Abstract
To determine the prevalence of bovine giardiasis in Heilongjiang Province in China and to molecularly characterize Giardia duodenalis, feces were collected from 814 dairy and beef cattle ranging in age from 6 days to 9 years. Clinical symptoms of diarrhea were recorded at the time of sampling. The G. duodenalis infection rate in cattle was 5.2 % (42/814) as determined by Lugol's iodine staining. G. duodenalis assemblages and subtypes were genetically diagnosed by sequence analysis of the triosephosphate isomerase (TPI) gene. Three assemblages were identified, representing A (n = 1), B (n = 18), and E (n = 24), with a mixed infection case of assemblages A and E. High heterogeneity was also observed within assemblages B and E at the TPI locus. Among the assemblages, eight subtypes of assemblage B and three subtypes of assemblage E were found to be novel subtypes. Findings on assemblages A and B are of public health importance. The zoonotic potential of bovine giardiasis needs to be further assessed by extensive genetic data of assemblages A and B from humans at the subtype level. The newly found subtypes of assemblages B and E imply that the evaluation of geographically distributed subtypes is of importance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Giardia, one of the most common intestinal protozoan parasites worldwide, can infect humans, domestic livestock, and wildlife. Among the six known Giardia species, only Giardia duodenalis (syn. Giardia intestinalis, Giardia lamblia) has been found to infect humans and mammals. Seven assemblages (A to G) of G. duodenalis have been identified and classified based on their genetic analysis and host specificity. Assemblages A and B are infectious to humans and mammals, while assemblages C to F are mostly seen in animals but are occasionally found in humans (Sprong et al. 2009). Assemblage H was recently identified in marine vertebrates (Lasek-Nesselquist et al. 2010).
Diarrhea is the common clinical symptom in human and animal giardiasis. Giardia is responsible for abdominal cramps, bloating, and malabsorption in children (Carvalho-Costa et al. 2007; Nematian et al. 2008), which result in growth and development retardation, even in asymptomatic cases (Prado et al. 2005). Numerous human outbreaks caused by Giardia via waterborne routes have raised concerns (Karanis et al. 2007). Some studies have revealed that close contact with farm animals is associated with the high prevalence of human giardiasis (Hoque et al. 2002, 2003). Due to the great number of cattle, the large feces output, and the high prevalence of Giardia infection, bovine giardiasis is of great concern. Molecular studies have confirmed that the prevalence of bovine giardiasis ranges from 2.2 to 50.7 % worldwide (Feng and Xiao 2011), and genetic data have revealed that the two common assemblages of G. duodenalis in cattle are assemblages A and E (Xiao and Fayer 2008; Feng and Xiao 2011). Although assemblage E was found to be the predominant genotype in cattle, assemblage A is of importance for public health. Assemblage B can also be isolated from cattle in some countries and regions; in fact, it is the most common species in Canada and New Zealand (Learmonth et al. 2003; Coklin et al. 2007).
In China, the true prevalence and assemblage distribution of G. duodenalis in humans and animals remain unclear. It is estimated that the number of human giardiasis cases is about 28.5 million, with an average infection rate of 2.52 % (Jiang et al. 1997). Large-scale investigations of G. duodenalis in humans showed infection rates of 2.55 % (620/24348) in Henan Province and 6.25 % (102/1632) in Sichuan Province (Li 2003; Su et al. 2010). In animals, infection rates of G. duodenalis in Henan have been reported to be 5.27 % for rabbits and 20.73 % for rodents (Qi et al. 2010; Shi et al. 2010). Molecular methods have been used in several studies, and it has been verified that assemblages A and B have been isolated from humans (Yong et al. 2000; Chen et al. 2001; Lu et al. 2002; Wang et al. 2011) and an assemblage A and an assemblage E have been found in a dog and a calf, respectively (Xiao et al. 2006; Zhu et al. 2011). It is essential to understand the potential transmission of cross-assemblages of G. duodenalis between humans and animals in China. An important aspect of the giardiasis epidemiology is to determine the zoonotic potential of G. duodenalis infections in animals. This would contribute to the assessment of the human giardiasis burden of animal origin. Assemblages A and B were recently found in wastewater plants in Harbin City (Liu et al. 2011). To trace G. duodenalis contamination sources in wastewater, the prevalence of bovine giardiasis was investigated in the present study, G. duodenalis isolates were identified molecularly, and the genetic variation of assemblages was analyzed. The zoonotic potential of G. duodenalis genotypes and subtypes to humans in the area was further assessed by aligning obtained sequences with those from GenBank.
Materials and methods
Specimen collection
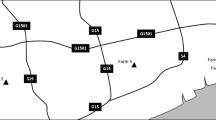
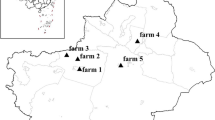
Between July 2009 and May 2011, 814 fresh fecal specimens were randomly collected from the rectum of cattle (605 from dairy cattle and 209 from beef cattle), with 10–15 g from the preweaned calves and 30–50 g from the other cattle on seven farms (respectively assigned as farms 1 to 7) located in Heilongjiang Province, China. Their ages ranged from 6 days to 9 years. Diarrheic or nondiarrheic clinical symptoms of all the cattle were recorded at the time of sampling. During specimen collection, all animal work followed guidelines in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals and approved by the Animal Ethical Committee of Harbin Medical University. Microscopic examinations were performed on all fecal specimens within 48 h after collection. Fats were removed by formalin–ethyl acetate sedimentation from approximately 10 g fecal specimens obtained from the preweaned calves prior to iodine wet mount staining. According to fecal consistency, the other cattle fecal specimens of approximately 100 mg were directly used to smear two or three slides for each specimen for iodine wet mount staining. All G. duodenalis-positive specimens were stored in 2.5 % potassium dichromate solution at 4°C prior to DNA extraction.
DNA extraction
After washing the G. duodenalis-positive fecal specimens twice with distilled water, genomic DNA was directly extracted from the fecal pellet using a QIAamp DNA Stool Mini Kit (QIAgen, Hilden, Germany) according to manufacturer-recommended procedures. DNA was eluted in 200 μL of AE and stored at −20°C prior to PCR analysis.
G. duodenalis genotyping and subtyping
G. duodenalis cysts in the specimens were molecularly diagnosed by nested PCR amplification of about a 530 bp fragment at the triosephosphate isomerase (TPI) locus (Sulaiman et al. 2003). All secondary PCR products were purified and directly sequenced to identify genotypes and subtypes of G. duodenalis-positive specimens. Each specimen was analyzed at least twice by PCR.
DNA sequence analysis
All secondary PCR products were sequenced on an ABI PRISMTM 3730 DNA Analyzer (Applied Biosystems, USA), using the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA). Sequence accuracy of PCR products was confirmed by sequencing directly in both directions and by sequencing a new PCR product as necessary. Nucleotide sequences obtained in the present study were aligned with G. duodenalis reference nucleotide sequences from GenBank and analyzed using ClustalX 1.83. Representative nucleotide sequences obtained were deposited in the GenBank database under accession numbers JN162347 to JN162361.
Results
Prevalence of G. duodenalis on cattle farms
In the present study, 814 bovine fecal specimens from seven farms located in Heilongjiang Province were examined by microscopy. Among the specimens, 42 were positive for G. duodenalis. The overall prevalence of bovine giardiasis was 5.2 % (42/814), with 16.1 % (22/137) for less than 2-month-old calves, 5.2 % (16/307) for 3- to 11-month-old heifers, 1.5 % (3/199) for 12- to 24-month-old cattle, and 0.6 % (1/171) for more than 24-month-old cattle. The infection rates were 7.8 % (12/154), 11.2 % (12/107), 2.9 % (4/136), 4.0 % (5/124), 3.6 % (3/84), 0.7 % (1/143), and 7.6 % (5/66), respectively, on farms 1 to 7 (Table 1).
Assemblage distribution of G. duodenalis on cattle farms
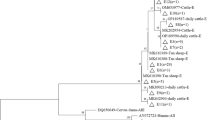
From the 42 G. duodenalis-positive specimens, 43 TPI gene sequences were obtained due to one mixed infection case. The sequences obtained were aligned with reference sequences and identified as three G. duodenalis assemblages: one was assemblage A, 18 were assemblage B, and 24 were assemblage E. The assemblage percentages in the study area were 2.3 % for assemblage A, 41.9 % for assemblage B, and 55.8 % for assemblage E; assemblage E was more prevalent than assemblage B in cattle in the study area. The distribution of assemblage E was the most widespread as it was found on all seven farms, while assemblages A and B were found only on one farm and five farms, respectively (Table 1). Assemblage A was positively identified in only one 6-month-old calf with assemblage E.
Subtypes of assemblages B and E
Both G. duodenalis assemblages B and E showed high polymorphism at the TPI locus in the present study. The intragenotypic diversity of G. duodenalis assemblage B was observed in the 18 TPI nucleotide sequences obtained. Using the sequence AY368171 as a reference sequence, single nucleotide polymorphisms were found in the nine representative sequences obtained (respectively named as subtypes B-I to B-IX for convenient description), with one- to five-base variations noted at 18 different nucleotide sites (Table 2). Among the subtypes, B-I was identical to assemblage B isolates from rabbits (HQ397719) and wastewater (HQ603781) in the study area. It was also the most common subtype in the study area, accounting for a high percentage of 33.3 % (6/18). The remaining eight subtypes (B-II to B-IX) have never been described. The percentages of assemblage B positive isolates were 16.7 % (3/18) for B-VIII, 11.1 % (2/18) for B-II and B-IV, and 5.6 % (1/18) for B-III, B-V, B-VI, B-VII, and B-IX.
Sequence analysis of the TPI gene of G. duodenalis revealed the presence of six subtypes (respectively named as E-I to E-VI for convenient description) in 24 assemblage E isolates. Using the sequence EF654682 as a reference sequence, genetic polymorphism was observed with one- to two-base variations at seven nucleotide sites (Table 3); 17 of them, including subtypes E-I (n = 9), E-II (n = 4), and E-III (n = 4), had 100 % similarity with the previous cattle-derived sequences; EF654682, AY228646, and AY655706 for subtype E-I; AY655705, EF654692, and AY228645 for subtype E-II; and EF654684 and AB569406 for subtype E-III. Among the subtypes, E-I was the most common, accounting for the highest percentage of 37.5 % (9/24). The percentages of the remaining seven sequences representing three new subtypes were 16.7 % (4/24) for E-IV, 4.2 % (1/24) for E-V, and 8.3 % (2/24) for E-VI. High diversities of assemblages B and E were commonly found on some individual farms (Table 4).
Correlation between diarrhea and G. duodenalis assemblages
Among the 42 G. duodenalis-positive specimens, 16 diarrheic cases and 26 nondiarrheic cases were observed. Of the diarrheic cases, seven were identified as assemblage B (7/16, 43.8 %) and nine were identified as assemblage E (9/16, 56.3 %). Of the nondiarrheic cases, 11 were identified as assemblage B (11/26, 42.3 %) and 15 were identified as assemblage E (15/26, 57.7 %). No obvious correlation was found between G. duodenalis assemblages and diarrhea in cattle (X 2 = 0.0084, P = 0.9269).
Discussion
The present study is the first to report the prevalence and molecular characterization of G. duodenalis in cattle in Heilongjiang Province, China. Infection rates of G. duodenalis in cattle vary significantly between epidemiological areas as well as age groups of animals. The average infection rate of G. duodenalis was 5.2 % in the study area, relatively lower than those in Portugal (9.0 %) (Mendonca et al. 2007), India (12.2 %) (Khan et al. 2011), and USA (26.6 %) (Trout et al. 2007). The highest infection rate (16.1 %) was found in preweaned calves, similar to the findings from previous studies. Giardia tends to be more prevalent in young animals than in adults. In a longitudinal study of preweaned calves in Western Australia, Giardia cysts were detected in 89 % of specimens (Becher et al. 2004). An infection rate of 49.0 % was described in an investigation on the prevalence of G. duodenalis in less than 6-month-old calves in Norway (Hamnes et al. 2006). In another longitudinal study of dairy calves in USA, the highest prevalence of infection, with 25 of 30 calves infected, was found in calves at 4 and 5 weeks of age (Santín et al. 2009). Due to the lack of epidemiological data on bovine giardiasis, we could not find the true reasons for the low prevalence in the current study areas. Infection rates are complicated and often related to many factors, including the sensitivity and specificity of diagnostic techniques, the size and structure of specimens, and the age and health status of the infected host. Compared to molecular methods, traditional microscopy methods have yielded false negatives for fecal specimens with a low intensity of G. duodenalis oocysts (Traub et al. 2009). In the present study, the low prevalence observed might be mainly related to the large percentage of mature and healthy animals in the sample population as well as the morphology method of detection used.
In the present study, of 43 positive specimens, 41.9 and 55.8 % were of assemblages B and E, respectively. Assemblage E was more prevalent and widespread than assemblage B in cattle. Assemblage B was detected on five farms, and assemblage E was detected on all the seven farms (Table 1). One assemblage A isolate (belonging to A-I) was identified from a specimen mixed with assemblage E. Previous molecular epidemiological studies in Denmark, USA, Canada, and Australia have shown that assemblage E is the predominant G. duodenalis genotype in cattle, with a percentage of 80–100 % out of all G. duodenalis-positive specimens (Appelbee et al. 2003; Becher et al. 2004; Trout et al. 2006, 2007; Langkjaer et al. 2007; Feng et al. 2008). Our findings imply that the investigated farms were polluted by assemblage E, and the cattle posed a serious threat to susceptible animals, such as other cattle, sheep, goats, and pigs. Some studies have revealed that G. duodenalis infections have a significant impact on growth and performance of ruminants, but did not demonstrate the relationship between the assemblages and virulence (O'Handley and Olson 2006). Little information is available on the severity of animal giardiasis caused by assemblage E. While assemblage E has only been isolated from three Egyptian giardiasis patients (Foronda et al. 2008), its host specificity/range still needs to be confirmed by further study data.
Assemblage B, one of the two major assemblages causing human giardiasis, exhibits a broad host range, including cattle, sheep, pigs, horses, dogs, cats, and rabbits (Feng and Xiao 2011). Only individual studies have found a relatively high percentage of assemblage B in cattle in Canada (58.3 %, 35/60) and New Zealand (45.8 %, 22/48) (Learmonth et al. 2003; Coklin et al. 2007). Our findings were similar to the above two studies. Variations in the distributions and percentages of G. duodenalis assemblages on cattle farms might be related to the transmission dynamics of G. duodenalis. The reasons for the predominance of G. duodenalis assemblages in cattle are still unclear. While no strong evidence has supported the direct zoonotic transmission of G. duodenalis from animals to humans, case-control studies have shown that the increased infection rate of human giardiasis is related to farm animal contact (Hoque et al. 2002, 2003). Thus, the cattle infected with assemblage B pose significant threats to local inhabitants and are of public health importance.
Assemblage A, another major assemblage of G. duodenalis found in humans, has also been found in cattle and is common in some countries and regions, accounting for the percentage of 22.4 % in Europe (Sprong et al. 2009) and 50 % in Italy (Lalle et al. 2005). In New Zealand, assemblage A was found to be the predominant G. duodenalis genotype (54.2 to 87.5 %) with an absence of assemblage E in cattle (Hunt et al. 2000; Learmonth et al. 2003; Winkworth et al. 2008). However, in the present study, only one subtype A-I isolate from the G. duodenalis-positive specimens was detected. In comparison with other regions of the world, the lower frequency of subtype A-I appeared to be the endemic characterization of the G. duodenalis population structure in Heilongjiang. Possibly, this finding was related to the limited number of specimens in the present work. Although humans are more commonly infected with subtype A-II than subtype A-I, the public health significance of both subtypes needs to be assessed by the data from extensive investigations with a large number of specimens from animals and humans, for subtype A-I has been detected in humans in some areas (Xiao and Fayer 2008; Feng and Xiao 2011). The true prevalence of subtype A-I in humans in the study area remains unclear due to the lack of the data on human giardiasis.
High genetic polymorphism was observed within both assemblages B and E by subtyping TPI sequences in the present study. The intragenotype diversity of G. duodenalis assemblage B has been confirmed by many reports on human giardiasis (Feng and Xiao 2011; Wang et al. 2011). In China, six human isolates of assemblage B were recently found to belong to six distinct subtypes (Wang et al. 2011). However, no reports have described the genetic diversity of cattle-derived isolates of G. duodenalis assemblage B in TPI sequences. Based on the β-giardin gene, sequence heterogeneity was found in assemblage B isolates in humans and cattle in Italy (Lalle et al. 2005). In the present study, nine subtypes were obtained from 18 assemblage B isolates. The most common subtype B-I (JN162353) (33.3 %, 6/18) showed 100 % similarity with the assemblage B sequences from rabbits (HQ397719) and wastewater (HQ603781) in the study area. Homology analysis indicated that there was no host specificity associated with subtype B-I. The true host range of subtype B-I could be defined by subtyping a large number of assemblage B isolates from different areas and hosts. We could not draw definitive conclusions because of the lack of human and other animal giardiasis data. The remaining eight subtypes (B-II to B-IX) were novel and low in incidence; their sequences based on TPI were not identical to any known subtype of assemblage B. Subtypes of assemblage B might represent the endemic genetic characteristics of G. duodenalis. The risk of cattle with assemblage B infecting humans needs to be assessed in subsequent studies by molecular epidemiology and genetics of giardiasis in local inhabitants.
Few studies have described the intragenotype diversity of assemblage E in cattle (Feng et al. 2008). In the present study, six subtypes of assemblage E were found at the TPI locus, three of which showed a large percentage (70.8 %, 17/24) in the study area, including E-I (n = 9), E-II (n = 4), and E-III (n = 4). These subtypes have also been found in cattle in other studies (Sulaiman et al. 2003; Trout et al. 2004; Feng et al. 2008) and are worldwide with no apparent differences in geographical distribution. The remaining three novel subtypes, defined as E-IV (n = 4), E-V (n = 1), and E-VI (n = 2), have never been reported and might be subtypes with characteristic geographical distributions. More data from large-scale investigations regarding the new subtypes will contribute to the study of geographical distributions. Moreover, the transmission dynamics and public health significance of the new subtypes need to be studied extensively.
Clinical symptoms of giardiasis are highly variable, mainly ranging from asymptomatic infection to acute or chronic diarrhea. The correlation between clinical symptoms and G. duodenalis assemblages has always been disputed. Some studies on human giardiasis reveal that assemblage B is associated with clinical symptoms (Gelanew et al. 2007; Pelayo et al. 2008; Mohammed Mahdy et al. 2009; Al-Mohammed 2011). In the present study, we only recorded the presence or absence of diarrhea, the most common clinical symptom of cattle infected with G. duodenalis, but did not examine other pathogens existing in the intestinal tract. The percentages of diarrheic cases were 43.8 % (7/16) for assemblage B and 53.6 % (9/16) for assemblage E. No obvious correlations were found between diarrhea and the G. duodenalis assemblages. Occurrence of diarrhea and the severity of giardiasis in animals are complicated, often involving the immune status of the infected host, the virulence of different G. duodenalis assemblages, the infective dose of the parasite, and the presence or absence of other intestinal pathogens. It is possible that they were also related with the different subtypes within assemblages B and E. Also, changes in diet can affect fecal consistency.
In conclusion, our findings showed that assemblages A, B, and E of G. duodenalis were found in cattle in the study area; assemblage E was more prevalent and widespread than assemblage B. Cattle infected with assemblage B are highly significant to public health. To better understand the transmission dynamics of G. duodenalis in China and assess the human burden of giardiasis caused by animals, large-scale molecular epidemiological investigations of humans and animals should be conducted, and the relationships between clinical symptoms and assemblages need to be studied at the subtype level.
References
Al-Mohammed HI (2011) Genotypes of Giardia intestinalis clinical isolates of gastrointestinal symptomatic and asymptomatic Saudi children. Parasitol Res 108:1375–1381
Appelbee AJ, Frederick LM, Heitman TL, Olson ME (2003) Prevalence and genotyping of Giardia duodenalis from beef calves in Alberta, Canada. Vet Parasitol 112:289–294
Becher KA, Robertson ID, Fraser DM, Palmer DG, Thompson RC (2004) Molecular epidemiology of Giardia and Cryptosporidium infections in dairy calves originating from three sources in Western Australia. Vet Parasitol 123:1–9
Carvalho-Costa FA, Goncalves AQ, Lassance SL, Silva Neto LM, Salmazo CA, Bóia MN (2007) Giardia lamblia and other intestinal parasitic infections and their relationships with nutritional status in children in Brazilian Amazon. Rev Inst Med Trop Sao Paulo 49:147–153
Chen XN, Lu SQ, Li JH, Wang FY, Wang NX, Wang F (2001) Isolation of Hebei isolates of Giardia lamblia and investigation on their genotypes. Chin J Parasitic Dis Con 14:100–102 (in Chinese)
Coklin T, Farber J, Parrington L, Dixon B (2007) Prevalence and molecular characterization of Giardia duodenalis and Cryptosporidium spp. in dairy cattle in Ontario, Canada. Vet Parasitol 150:297–305
Feng Y, Ortega Y, Cama V, Terrel J, Xiao L (2008) High intragenotypic diversity of Giardia duodenalis in dairy cattle on three farms. Parasitol Res 103:87–92
Feng Y, Xiao L (2011) Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev 24:110–140
Foronda P, Bargues MD, Abreu-Acosta N, Periago MV, Valero MA, Valladares B, Mas-Coma S (2008) Identification of genotypes of Giardia intestinalis of human isolates in Egypt. Parasitol Res 103:1177–1181
Gelanew T, Lalle M, Hailu A, Pozio E, Caccio SM (2007) Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Trop 102:92–99
Hamnes IS, Gjerde B, Robertson L (2006) Prevalence of Giardia and Cryptosporidium in dairy calves in three areas of Norway. Vet Parasitol 140:204–216
Hoque ME, Hope VT, Kjellstrom T, Scragg R, Lay-Yee R (2002) Risk of giardiasis in Aucklanders: a case-control study. Int J Infect Dis 6:191–197
Hoque ME, Hope VT, Scragg R, Kjellstrom T (2003) Children at risk of giardiasis in Auckland: a case-control analysis. Epidemiol Infect 131:655–662
Hunt CL, Ionas G, Brown TJ (2000) Prevalence and strain differentiation of Giardia intestinalis in calves in the Manawatu and Waikato regions of North Island, New Zealand. Vet Parasitol 91:7–13
Jiang Z, Xu L, Xu S, Li B, Fang Y, Li Y, Liu C, Xu X, Chen S, Zhang X (1997) Epidemiology of giardiasis in China. Chin J Public Health 13:407–408 (in Chinese)
Karanis P, Kourenti C, Smith H (2007) Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J Water Health 5:1–38
Khan SM, Debnath C, Pramanik AK, Xiao L, Nozaki T, Ganguly S (2011) Molecular evidence for zoonotic transmission of Giardia duodenalis among dairy farm workers in West Bengal, India. Vet Parasitol 178:342–345
Lalle M, Pozio E, Capelli G, Bruschi F, Crotti D, Cacciò SM (2005) Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int J Parasitol 35:207–213
Langkjaer RB, Vigre H, Enemark HL, Maddox-Hyttel C (2007) Molecular and phylogenetic characterization of Cryptosporidium and Giardia from pigs and cattle in Denmark. Parasitology 134:339–350
Lasek-Nesselquist E, Welch DM, Sogin ML (2010) The identification of a new Giardia duodenalis assemblage in marine vertebrates and a preliminary analysis of G. duodenalis population biology in marine systems. Int J Parasitol 40:1063–1074
Learmonth JJ, Ionas G, Pita AB, Cowie RS (2003) Identification and genetic characterization of Giardia and Cryptosporidium strains in humans and dairy cattle in the Waikato Region of New Zealand. Water Sci Technol 47:21–26
Li Z (2003) Investigation of Giardia lamblia in humans in Huaying. Sichuan Province. J Practical Parasitic Dis 1:34–35 (in Chinese)
Liu A, Ji H, Wang E, Liu J, Xiao L, Shen Y, Li Y, Zhang W, Ling H (2011) Molecular identification and distribution of Cryptosporidium and Giardia duodenalis in raw urban wastewater in Harbin. China Parasitol Res. doi:10.1007/s00436-011-2333-4
Lu SQ, Li JY, Wen JF, Shen JZ, Zhu H, Wang ZY, Li JH, Wang FY, Guo ZZ (2002) Study of Chinese Giardia lamblia isolates culture, genotype and nucleus. J Med Res 31:19–20 (in Chinese)
Mendonca C, Almeida A, Castro A, de Lurdes DM, Soares S, da Costa JM, Canada N (2007) Molecular characterization of Cryptosporidium and Giardia isolates from cattle from Portugal. Vet Parasitol 147:47–50
Mohammed Mahdy AK, Surin J, Wan KL, Mohd-Adnan A, Al-Mekhlafi MS, Lim Y (2009) Giardia intestinalis genotypes: risk factors and correlation with clinical symptoms. Acta Trop 112:67–70
Nematian J, Gholamrezanezhad A, Nematian E (2008) Giardiasis and other intestinal parasitic infections in relation to anthropometric indicators of malnutrition: a large, population-based survey of schoolchildren in Tehran. Ann Trop Med Parasitol 102:209–214
Pelayo L, Nunez FA, Rojas L, Hansen EF, Gjerde B, Wilke H, Mulder B, Robertson L (2008) Giardia infections in Cuban children: the genotypes circulating in a rural population. Ann Trop Med Parasitol 102:585–595
Prado MS, Cairncross S, Strina A, Barreto ML, Oliveira-Assis AM, Rego S (2005) Asymptomatic giardiasis and growth in young children; a longitudinal study in Salvador, Brazil. Parasitology 131:51–56
O'Handley RM, Olson ME (2006) Giardiasis and cryptosporidiosis in ruminants. Vet Clin North Am Food Anim Pract 22:623–643
Qi M, Xi J, Lv C, Zhao Z, Jian F, Zhang L, Ning C (2010) Survey of Giardia infection in rodent pets. Chin J Zoonoses 26:613–614 (in Chinese)
Santín M, Trout JM, Fayer R (2009) A longitudinal study of Giardia duodenalis genotypes in dairy cows from birth to 2 years of age. Vet Parasitol 162:40–45
Shi K, Ren X, Wang Q, Qi M, An C, Chen L, Jian F, Zhang L, Ning C (2010) Survey of intestinal parasites in rabbits in Henan Province. Anim Husban Vet Med 42:68–70 (in Chinese)
Sprong H, Caccio SM, van der Giessen JW (2009) Identification of zoonotic genotypes of Giardia duodenalis. PLoS Negl Trop Dis 3:e558
Su Y, He L, Yan Q, Liu H, Zhang H (2010) Survey of current prevalence of human intestinal protozoa in Henan Province. Chin Trop Med 10:398–399 (in Chinese)
Sulaiman IM, Fayer R, Bern C, Gilman RH, Trout JM, Schantz PM, Das P, Lal AA, Xiao L (2003) Triosephosphate isomerase gene characterization and potential zoonotic transmission of Giardia duodenalis. Emerg Infect Dis 9:1444–1452
Traub RJ, Inpankaew T, Reid SA, Sutthikornchai C, Sukthana Y, Robertson ID, Thompson RC (2009) Transmission cycles of Giardia duodenalis in dogs and humans in Temple communities in Bangkok—a critical evaluation of its prevalence using three diagnostic tests in the field in the absence of a gold standard. Acta Trop 111:125–132
Trout JM, Santin M, Greiner EC, Fayer R (2004) Prevalence of Giardia duodenalis genotypes in pre-weaned dairy calves. Vet Parasitol 124:179–186
Trout JM, Santin M, Greiner EC, Fayer R (2006) Prevalence and genotypes of Giardia duodenalis in 1–2 year old dairy cattle. Vet Parasitol 140:217–222
Trout JM, Santin M, Fayer R (2007) Prevalence of Giardia duodenalis genotypes in adult dairy cows. Vet Parasitol 147:205–209
Wang R, Zhang X, Zhu H, Zhang L, Feng Y, Jian F, Ning C, Qi M, Zhou Y, Fu K, Wang Y, Sun Y, Wang Q, Xiao L (2011) Genetic characterizations of Cryptosporidium spp. and Giardia duodenalis in humans in Henan, China. Exp Parasitol 127:42–45
Winkworth CL, Learmonth JJ, Matthaei CD, Townsend CR (2008) Molecular characterization of Giardia isolates from calves and humans in a region in which dairy farming has recently intensified. Appl Environ Microbiol 74:5100–5105
Xiao L, Fayer R (2008) Molecular characterisation of species and genotypes of Cryptosporidium and Giardia and assessment of zoonotic transmission. Int J Parasitol 38:1239–1255
Xiao S, Li G, Wang B, Yang J, Li W (2006) Molecular identification of the first isolate of calf-derived Giardia from mainland of China. Chin J Zoonoses 22:861–863
Yong TS, Park SJ, Hwang UW, Yang HW, Lee KW, Min DY, Rim HJ, Wang Y, Zheng F (2000) Genotyping of Giardia lamblia isolates from humans in China and Korea using ribosomal DNA sequences. J Parasitol 86:887–891
Zhu H, Li G, Zhang P, Li J, Li J, Lin Z (2011) Genotype identification of Giardia from dogs in Guangdong. Chin Vet Sci 2:143–147
Acknowledgments
This work was supported by the Natural Science Foundation of Heilongjiang Province (grant D200628), China and the Chinese Special Program for Scientific Research of Public Health (No. 200802012).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Aiqin Liu and Xiaoyun Zhang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, A., Zhang, X., Zhang, L. et al. Occurrence of bovine giardiasis and endemic genetic characterization of Giardia duodenalis isolates in Heilongjiang Province, in the Northeast of China. Parasitol Res 111, 655–661 (2012). https://doi.org/10.1007/s00436-012-2883-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-012-2883-0