Abstract
We describe a new species of myxozoan, Henneguya mauritaniensis n. sp., extracted from the arterial bulb of the bluespotted seabream, Pagrus caeruleostictus (Valenciennes, 1830), collected in Mauritanian waters. Out of the 209 individuals examined, 30.1 % were infected with this new taxon. Spore total length ranged from 15.0 to 20.5 μm with a mean of 17.9 μm. The two polar capsules were equal in size, and pyriform and caudal appendages joined until mid-length. Morphometric analysis revealed significant differences between H. mauritaniensis n. sp. and morphologically similar species from this region as well as congeners known from other sparid hosts. Phylogenetic analysis of 18 S rDNA indicated that this new species is closely related to Henneguya pagri, reported recently from Pagrus major off Japan. Bayesian inference and maximum likelihood analyses of the 18 S rDNA dataset also revealed that species of marine Henneguya reported forming pseudocysts in the hearts of their fish hosts were closely related. Histological analysis of the H. mauritaniensis n. sp. pseudocysts embedded in the arterial bulb of P. caeruleostictus suggests that these parasites may cause considerable pathology, which may impact negatively on the health of the fish host. Finally, we discussed the importance of a combination of morphological and molecular analysis for species description because of high variability in size within the same taxa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The exclusive economic zone along the coast of Mauritania is considered one of the richest in the world in relation to fisheries stocks due to intense deep-water upwelling, which results in a dramatic increase in productivity in its waters (Domain 1986). As a consequence, coastal fisheries form a significant component of the Mauritanian economy. One species in particular, the bluespotted seabream, Pagrus caeruleostictus (Valenciennes, 1830), is an important part of the artisanal and commercial coastal fishery of Mauritania. This sparid contributes considerably to the local economies as this species is a valuable export to the European fresh fish markets (IMROP 2004). P. caeruleostictus inhabits mainly on rocky or coarse shell sand seabeds at depths of 20–150 m in the eastern Atlantic region from Angola to the Mediterranean (Froese and Pauly 2011). The diet of this species consists primarily of bivalves and crustaceans, although they are known to prey on fish occasionally (Domain et al. 2000; Chakroun-Marzouk and Karta 1987).
There is the growing interest in understanding how parasites impact on ecosystem functioning as a whole, particularly in the context of the rapidly changing marine ecosystems (Hudson et al. 2006). Much of this interest is on how parasites and outbreaks of disease in potentially vulnerable fisheries may affect populations, although much work is needed (often on a case-by-case basis for each fishery) to integrate models of parasite impacts into management practices and stock assessments (Chavez et al. 2007). Currently, there is little data on parasites of fish in Mauritanian waters, so efforts are being made to document and report parasitic infections that may have an impact on the export of high quality fishes to the European markets (Brian 1924; FAO 2001). One group of parasites that has the potential to impact negatively on the Mauritanian economy due to fisheries health decline in this region is the myxozoans. Myxozoans are a group of microscopic pseudocyst-forming parasites known to infect a wide range of marine and freshwater fish species (Lom and Dykova 1992). Their life cycles generally consist of two hosts (often and annelid worm and fish), particularly for marine species, although rare cases of direct transmission have been reported (Kent et al. 2001; Redondo et al. 2004). Many of these parasites are also known to cause significant pathology leading to debility, reduced fecundity or mortality in their fish hosts (Kent et al. 2001). However, several host species seem to be able to encapsulate the plasmodia in order to prevent further dispersal (Sitja-Babadilla 2008), and even some more species seem to show very little effect on hosts (Barassa et al. 2012). They can be found in almost every tissue within a fish host, but many show predilections for a single small group of tissues including the brain, cranial cartilage, gills, heart, muscle, kidneys, ovaries and intestine (Eiras 2002).
Henneguya Thelohan, 1892 is one of the largest myxosporean genera, with around 200 species described from freshwater and marine fishes worldwide (Eiras 2002). Recent molecular phylogenetic analyses suggest that this genus is polyphyletic, similar to that observed for many other large myxozoan genera (Fiala and Bartošová 2010; U-taynapun et al. 2011). The taxonomic complexities associated with this and other myxozoan groups coupled with the paucity of characters available for detailed morphological comparative analysis make the inclusion of molecular as well morphological data the most robust way to distinguish species and unravel their relationships. Here, we present a morphological and molecular study of a new species of Henneguya collected from the arterial bulb, in the hearts of P. caeruleostictus off Mauritania.
Material and methods
Host and parasite sampling
Between April and August 2005 and in subsequent collections in August 2009 and February 2010, 209 P. caeruleostictus (Valenciennes, 1830) were obtained randomly at the fish market in Nouakchott, Mauritania (18°05′ N, 15°58′ W), from samples collected just off the coast. Fish were kept in a cooler until examination in the lab. After dissection, the organs (heart, gall bladder, kidney, liver and intestine) were removed and observed macroscopically for any abnormalities or signs of myxosporean pseudocysts. Fresh smears were done on cysts collected on dilacerated organs in a drop of sterilised physiological water and observed under a light microscope. Parasites were photographed and drawn with a camera lucida. Infected organs were processed for histopathology and stained with hematoxylin and eosin according to Martoja and Martoja (1967). Spores were measured under a photonic microscope connected to a digital imaging software. Morphometric measurements taken from fresh spores included length and width of spores and polar capsules as well as the length of the polar filament. Type specimens were deposited at the Natural History Museum in Paris.
Molecular sample processing
Comparative DNA analyses
A BLAST sequence similarity search of the GenBank database (Altschul et al. 1990) using the partial SSU rDNA region sequenced for the Henneguya species recovered from P. caeruleostictus off French Polynesia reported here was performed to identify similar sequences and to characterise this new taxon relative to other myxobolids. The top 67 sequences returned from the BLAST search based on percentage similarity and query coverage were selected for inclusion in subsequent phylogenetic analyses (a number of identical replicate sequences for some taxa or sequences of dubious origin were excluded from the phylogenetic analyses performed here). We also included sequence data for an undescribed Henneguya species collected from the heart of the lethrinid, Gymnocranius audleyi, off Heron Island on the Great Barrier Reef, Australia (23°27′ S, 151°52′ E). The sequence data for this specimen were generated using the same protocols as above. The partial SSU rDNA sequence generated here and those obtained from GenBank were initially aligned using MUSCLE version 3.7 (Edgar 2004) with ClustalW sequence weighting and UPGMA clustering for iterations 1 and 2. The resultant alignments were refined by eye using MESQUITE v. 2.75 (Maddison and Maddison 2009). After alignment of the SSU rDNA, the dataset was edited and the ends of each fragment were trimmed to match the shortest sequence in the alignment.
The software jModelTest version 0.1.1 (Guindon and Gascuel 2003; Posada 2008) was used to estimate the best nucleotide substitution models for the dataset. Bayesian inference analysis of the partial SSU rDNA dataset was then performed using MrBayes version 3.1.2 (Ronquist and Huelsenbeck 2003) run on the CIPRES portal (Miller et al. 2009) to explore the relationships among these taxa. Bayesian inference analysis was conducted on the SSU dataset using the TIM1 + I + G model predicted as the best estimator by the Akaike information criterion and Bayesian information criterion in jModelTest. Bayesian inference analysis was run over 10,000,000 generations (ngen = 10,000,000) with two runs each containing four simultaneous Markov chain Monte Carlo chains (nchains = 4) and every 100th tree saved (samplefreq = 1,000). Bayesian analyses used the following parameters: nst = 6, rates = invgamma and ngammacat = 4, and the prior parameters of the combined dataset were set to ratepr = variable. Samples of substitution model parameters and tree and branch lengths were summarised using the parameters ‘sump burnin = 3,000’ and ‘sumt burnin = 3,000’. These ‘burnin’ parameters were chosen because the log likelihood scores ‘stabilised’ well before 300,000 replicates in the Bayesian inference analyses.
Maximum likelihood analyses were performed on the SSU rDNA dataset using the RAxML algorithm (Stamatakis et al. 2008) on the CIPRES portal with the gamma rate model of heterogeneity and maximum likelihood search estimating the proportion of invariable site parameters. Nodal support was inferred based on 100 bootstrap replicates.
Results
Henneguya mauritaniensis n. sp.
Type-host: P. caeruleostictus (Valenciennes, 1830), bluespotted seabream (Sparidae).
Type-locality: Fish obtained at market in Nouakchott (18°05′ N, 15°58′ W) from individuals collected off the coast of Mauritania, Africa.
Site in host: Arterial bulb (bulbus arteriosus) of heart.
Prevalence: 63 of 209 P. caeruleostictus (30.1 %).
Specimens deposited: National Museum of Natural History Paris, slides N° ZS108 to ZS 111..
GenBank accession number: JQ687060
Etymology: The epithet ‘mauritaniensis’ refers to the coastal waters of Mauritania where this new species was found.
Description
Plasmodia
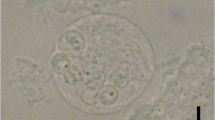
Plasmodia present as white subspherical to spherical pseudocysts approximately 0.1 mm in diameter, embedded in or around the arterial bulb tissue of the heart (Fig. 1).
Mature spores
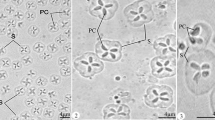
[Based on 30 specimens] Spores ovoid to ellipsoid with a rounded anterior extremity (Figs. 2 and 3). Sutural folds absent. Spore main body smooth, bilaterally symmetrical, length 12.3 ± 0.6 μm, width 8.0 ± 0.4 μm. The total spore length 17.9 (15.0–20.5). Two polar capsules equal, pyriform, anteriorly converged, terminating below spore apex, occupying approximately half of the spore cavity, 4.1 ± 0.2 μm long by 3 μm wide. Polar filament coil number could not be determined. Caudal appendages tapered, joined until mid-length and flexible distally, 25.3 ± 3.2 μm long (Table 1). Diagnosis consistent with the characters of this genus (Fomena and Bouix 1997; Eiras 2002).
Based on the site of infection, it seems more than probable that the parasite may affect the fish health. Histological information gives only some evidence of cell modification around the cysts (Fig. 4).
Comparative DNA analyses
Alignment of the SSU rDNA region for the myxobolid taxa examined here yielded 1,268 characters for analysis. Bayesian inference and maximum likelihood analyses resulted in phylograms with nearly identical topology (Fig. 5). All of the marine myxobolids also formed a relatively well-supported clade in these analyses, the only exceptions being the sequence data for Myxobolus osburni from the freshwater perciform, Lepomis gibbosus, from Canada being embedded with the ‘marine’ clade observed in both the Bayesian inference and maximum likelihood analyses (Fig. 5). This marine clade, which consisted of Henneguya and Myxobolus species and actinosporean types recovered from a wide range of hosts and geographic localities, was a sister to the remainder of the freshwater myxobolid taxa examined here. H. mauritaniensis n. sp. was grouped as a sister taxon to Henneguya pagri (also from a sparid, Pagrus major) and Henneguya tunisiensis in these analyses. These species together also formed a distinct and well-supported clade with Henneguya akule, Henneguya cynoscioni, Henneguya lateolabracis and the undescribed species of Henneguya from the heart of G. audleyi collected off Heron Island on the Great Barrier Reef (Fig. 5). The only non-heart infecting species observed within this clade was H. tunisiensis. H. mauritaniensis n. sp. differed from H. pagri by 6.0 %, H. tunisiensis by 6.6 %, Henneguya cynoscioni by 8.3 %, H. akule by 8.6 %, the undescribed Henneguya sp. ex G. audleyi by 9.2 %, H. lateolabracis by 9.3 % and over the partial SSU rDNA dataset analysed here.
Phylogenetic relationships between H. mauritaniensis n. sp. and the remainder of the myxobolid taxa and outgroups examined here based on Bayesian inference analysis of the partial SSU rDNA dataset. Posterior probability and bootstrap support values are given at the nodes, with values less than 50 % indicated with an asterisk
Discussion
Differential diagnosis
H. mauritaniensis n. sp. can be distinguished from all the other species of Henneguya based on a combination of morphometric measurements, predilection for infecting the heart tissue (arterial bulb) of their marine fish host, biogeographic distribution, caudal appendages that are joined until near mid-length and the distinguishing partial SSU rDNA sequence data relative to other taxa. Several species of Henneguya have also been reported from the heart tissue of their host fishes, including H. pagri Yokoyama et al. 2005; H. lateolabracis Yokoyama et al. 2003; Henneguya otolithi Gnapati, 1941; H akule Work et al. 2008; Henneguya sebasta Moser and Love 1975; and more recently H. cynoscioni Dyková et al. 2011. Another species, Henneguya yoffensis Kpatcha et al. 1997, was described from the same host as the new taxon described here, P. caeruleostictus, off Senegal (Kpatcha et al. 1997). This species was reported from the branchial lamellae of P. caeruleostictus, but the authors also briefly stated that some cysts had been observed on the heart without providing additional details or description of the spores found on the heart (Kpatcha et al. 1997). We did not observe pseudocysts of H. mauritaniensis n. sp. on the gills of the many P. caeruleostictus individuals examined here. H. mauritaniensis n. sp. can be distinguished from H. yoffensis based on the smaller spore body size, polar capsules which are larger relative to H. yoffensis and caudal appendages which are joined until mid-length as opposed to completely disjoined; the latter character was considered a sufficient criterion for describing a new species according to Kpatcha et al. (1997). The next most similar species based on morphology and host distribution is that of an undescribed species of Henneguya reported from Sparus aurata off Italy by Caffara et al. (2003). The spores and polar capsule sizes are similar to H. mauritaniensis n. sp.; the only apparent difference is in the slightly shorter caudal appendage length (Table 1). No molecular data are available for these specimens for comparative purposes, so for now it is impossible to conclude that these may be conspecific, particularly in light of the strict host specificity observed in other myxobolids (Tajdari et al. 2005). Dyková et al. (2011) recently described a new species discovered in the bulbus arteriosus of the sciaenid, Cynoscion nebulosus, from the western Atlantic and Gulf of Mexico with comparable measurements to the species reported here. However, the spore and polar capsules are distinctly larger in H. mauritaniensis n. sp., and the molecular analyses clearly indicate that H. cynoscioni and H. mauritaniensis n. sp. are different species.
Phylogenetic analyses
Phylogenetic analyses of the SSU data examined here for H. mauritaniensis n. sp. and other closely related taxa revealed the group of Myxobolidae reported from marine ecosystems formed a relatively well-supported clade. The only exceptions to this were three species reported from the gills of freshwater fishes (Perca flavescens and freshwater gobiids), which formed a clade with Henneguya lesteri, reported from the pseudobranchs of sand whiting Sillago analis (Hallett and Diamant 2001). These analyses further suggest that host habitat, (i.e. freshwater vs. seawater) and the tissue where the parasite is found may reveal patterns of relationship within some myxosporean groups (Eszterbauer 2004).
These analyses also highlighted the existence of a group of closely related heart-infecting species of Myxobolidae. These taxa were from widely separated biogeographic localities, including sites off the Great Barrier Reef, Japan and the west coast of Africa. H. mauritaniensis n. sp. was observed as a sister taxon to H. pagri reported from the heart of another sparid species, the red seabream P. major, off Japan and H. tunisiensis, reported from Symphodus tinca off Tunisia. The only exception observed in the ‘heart-infecting’ clade was H. tunisiensis, which was reported to infect the gills (Bahri et al. 2010). This result seems consistent with the fact that many species found in the heart were previously found in the gills, while the opposite is often not necessarily true (Dyková et al. 2011). It is possible that H. tunisiensis could also infect the heart but was not found during necropsy by the authors Bahri et al. (2010). This again seems to highlight the relationship between the site of infection or tissue predilection and the phylogenetic relationships observed between some groups of myxosporeans.
Multiple species of Henneguya have in some cases been reported from the same host and even tissue, highlighting the potential diversity of myxosporeans in fish, e.g. the species Henneguya bopeleti and Henneguya chrysichthyi from Chrysichthys nigrodigitatus (see Kostoïngue et al. 2001). Species of the genus Myxobolus have also been described on the same host species and even on the same tissue in Mullet (U-taynapun et al. 2011). In this work again, despite closely related morphological features, molecular analysis confirmed that they were separate species. Another recent work shows that temporal variation in morphology may also occur (Abdel-Baki et al. 2011); this may complicate species identification without molecular analysis. Further study and molecular characterization of the myxobolid fauna infecting the hearts of fishes, particularly those of sparids or closely related fishes, will help in our understanding in the species diversity, the host specificity and the evolutionary history of these parasites.
References
Abdel-Baki AS, Sakran Th, Zayed E (2011) Validity, impacts and seasonal prevalence of Henneguya species infecting catfish Clarias gariepinus from River Nile, Egypt. Parasitol Research 109:119–123
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bahri S, Hassine OKB, Marques A (1996) Henneguya sp. (Myxosporea, Bivalvulida) infecting the gills of wild gilthead sea bream Sparus aurata L., from the coast of Tunisia. Bull Eur Assoc Fish Pat 16:51–53
Bahri S, Marton S, Marques A, Eszterbauer E (2010) Henneguya tunisiensis n. sp. (Myxosporea: Bivalvulida), a new gill parasite of Symphodus tinca (L.) (Teleostei: Labridae) off Tunisia. Syst Parasitol 76:93–101. doi:10.1007/s11230-010-9237-z
Barassa B, Adriano EA, Cordeiro NS, Arana S, Ceccarelli PS (2012) Morphology and host-parasite interaction of Henneguya azevedoi n.sp., parasite of gills of Leporinus obtusidens from Mogi-Guaçu River, Brazil. Parasitol Res 110:887–894
Brian A (1924) Arthropoda (1re partie) Copepoda. Copépodes commensaux et parasites des côtes mauritaniennes. Bull Com Etud Hist Sci Afr Occid Fr 7:365–427
Caffara M, Marcer F, Florio D, Quaglio F, Fioravanti ML (2003) Heart infection due to Henneguya sp. (Myxozoa, Myxosporea) in gilthead sea bream (Sparus aurata) cultured in Italy. Bull Eur Assoc Fish Pat 23:108–112
Chakroun-Marzouk N, Kartas F (1987) Denture et régime alimentaire des espèces du genre Pagrus (Pisces, Sparidae) des côtes Tunisiennes. Cybium 11:3–19
Chavez RA, Valdivia IM, Oliva ME (2007) Local variability in metazoan parasites of the pelagic fish species, Engraulis ringens: implications for fish stock assessment using parasites as biological tags. J Helminthol 81:113–116
Domain F (1986) Les fonds de pêche et les ressources. In: josse e, garcia s (eds) Description et évaluation des ressources halieutiques de la ZEE mauritanienne : rapport du groupe de travail CNROP/FAO/ORSTOM. Rome: FAO, 1986, p. 1-28. Groupe de Travail CNROP/FAO/ORSTOM sur la Description et Evaluation des Ressources Halieutiques de la ZEE Mauritanienne, Nouadhibou (MAU), 1985/09/16-27
Domain F, Keita M, Morize E (2000) Typologie générale des ressources démersales du plateau continental. In: Domain F, Chavance P, Diallo A (eds) La pêche côtière en Guinée: ressources et exploitation. CNSHB, Paris, pp 53–85
Dyková I, de Buron I, Roumillat WA, Fiala I (2011) Henneguya cynoscioni sp. n. (Myxosporea: Bivalvulida), an agent of severe cardiac lesions in the spotted seatrout, Cynoscion nebulosus (Teleostei: Sciaenidae). Folia Parasitol 58:169–177
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Eiras JC (2002) Synopsis of the species of the genus Henneguya Thélohan, 1892 (Myxozoa: Myxosporea: Myxobolidae). Syst Parasitol 52:43–45
Eszterbauer E (2004) Genetic relationship among gill-infecting Myxobolus species (Myxosporea) of cyprinids: molecular evidence of importance of tissue-specificity. Dis Aquat Organ 58:35–40
FAO (2001) Rapport du Groupe de Travail de la FAO sur l'Évaluation des Petits Pélagiques au Large de l'Afrique Nord-Occidentale, Nouadhibou, Mauritania, 24-31 mars 2001. Rapport sur les pêches. N 657 Rome, Italie
Fiala I, Bartošová P (2010) History of myxozoan character evolution on the basis of rDNA and EF-2 data. BMC Evol Biol 10:228. doi:10.1186/1471-2148-10-228
Fomena A, Bouix G (1997) Myxosporea (Protozoa: Myxozoa) of freshwater fishes in Africa: keys to genera and species. Syst Parasitol 37:161–178
Froese R, Pauly D (2011) FishBase. World Wide Web electronic publication. www.fishbase.org, version (10/2011)
Ganapati PN (1941) On a new myxosporidian Henneguya otolithi n. sp., a tissue parasite from the bulbus arteriosus of two species of fish of the genus Otolithus. Proc Indian Acad Sci 13:135–150
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Hallett SL, Diamant A (2001) Ultrastructure and small-subunit ribosomal DNA sequence of Henneguya lesteri n. sp. (Myxosporea), a parasite of sand whiting Sillago analis (Sillaginidae) from the coast of Queensland, Australia. Dis Aquat Organ 46:197–212
Hudson PJ, Dobson AP, Lafferty KD (2006) Is a healthy ecosystem one that is rich in parasites? TREE 21:381–385
IMROP (2004) Rapport du 5ème groupe de travail IMROP. Evaluation des stocks et aménagement des pêcheries de la ZEEM. Nouadhibou, Mauritanie. 7-17 Décembre 2002. p205
Kent ML, Andree KB, Bartholomew JB, El-Matbouli M, Desser SS, Devlin RH, Feist SW, Hedrick RP, Hoffman RW, Khattra J, Hallett SL, Lester JG, Longshaw M, Palenzuela O, Siddall ME, Xiao C (2001) Recent advances in our knowledge of the Myxozoa. J Eukaryot Microbiol 48:395–413
Kostoïngue B, Diebakate C, Faye N, Toguebaye BS (2001) Presence of Myxosporidea (Myxozoa: Myxosporea) of the genus Henneguya Thelohan, 1892 in freshwater fishes from Chad (Central Africa). Acta Protozool 40:117–123
Kpatcha TK, Faye N, Diebakate C, Fall M, Toguebaye BS (1997) Nouvelles espèces d’Henneguya Thélohan, 1895 (Myxozoa, Myxosporea) parasite des poisons marins du Sénégal: étude en microscopie photonique et électronique. Ann Sci Nat Zool Paris 18:81–91
Laird M (1950) Henneguya vitiensis n.sp., a myxosporidian from a Fijian marine fish, Leiognathus fasciatus (Lacépède, 1803). J Parasitol 36:285–292
Lom J, Dykova I (1992) Protozoan parasite of fishes. Development in aquaculture and fisheries science. Elsevier, Amesterdam
Maddison WP, Maddison DR (2009) Mesquite: a modular system for evolutionary analysis. Version 2.72 http://mesquiteproject.org
Martoja RA, Martoja-Pierson M (1967) Initiation aux Techniques d’histologie animale. Masson et Cie Paris
Miller MA, Holder MT, Vos R, Midford PE, Liebowitz T, Chan L, Hoover P, Warnow T (2009) The CIPRES Portals. CIPRES. 2009-08-04 URL:http://www.phylo.org/sub_sections/portal. Accessed: 2009-08-04. (Archived by WebCite(r) at http://www.webcitation.org/5imQlJeQa)
Moser M, Love MS (1975) Henneguya sebasta sp. n. (Protozoa, myxosporida) from California rockfish, Sebastes spp. J Parasitol 61:481–483
Posada D (2008) jModelTest: phylogenetic model averaging. Mol Biol Evol 25:1253–1256
Redondo MJ, Palenzuela P, Alvarez-Pellitero P (2004) Studies on transmission and life cycle of Enteromyxum scophthalmi (Myxozoa), an enteric parasite of turbot Scophthalmus maximus. Folia Parasitol 51:188–198
Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Sitja-Bobadilla A (2008) Fish immune response to myxozoan parasites. Parasite 15:420–425
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol 57:758–771
Tajdari J, Matos E, Mendonça I, Azevedo C (2005) Ultrastructural morphology of Myxobolus testicularis sp. n., parasite of the testis of Hemiodopsis microlepis (Teleostei: Hemiodontidae) from the NE of Brazil. Acta Protozool 44:377–384
U-taynapun K, Penprapai N, Bangrak P, Mekata T, Itami T, Tantikitti C (2011) Myxobolus supamattayai n. sp. (Myxosporea: Myxobolidae) from Thailand parasitizing the scale pellicle of wild mullet (Valamugil seheli). Parasitol Res 109:81–91
Work TM, Takata G, Whipps CM, Kent ML (2008) A new species of Henneguya (Myxozoa) in the big-eyed scad (Selar crumenophthalmus) from Hawaii. J Parasitol 81:524–529
Yokoyama H, Kawakami H, Yasuda H, Tanaka S (2003) Heneguya lateolabracis sp. n. (Myxozoa: Myxosporea the causative agent of cardiac henneguyosis in Chinese sea bass Lateolabrax sp. Fish Sci 69:1116–1120
Yokoyama H, Itoh N, Tanaka S (2005) Henneguya pagri n. sp. (Myxozoa: Myxosporea) causing cardiac henneguyosis in red sea bream, Pagrus major (Temminck & Schlegel). J Fish Dis 28:479–487
Acknowledgments
This study was partially funded by and is a contribution from the Australian node of the CReefs global research initiative (grant number 209/29), a partnership between the ABRS, BHP Billiton, the Great Barrier Reef Foundation, the Census of Marine Life and the Australian Institute of Marine Science (AIMS). The CReefs Australia Project is generously sponsored by BHP Billiton in partnership with The Great Barrier Reef Foundation, the Australian Institute of Marine Science, the Australian Biological Resources Study and the Alfred P. Sloan Foundation. CReefs is a field program of the Census of Marine Life.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khlifa, S., Miller, T.L., Adlard, R.D. et al. Henneguya mauritaniensis n. sp. (Myxozoa) from the arterial bulb of Pagrus caeruleostictus (Valenciennes, 1830) off Mauritania. Parasitol Res 111, 1287–1294 (2012). https://doi.org/10.1007/s00436-012-2963-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-2963-1