibrahim ME, Kamil AA, Elsheikh M, Babiker A et al (2000) Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomised, double-blind, BCG-controlled trial in Sudan. Lancet 356(9241):1565–1569
Khamesipour A, Dowlati Y, Asilian A, Hashemi-Fesharki R, Javadi A, Noazin S, Modabber F (2005) Leishmanization: use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine 23(28):3642–3648
Khamesipour A, Rafati S, Davoudi N, Maboudi F, Modabber F (2006) Leishmaniasis vaccine candidates for development: a global overview. Indian J Med Res 123(3):423–438
Krieg AM (1999) Mechanisms and applications of immune stimulatory CPG oligodeoxynucleotides. Biochim Biophys Acta BBA Gene Struct Expr 1489(1):107–116
Krieg AM (2002) CPG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 20:709–760
Mauel J (2002) Vaccination against Leishmania infections. Curr Drug Targets Immune Endocr Metabol Disord 2(3):201–226
McCluskie MJ, Davis HL (1998) CPG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J Immunol (1950) 161(9):4463–4466
Mendez S, Tabbara K, Belkaid Y, Bertholet S, Verthelyi D, Klinman D, Seder RA, Sacks DL (2003) Coinjection with CPG-containing immunostimulatory oligodeoxynucleotides reduces the pathogenicity of a live vaccine against cutaneous leishmaniasis but maintains its potency and durability. Infect Immun 71(9):5121–5129
Misra A, Dube A, Srivastava B, Sharma P, Srivastava JK, Katiyar JC, Naik S (2001) Successful vaccination against Leishmania donovani infection in Indian langur using alum-precipitated autoclaved Leishmania major with BCG. Vaccine 19(25–26):3485–3492
Modabber F (1995) Vaccines against leishmaniasis. Ann Trop Med Parasitol 89(Supplement 1):83–88
Mohebali M, Khamesipour A, Mobedi I, Zarei Z, Hashemi-Fesharki R (2004) Double-blind randomized efficacy field trial of alum precipitated autoclaved Leishmania major vaccine mixed with BCG against canine visceral leishmaniasis in Meshkin-Shahr District, I.R. Iran. Vaccine 22(29–30):4097–4100
Newman KD, Sosnowski DL, Kwon GS, Samuel J (1998) Delivery of muc1 mucin peptide by poly(d, l-lactic-co-glycolic acid) microspheres induces type 1 T helper immune responses. J Pharm Sci 87(11):1421–1427
Newman KD, Elamanchili P, Kwon GS, Samuel J (2002) Uptake of poly(d, l-lactic-co-glycolic acid) microspheres by antigen-presenting cells in vivo. J Biomed Mater Res 60(3):480–486
Noazin S, Modabber F, Khamesipour A, Smith PG, Moulton LH, Nasseri K, Sharifi I, Khalil EA, Bernal ID, Antunes CM, Kieny MP, Tanner M (2008) First generation leishmaniasis vaccines: a review of field efficacy trials. Vaccine 26(52):6759–6767
Rebelatto MC, Guimond P, Bowersock TL, HogenEsch H (2001) Induction of systemic and mucosal immune response in cattle by intranasal administration of pig serum albumin in alginate microparticles. Vet Immunol Immunopathol 83(1–2):93–105
Reiner SL, Locksley RM (1995) The regulation of immunity to Leishmania major. Annu Rev Immunol 13:151–177
Rhee EG, Mendez S, Shah JA, Wu CY, Kirman JR, Turon TN, Davey DF, Davis H, Klinman DM, Coler RN, Sacks DL, Seder RA (2002) Vaccination with heat-killed Leishmania antigen or recombinant leishmanial protein and CPG oligodeoxynucleotides induces long-term memory cd4+ and cd8+ T cell responses and protection against Leishmania major infection. J Exp Med 195(12):1565–1573
Sacks D, Noben-Trauth N (2002) The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol 2(11):845–858
Satti IN, Osman HY, Daifalla NS, Younis SA, Khalil EA, Zijlstra EE, El Hassan AM, Ghalib HW (2001) Immunogenicity and safety of autoclaved Leishmania major plus BCG vaccine in healthy Sudanese volunteers. Vaccine 19(15–16):2100–2106
Scott P, Pearce E, Natovitz P, Sher A (1987) Vaccination against cutaneous leishmaniasis in a murine model. I. Induction of protective immunity with a soluble extract of promastigotes. J Immunol 139(1):221–227
Shimizu Y, Yamakami K, Gomi T, Nakata M, Asanuma H, Tadakuma T, Kojima N (2003) Protection against Leishmania major infection by oligomannose-coated liposomes. Bioorg Med Chem 11(7):1191–1195
Shimizu Y, Takagi H, Nakayama T, Yamakami K, Tadakuma T, Yokoyama N, Kojima N (2007) Intraperitoneal immunization with oligomannose-coated liposome-entrapped soluble leishmanial antigen induces antigen-specific T-helper type immune response in BALB/c mice through uptake by peritoneal macrophages. Parasite Immunol 29(5):229–239
Sinha VR, Trehan A (2003) Biodegradable microspheres for protein delivery. J Control Release 90(3):261–280
Tafaghodi M, Sajadi Tabassi SA, Jaafari M-R, Zakavi SR, Momen Nejad M (2004) Evaluation of the clearance characteristics of various microspheres in the human nose by gamma-scintigraphy. Int J Pharm 280:125–135
Tewary P, Pandya J, Mehta J, Sukumaran B, Madhubala R (2004) Vaccination with Leishmania soluble antigen and immunostimulatory oligodeoxynucleotides induces specific immunity and protection against Leishmania donovani infection. FEMS Immunol Med Microbiol 42(2):241–248
Venkataprasad N, Coombes AGA, Singh M, Rohde M, Wilkinson K, Hudecz F, Davis SS, Vordermeier HM (1999) Induction of cellular immunity to a mycobacterial antigen adsorbed on lamellar particles of lactide polymers. Vaccine 17(15–16):1814–1819
Verthelyi D, Kenney RT, Seder RA, Gam AA, Friedag B, Klinman DM (2002) Cpg oligodeoxynucleotides as vaccine adjuvants in primates. J Immunol (1950) 168(4):1659–1663
Waterborg JH (2002) Quantitation of proteins. In: Walker J (ed) The protein protocols handbook, 2nd edn. Humana, New Jersey, pp 3–36
Wu W, Weigand L, Belkaid Y, Mendez S (2006) Immunomodulatory effects associated with a live vaccine against Leishmania major containing CPG oligodeoxynucleotides. Eur J Immunol 36(12):3238–3247
Acknowledgements
This study was part of a research project which was financially supported by the vice chancellor for research of Mashhad University of Medical Sciences. We acknowledge the excellent technical assistance of Afshin Samiei and Dina Soroush.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tafaghodi, M., Khamesipour, A. & Jaafari, M.R. Immunization against leishmaniasis by PLGA nanospheres encapsulated with autoclaved Leishmania major (ALM) and CpG-ODN. Parasitol Res 108, 1265–1273 (2011). https://doi.org/10.1007/s00436-010-2176-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-2176-4