Abstract
The parasitic nematode Trichinella spiralis can cause trichinellosis, which leads to pathological processes in the intestine and muscle. The intestinal invasion determines the development, subsequent course, and consequences of the disease. Gastrointestinal nematode infection, including with T. spiralis, is accompanied by a rapid and reversible expansion of mucosal mast cell and goblet cell in the intestinal epithelium, which play important roles in the host immune response to parasite and worm expulsion from the intestine. Taurine and its derivatives have anti-infection and anti-inflammatory properties. We investigated whether taurine supplementation in mice could influence the development and pathological processes of infection with T. spiralis. Supplementing 1 % taurine in drinking water in mice infected with T. spiralis could alleviate the burden of intestinal adult worms on days 7 and 10 postinfection (all p < 0.01) and the formation of infective muscle larvae in striated muscle during T. spiralis infection (p < 0.01). As compared with T. spiralis infection alone, taurine treatment increased the number of goblet cells on days 7, 10, and 15 (p < 0.01 and p < 0.05) and alleviated intestinal mucosal mast cell hyperplasia on days 10 and 15 (all p < 0.01). So taurine supplementation in drinking water increased infection-induced intestinal goblet cell hyperplasia and ameliorated mucosal mastocytosis. Thus, taurine can ameliorate the pathological processes of trichinellosis and may be of great value for the treatment and prevention of infection with T. spiralis and other gastrointestinal nematodes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trichinellosis is a parasitic zoonosis with pathological processes occurring in the intestines and muscles. Intestinal invasion is an important stage of trichinellosis because it determines the development and subsequent course of the disease and its consequences. The first-stage larvae of Trichinella spiralis initiate infection in the intestine, colonizing the epithelium where they molt and develop into adults that mate and reproduce within 3 to 7 days, thus yielding the next generation of larvae. Adult worms are expelled within 10 to 15 days postinfection. The life cycle of T. spiralis is complete when newborn larvae develop into muscle larvae and induce the transformation of muscle cells into nurse cells (Gottstein et al. 2009; Thrasher et al. 2013; Ren et al. 2013). Previous studies showed that infective larvae invade the intestinal epithelial cells and then migrate within the epithelium, continually invading and occupying the cytoplasm of new cells (Wright et al. 1987). In the intestine, the larvae and adult parasites localize to the crypt–villus junction and establish an intramulticellular niche composed of numerous epithelial cells.
Generally, the mechanism by which intestinal nematodes invade intestinal mucosa may involve both mechanical and chemical damage. T. spiralis larvae do not possess oral appendages or a spike, so the invasion of intestinal epithelial cells may not simply result from mechanical penetration but may be mediated by surface molecules and secretions of parasites and host. However, until now, the mechanisms have not been well elucidated. Previous studies showed that gastrointestinal infection with T. spiralis is accompanied by a rapid and reversible expansion of mucosal mast cell and goblet cell populations in the intestinal epithelium, which is associated with release of their mediators into the gut lumen (Knight et al. 2008). In addition, T helper type 2 (Th2) cytokines interleukin (IL)-4 and IL-13 regulate the development of goblet cell hyperplasia in the gut during nematode infection via activating signal transducer and activator of transcription factor 6 (Stat6) and that the increased number of goblet cells plays an important role in host protective immunity against the infection (Khan et al. 2001; Finkelman et al. 2004; Khan 2008). Mice deficient in mast cells or lacking the gene for mouse mast cell protease-1 (mMCP-1), the protease that is unique to mucosal mast cells of mice, showed a compromised process of adult worm expulsion and an increased number of encysted larvae as compared with wild-type animals (Knight et al. 2000). Mast cell hyperplasia is known to create an unsuitable environment for the worm in infection with T. spiralis (Suzuki et al. 2008). Thus, intestinal goblet cell and mucosal mast cell play an important role in the invasion and expulsion of T. spiralis in the intestine.
Taurine (2-aminoethanesulfonic acid), a semi-essential sulfur-containing β-amino acid, is the most abundant free amino acid in most animal tissue and plays an important role in several essential biologic processes such as bile acid conjugation, maintenance of calcium homeostasis, osmoregulation, and membrane stabilization (Marcinkiewicz and Kontny 2012). A large number of reports suggest a key role for taurine and its derivatives in the innate immune response and its potential use in preventing and treating various topical infections and chronic inflammatory diseases. Taurine was used to regulate the immune response following infection with Streptococcus uberis and prevented mammary tissue damage by increasing T regulatory cells in rat (Miao et al. 2012). Dietary taurodeoxycholic acid supplementation alleviated mucosal damage and improved survival with lipopolysaccharide-induced intestinal injury (Perrone et al. 2010). N-chlorotaurine shows high in vitro activity against promastigotes and amastigotes of Leishmania species (Fürnkranz et al. 2009). These findings suggest that taurine and its derivatives possess anti-infection and anti-inflammatory properties.
Whether taurine supplementation can influence the development and pathological processes of infection with T. spiralis is not known. We aimed to determine the effect of taurine on nematode invasion and pathogenesis during T. spiralis infection in mice. Taurine supplementation in drinking water could alleviate the burden of intestinal adult worms and muscle larvae during the infection, which was accompanied by enhanced intestinal goblet cell hyperplasia and ameliorated mucosal mastocytosis. These results will be of great value for preventing and treating infection with T. spiralis and other intestinal nematodes.
Materials and methods
Animals and materials
Female ICR mice, 6–8 weeks old, were purchased from the Department of Laboratory Animal Science of Peking University Health Science Center (Beijing). All animal husbandry and experimental procedures were performed in accordance with the Chinese Animal Management Ordinance (People’s Republic of China Ministry of Health, document no. 55, 2001), and the animal experiment standards, approved by the Animal Management Committee of Peking University Health Science Center.
Taurine (2-aminoethanesulfonic acid), pepsin, and other agents were from Sigma (St. Louis, MO, USA).
Parasite, experimental groups, and infection
Parasite T. spiralis, strain ISS533, was a gift from Professor Xin-Ping Zhu (Department of Parasitology, School of Basic Medical Sciences, Capital Medical University, People’s Republic of China) and maintained by serial passage in female ICR mice. Muscle larvae were recovered from infected mice by the standard pepsin digestion method (Dennis et al. 1970). Experimental mice were each infected with 400 infective T. spiralis muscle larvae by oral gavage on day 0. In all, 200 female ICR mice were randomly divided into four groups for treatment (eight to ten mice per group per time): (1) uninfected control: not infected with T. spiralis and normally fed; (2) taurine control: not infected with T. spiralis but fed 1 % taurine in drinking water for 30 days from day 0; (3) T. spiralis infection: infected with T. spiralis and normally fed; (4) taurine treatment: infected with T. spiralis and fed 1 % taurine in drinking water for 30 days from day 0. Mice were killed by cervical dislocation at the times indicated.
Assessment of burden of intestinal adult worms and muscle larvae
The number of adult worms in the small intestine was assessed on days 3, 7, 10, and 15 postinfection. Briefly, the duodenum–ileum region of the small intestine was sectioned into 1- to 2-cm pieces and dissected longitudinally in a 100-mm Petri dish containing 20 ml phosphate buffer solution. After rocking the dish slowly at 37 °C for 4 h, the adult worms were collected and counted under a dissecting microscope (Zeiss, German).
The burden of muscle larvae was determined on day 30 postinfection. Carcasses of infected mice were skinned, eviscerated, minced, and digested in separate flasks containing 200 ml of 1 % pepsin–HCl at 37 °C for 4 h. Then the mixture was filtered through a 60-mesh screen, and larvae concentrated by sedimentation were washed and counted.
Histology and determination of the number of goblet cells and mast cells
A 2-cm-long segment of the jejunum 10 cm from the pyloric sphincter was taken from each mouse for histological examination. The intestine was fixed in neutral buffered 10 % formaldehyde solution for 24 h before being embedded in paraffin and histologically processed by standard methods; 5-μm sections were stained for mast cells (0.5 % toluidine blue, followed by eosin) and goblet cells (periodic acid-Schiff). The number of goblet cells and mucosa mast cells per ten randomly selected villus–crypt units (VCU) were determined by microscopy (Leica DMI 3000B, German) from at least two sections per animal.
Mouse tongues were fixed and embedded as mentioned above and stained with hematoxylin and eosin (H&E) for the assessment of the burden of muscle larvae.
Statistical analysis
Results are presented as mean ± SD. Statistical analysis involved the use of GraphPad Prism 5 for Windows (GraphPad Software, San Diego, CA, USA). Two-way ANOVA was used for the analysis. A p < 0.05 was considered statistically significant.
Results
Taurine supplementation in drinking water alleviated the burden of intestinal adult worms during T. spiralis infection in mice
To elucidate the role of taurine in T. spiralis infection, mice infected with T. spiralis, with or without taurine in drinking water, were killed on different days after infection. Small intestinal adult worms were observed on days 3, 7, 10, and 15 postinfection. The maximal worm burden was on day 7 for T. spiralis-infected mice with and without taurine treatment (Fig. 1). Expulsion of worms was indicated by a decreased number of adult worms on day 10, with almost full clearance on day 15. However, as compared with no taurine treatment, taurine-treated mice showed significantly lower worm burden on days 7 and 10 postinfection (all p < 0.01) (Fig. 1). Therefore, taurine supplementation in drinking water could reduce the burden of intestinal adult worms in T. spiralis-infected mice.
Worm burden in small intestine after infection with T. spiralis and taurine treatment in mice. Mice were infected with 400 muscle larvae. The number of worms is mean ± SD of eight to ten mice/group. T.s indicates T. spiralis infection; T.s + Tau indicates taurine treatment; *p < 0.05, **p < 0.01 vs T.s
Taurine supplementation in drinking water alleviated the burden of infective muscle larvae in striated muscle during T. spiralis infection
Next, we assessed the muscle larvae burden on day 30 postinfection. The number of larvae in striated muscle was significantly lower with than without taurine treatment in T. spiralis-infected mice (p < 0.01) (Fig. 2a). H&E-stained tongue tissue showed the same tendency: encysted muscle larvae were rarely seen in tongues of T. spiralis-infected mice with taurine supplementation (Fig. 2b–e). Thus, taurine supplementation could reduce the formation of infective T. spiralis muscle larvae in striated muscle.
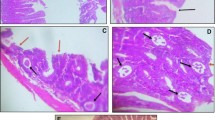
Muscle larvae burden in striated muscle at day 30 after infection with T. spiralis and taurine treatment in mice. Mice were infected with 400 muscle larvae. The number of larvae is mean ± SD of eight to ten mice/group. T.s indicates T. spiralis infection; T.s + Tau indicates taurine treatment; **p < 0.01 vs T.s (a). Hematoxylin and eosin-stained muscle larvae (arrowhead) of tongue tissue with T. spiralis infection alone (b, c), and T. spiralis infection with taurine treatment (d, e). c and e Amplification of boxes of b and d, respectively. Scale bars = 100 μm
Taurine supplementation in drinking water increased intestinal goblet cell hyperplasia during T. spiralis infection
To investigate how taurine drinking alleviated the burden of intestinal worms and muscle larvae during T. spiralis infection, we observed goblet cell hyperplasia of intestinal epithelium, a characteristic of intestinal nematode infection. The number of periodic acid-Schiff (PAS)-reactive intestinal goblet cells increased on days 7, 10, and 15 in T. spiralis-infected mice as compared with noninfected mice (all p < 0.01). Further, the number of goblet cells was markedly increased in the intestine of taurine-drinking infected mice on days 3, 7, 10, and 15 (p < 0.05 and p < 0.01) (Fig. 3). As compared with T. spiralis infection alone, taurine treatment increased the number of goblet cells on days 7, 10, and 15 (p < 0.01 and p < 0.05) (Fig. 3). Therefore, taurine could increase intestinal goblet cell hyperplasia induced by the infection.
Effect of taurine treatment on goblet cell hyperplasia of intestinal epithelium after infection with T. spiralis. Mice were infected with 400 muscle larvae. a–d Small intestine section stained with periodic acid-Schiff on day 7 postinfection: a uninfected control (Con), b taurine control (Con + Tau), c T. spiralis infection (T.s), d taurine treatment (T.s + Tau); scale bars = 100 μm. e Number of goblet cells in ten villus–crypt units. Data are mean ± SD of six mice/group. *p < 0.05, **p < 0.01 vs Con; &p < 0.05, &&p < 0.01 vs T.s
Taurine supplementation in drinking water alleviated intestinal mucosal mast cell hyperplasia during T. spiralis infection
Mucosal mastocytosis is a significant histological feature of intestinal nematode infection. Intestinal worm expulsion depends on mast cells; so next, we assessed the effects of taurine drinking on jejunum mucosal mast cell hyperplasia. As compared with noninfected mice, T. spiralis-infected mice with and without taurine treatment showed pronounced mastocytosis in intestinal mucosa on days 10 and 15 (all p < 0.01), with lower mucosal mastocytosis with than without taurine treatment on days 10 and 15 (all p < 0.01) (Fig. 4). These results show that mucosal mastocytosis is a universal phenomenon and coincides with the expulsion of adult worms from the intestine during T. spiralis infection in mice, as seen in Fig. 1. Additionally, taurine supplementation can ameliorate mastocytosis but does not affect clearance of adult worms from the intestine.
Effect of taurine treatment on mucosal mast cell hyperplasia of the intestine after infection with T. spiralis. Mice were infected with 400 muscle larvae. The number of mast cell in ten VCU was counted. Data are mean ± SD of six mice/group. Con indicates uninfected control; Con + Tau indicates taurine control; T.s indicates T. spiralis infection; T.s + Tau indicates taurine treatment; **p < 0.01 vs Con, &&p < 0.01 vs T.s
Discussion
Here, we show for the first time that during T. spiralis infection in mice, taurine drinking can alleviate the burden of intestinal adult worms and muscle larvae, enhance intestinal goblet cell hyperplasia, and ameliorate mucosal mastocytosis. Taurine may participate in the immune response to infection by the parasite. In addition, these results confirm that the modulating influence of orally administered taurine on histological changes in the intestine may be associated with reduced intensity of invasion and pathological damage during experimental trichinellosis in mice.
The mucus layer overlying the intestinal epithelium has both protective and lubricative functions, protecting the mucosa against dehydration and mechanical damage and providing a physical barrier between the underlying epithelium and luminal contents, such as pathogenic bacteria, viruses, and parasites. During invasion by an intestinal nematode, the mucus secreted by goblet cells plays an important role in the defense response by dislodging the established worm or trapping the worm in the mucus and inhibiting parasite motility and feeding, thereby excluding the worm from its niche and favoring its expulsion (Khan 2008). Hyperplasia of mucin-secreting goblet cells has been described in a number of helminth infections, including that caused by T. spiralis (Ishikawa et al. 1997; Khan et al. 2001; Sagar et al. 2004). In addition, goblet cells are the source of a number of other molecules involved in host defense, including intestinal trefoil factor-3 (Artis and Grencis 2008). In this study, we observed goblet cell hyperplasia in the jejunum epithelium of mice infected with T. spiralis. Importantly, taurine drinking could increase the infection-induced goblet cell hyperplasia (Fig. 3), which may increase mucus secretion and enhance the defense response to helminth infection, ultimately reducing the burden of intestinal adult worms and muscle larvae (Figs. 1 and 2).
Mucosal mastocytosis is a significant histological feature of intestinal nematode infection, including T. spiralis infection. Peak mastocytosis coincides with the expulsion of the adult worms from the intestine (Ierna et al. 2009). Mucosal mast cells expand rapidly in the mucosa, predominantly within the epithelium, and release effector molecules such as histamine and cytokines and serine proteases such as mMCP-1, which can cause pathology, but also induce their role in defense against nematode infection (Pennock and Grencis 2006). mMCP-1 specifically impairs epithelial cell barriers by degrading the tight junction protein occludin and thereby increasing luminal fluid flow (McDermott et al. 2003; Anthony et al. 2007), potentially contributing to parasite expulsion. We found pronounced mastocytosis from day 10 postinfection (Fig. 4), which corresponds to the time of worm expulsion from the intestine (Fig. 1). In addition, taurine drinking could ameliorate T. spiralis-induced mastocytosis (Fig. 4), which did not delay the clearance of adult worms from the intestine. In mice infected with T. spiralis, ameliorated intestinal mucosal mastocytosis triggered by taurine drinking may alleviate intestine inflammatory pathophysiologic states without changing worm expulsion.
T. spiralis-induced mucosal mast cell and goblet cell hyperplasia depends on mucosal T cells and, more specifically, the Th2 cytokines IL-4 and IL-13 (Knight et al. 2008). We found that taurine supplementation could ameliorate the histological change in the T. spiralis-infected intestine, enhance goblet cell hyperplasia, and alleviate mastocytosis, ultimately ameliorating the development and consequence of trichinellosis. However, the mechanism of taurine ameliorating intestinal histology is not known. Taurine plays an important role in the immune system as an antioxidant to protect cells, including leukocytes, against oxidative stress (Schaffer et al. 2009; Wang et al. 2009). Therefore, the primary role of taurine is cytoprotection and maintaining the homeostasis of cells involved in acute and chronic inflammatory and infectious disease. Previous studies have clearly indicated that both taurine and its derivatives may have a place in therapy for various topical infection as well as chronic inflammatory diseases (Kim et al. 2006; Joo et al. 2009; Shimizu et al. 2009). So taurine may ameliorate intestinal histological changes during T. spiralis infection because of its anti-inflammatory properties and cytoprotective function. Further studies are needed to investigate the underlying mechanisms.
In conclusion, our results demonstrate that taurine supplementation can ameliorate the pathologic features and consequences of trichinellosis in mice. Taurine could attenuate the burden of intestinal adult worms and muscle larvae, enhance intestinal goblet cell hyperplasia, and ameliorate mucosal mastocytosis. Gastrointestinal parasites are probably the most important infectious agents in terms of their global prevalence and their ability for causing diseases. Our results may provide clues for the treatment and prevention of trichinellosis and suggest innovative new strategies for control of intestinal helminth infections.
References
Anthony RM, Rutitzky LI, Urban JF Jr, Stadecker MJ, Gause WC (2007) Protective immune mechanisms in helminth infection. Nat Rev Immunol 7(12):975–987
Artis D, Grencis RK (2008) The intestinal epithelium: sensors to effectors in nematode infection. Mucosal Immunol 1(4):252–264
Dennis DT, Despommier DD, Davis N (1970) Infectivity of the newborn larva of Trichinella spiralis in the rat. J Parasitol 56(5):974–977
Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF Jr (2004) Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev 201:139–155
Fürnkranz U, Nagl M, Gottardi W, Matt U, Aspöck H, Walochnik J (2009) N-Chlorotaurine shows high in vitro activity against promastigotes and amastigotes of Leishmania species. J Med Microbiol 58:1298–1302
Gottstein B, Pozio E, Nöckler K (2009) Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin Microbiol Rev 22(1):127–145
Ierna MX, Scales HE, Mueller C, Lawrence CE (2009) Transmembrane tumor necrosis factor alpha is required for enteropathy and is sufficient to promote parasite expulsion in gastrointestinal helminth infection. Infect Immun 77(9):3879–3885
Ishikawa N, Wakelin D, Mahida YR (1997) Role of T helper 2 cells in intestinal goblet cell hyperplasia in mice infected with Trichinella spiralis. Gastroenterology 113:542–549
Joo K, Lee Y, Choi D, Han J, Hong S, Kim YM, Jung Y (2009) An anti-inflammatory mechanism of taurine conjugated 5-aminosalicylic acid against experimental colitis: taurine chloramines potentiates inhibitory effect of 5-aminosalicylic acid on IL-1beta-mediated NFkappab activation. Eur J Pharmacol 618(1–3):91–97
Khan WI, Blennerhasset P, Ma C, Matthaei KI, Collins SM (2001) Stat6 dependent goblet cell hyperplasia during intestinal nematode infection. Parasite Immunol 23(1):39–42
Khan WI (2008) Physiological changes in the gastrointestinal tract and host protective immunity: learning from the mouse-Trichinella spiralis model. Parasitology 135(6):671–682
Kim C, Choi HS, Kim JW (2006) Taurine chloramine inhibits the production of nitric oxide and superoxide anion by modulating specific mitogen-activated protein kinases. Adv Exp Med Biol 583:493–498
Knight PA, Brown JK, Pemberton AD (2008) Innate immune response mechanisms in the intestinal epithelium: potential roles for mast cells and goblet cells in the expulsion of adult Trichinella spiralis. Parasitology 135(6):655–670
Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR (2000) Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med 192:1849–1856
Marcinkiewicz J, Kontny E (2012) Taurine and inflammatory diseases. Amino Acids. doi:10.1007/s00726-012-1361-4
McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK (2003) Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci USA 100(13):7761–7766
Miao J, Zhang J, Zheng L, Yu X, Zhu W, Zou S (2012) Taurine attenuates Streptococcus uberis-induced mastitis in rats by increasing T regulatory cells. Amino Acids 42(6):2417–2428
Pennock JL, Grencis RK (2006) The mast cell and gut nematodes: damage and defence. Chem Immunol Allergy 90:128–140
Perrone EE, Chen C, Longshore SW, Okezie O, Warner BW, Sun CC, Alaish SM, Strauch ED (2010) Dietary bile acid supplementation improves intestinal integrity and survival in a murine model. J Pediatr Surg 45(6):1256–1265
Ren HJ, Liu RD, Wang ZQ, Cui J (2013) Construction and use of a Trichinella spiralis phage display library to identify the interactions between parasite and host enterocytes. Parasitol Res 112(5):1857–1863
Sagar M, Padol I, Khan WI, Bonin RP, Blennerhassett PA, Hunt RH (2004) Establishment of T-Helper-2 immune response based gerbil model of enteric infection. Scand J Gastroenterol 39(7):668–673
Schaffer SW, Azuma J, Mozaffari M (2009) Role of antioxidant activity of taurine in diabetes. Can J Physiol Pharmacol 87(2):91–99
Shimizu M, Zhao Z, Ishimoto Y, Satsu H (2009) Dietary taurine attenuates dextran sulfate sodium (DSS)-induced experimental colitis in mice. Adv Exp Med Biol 643:265–271
Suzuki T, Sasaki T, Takagi H, Sato K, Ueda K (2008) The effectors responsible for gastrointestinal nematode parasites, Trichinella spiralis, expulsion in rats. Parasitol Res 103(6):1289–1295
Thrasher SM, Scalfone LK, Holowka D, Appleton JA (2013) In vitro modelling of rat mucosal mast cell function in Trichinella spiralis infection. Parasite Immunol 35(1):21–31
Wang L, Zhao N, Zhang F, Yue W, Liang M (2009) Effect of taurine on leucocyte function. Eur J Pharmacol 616:275–280
Wright KA, Weidman E, Hong H (1987) The distribution of cells killed by Trichinella spiralis in the mucosal epithelium of two strains of mice. J Parasitol 73(5):935–939
Acknowledgments
We thank Prof. X-P Zhu, Department of Parasitology, School of Basic Medical Sciences, Capital Medical University, for providing the T. spiralis strain. This work was supported by the National Natural Science Foundation of China (no. 30901247), National Quality Inspection Public welfare Scientific Research Project (no. 201110039), and Leading Academic Discipline Project of Beijing Education Bureau.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yu, YR., Liu, XC., Zhang, JS. et al. Taurine drinking attenuates the burden of intestinal adult worms and muscle larvae in mice with Trichinella spiralis infection. Parasitol Res 112, 3457–3463 (2013). https://doi.org/10.1007/s00436-013-3525-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3525-x