Abstract
Giardia duodenalis is a common parasitic protozoan in human and animals. Epidemiological and molecular data are available from dairy cattle in many industrialized countries, but information on genetic diversity at multiple genetic loci is limited, especially in pre-weaned dairy calves. In this study, 818 fecal specimens were collected from five dairy cattle farms located in suburbs of Shanghai, China, with two to five samplings per farm. G. duodenalis assemblages and subtypes were determined using multilocus genotyping (MLG) at the β-giardin (bg), glutamate dehydrogenase (gdh), and triosephosphate isomerase (tpi) loci. The overall prevalence was 60.1% (492/818) combining data from the three genetic loci. Three G. duodenalis assemblages were detected, including E (n = 482), A (n = 5), and B (n = 1), with the concurrence of A and E in a few animals (n = 4). Intra-genotypic sequence diversity was high for assemblage E, showing 12, 13, and 17 subtypes at the bg, gdh, and tpi loci, including four, six, and eight new subtypes, respectively. All dominant subtypes (E3, E2, and E8 at the bg locus; E1 and E3 at the gdh locus; and E11 and E3 at the tpi locus) were detected on all farms at most sampling occasions, and only limited differences in subtype distribution were observed among five farms. Altogether, 58 assemblage E MLGs were identified, all of which had not been reported before, and seven (MLG-E1–MLG-E7) were each seen on multiple farms. These results indicate a high occurrence of G. duodenalis in dairy calves, the existence of high genetic heterogeneity of assemblage E on five farms, and frequent exchange of parasite populations among farms within a small geographic area. The clinical and epidemiologic significance of these observations warrants further investigations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Giardia duodenalis is a common gastrointestinal protozoan and a causative agent of diarrhea in human and animals (Feng and Xiao 2011). Cattle, especially pre-weaned calves, are important G. duodenalis hosts because of the high prevalence of infection and intensive excretion of cysts. Concerns have also been raised about zoonotic potentials of G. duodenalis from cattle (Ryan and Caccio 2013).
Molecular analyses have been used in genotyping G. duodenalis (Thompson and Ash 2016). Currently, eight distinct assemblages (A–H) of G. duodenalis with different host range have been identified. Assemblages A and B infect humans and various mammals, thus are considered zoonotic genotypes, while the remaining assemblages are mostly host-specific (Feng and Xiao 2011). Numerous molecular characterizations of G. duodenalis have shown that assemblage E is the dominant genotype in cattle worldwide (Asher et al. 2016; Liu et al. 2015; Qi et al. 2016; Santin et al. 2012). In contrast, assemblages A and B have been reported in only a few cattle in most studies. Nevertheless, several studies conducted in China, New Zealand, Italy, and Canada have shown a common occurrence of assemblages A and B in dairy calves (Coklin et al. 2007; Lalle et al. 2005; Liu et al. 2015; Wang et al. 2014; Winkworth et al. 2008).
Most of the earlier studies had characterized G. duodenalis at individual genetic loci. Inconsistency in genotyping results have sometimes been observed among genetic loci (Feng and Xiao 2011). To improve the understanding of the genetic heterogeneity and zoonotic potential of G. duodenalis, the use of multilocus genotyping (MLG) is now recommended (Caccio et al. 2008). This approach has been used in recent studies of G. duodenalis infections in humans and animals (Karim et al. 2014; Lebbad et al. 2011; Scalia et al. 2016; Solarczyk et al. 2012; Ye et al. 2015).
MLG analysis has not been widely used in characterizations of G. duodenalis infections in pre-weaned dairy calves. A few studies conducted thus far are mostly from China (Qi et al. 2016; Wang et al. 2014, 2016b; Zhang et al. 2016). A total of 58 assemblage E MLGs and 5 assemblage A MLGs were observed among 174 G. duodenalis isolates characterized. Most of the assemblage E MLGs appear to be geographically isolated. As these studies were conducted in distant areas with very different animal management and none involved longitudinal sampling, the significance of these observations in giardiasis epidemiology is not clear. Elsewhere, the MLG approach was used in only one study of G. duodenalis in cattle in Ethiopia, but the sequence data generated were not used in the differentiation of MLGs within assemblage E (Wegayehu et al. 2016).
In this study, the MLG analysis was used in the characterizations of G. duodenalis isolates from pre-weaned calves on five dairy farms that are located in two neighboring suburb districts of Shanghai, China. Inter-farm transmission of pathogens and temporal changes in parasite populations on study farms were assessed by comparing assemblage E MLGs among farms and between sampling points. Results of the study have shown a high occurrence of G. duodenalis in dairy calves, high genetic heterogeneity of assemblage E in a small geographic area, and possible inter-farm transmission of G. duodenalis infection.
Materials and methods
Specimen collection
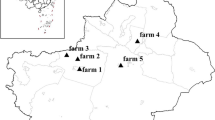
During April 2015 to March 2016, 818 fecal specimens were collected from Holstein dairy cattle on five dairy cattle farms located in the suburb of Shanghai, China, including the Fengxian District (farms 1, 2, 3, and 4) and Jinshan District (farm 5) (Table 1, Fig. 1). These farms were owned by one dairy enterprise and used the same animal management practices. Farm 5 differed from others in having a large, modern facility. Farm 3 was sampled five times at 2–3-month intervals, followed by four times for farm 1 and twice for farms 2, 4, and 5 (Table 1). Only pre-weaned dairy calves (age ≤9 weeks old) were included in this study. Fecal specimens were collected from the rectum using disposable gloves, transferred into 50-ml centrifuge tubes, and stored in 2.5% potassium dichromate at 4 °C.
DNA extraction
DNA was extracted from 200 mg of fecal specimens using the Fast DNA SPIN Kit for Soil (MP Biomedicals, Santa Ana, CA), after the specimens have been washed twice with distilled water by centrifugation. The extracted DNA was stored at −20 °C until being analyzed by PCR.
Detection of G. duodenalis by PCR
For the detection of G. duodenalis, partial β-giardin (bg), glutamate dehydrogenase (gdh), and triosephosphate isomerase (tpi) genes were amplified by nested PCR. The primers used were as previously reported (Caccio et al. 2008; Lebbad et al. 2010). The annealing temperatures of bg in primary and secondary PCR were 65 and 55 °C, respectively, compared to 54 and 58 °C for tpi, and 55 and 55 °C for gdh. The master mix of 50-μL bg and gdh PCR contained 1× PCR Master Mix (Thermo Scientific, Shenzhen, China), 0.25 μM (for primary PCR), or 0.5 μM (for secondary PCR) primers, 500 μg bovine serum albumin (Sigma-Aldrich, St. Louis, MO) (in primary PCR only), and 1 μL of DNA template (for primary PCR) or 2 μL of primary PCR products (for secondary PCR). The 25-μL tpi PCR reaction contained 1× PCR Buffer (TaKaRa, Dalian, China), 200 μM dNTP (TaKaRa), 0.4 μM primers, 1 U rTaq DNA polymerase (TaKaRa), 500 μg bovine serum albumin (in primary PCR only), and 1 μL of DNA template (for primary PCR) or 2 μL of primary PCR products (for secondary PCR). Duplicate PCR analyses were conducted for each specimen at each locus. The secondary PCR products were examined by electrophoresis in 1.5% agarose gels stained with ethidium bromide.
Genotyping and subtyping G. duodenalis by sequence analysis
All positive secondary PCR products were sequenced in both directions on an ABI 3730 autosequencer (Applied Biosystems, Foster City, CA) at the BioSune Biotechnology Company (Shanghai, China). To determine the genotypes or subtypes of G. duodenalis, sequences obtained were assembled using ChromasPro 1.5 (http://www.technelysium.com.au/ChromasPro.html), edited using BioEdit 7.1 (http://www.mbio.ncsu.edu/BioEdit/bioedit.html), and aligned with each other and reference sequences of each genetic target using Clustal X 1.81 (http://www.clustal.org/). Representative nucleotide sequences generated in this study were deposited in GenBank under accession numbers KY432834-KY432852, KY710741-KY710749, and KY769089-KY769103.
The nomenclature system used in previous reports was used in naming assemblage E subtypes at each genetic locus (Feng et al. 2008; Wang et al. 2014, 2016a). Novel subtypes identified in this study and published sequences with no subtype designations were named accordingly as E4–E12 at the bg locus, E8–E17 at the gdh locus, and E12–E24 at the tpi locus. The distribution of subtypes at three genetic loci was displayed by heatmap using Rstudio 0.99.447 (http://www.rstudio.com/Heatmap.html). Specimens that were successfully subtyped at all three loci were included in MLG analysis of G. duodenalis, with MLG types identified. The evolution of assemblage E MLGs was assessed by using eBURST 3.0 (http://eBURST.mlst.net/).
Statistical analysis
The χ 2 test implemented in SPSS version 17.0 (SPSS Inc., Chicago, IL, USA) was used to compare differences in infection rates between farms or age groups. Differences with P < 0.05 were considered significant.
Results
Occurrence of G. duodenalis on five farms
Based on PCR results at any of the three genetic loci, 492 (60.1%) of the 818 fecal specimens analyzed were positive for G. duodenalis. The infection rates ranged from 45.9% (95/207) to 72.5% (29/40). The lowest infection rate of 45.9% was seen on farm 5, which was significantly lower than infection rates of 67.3% on farm 3 and 72.5% on farm 4 (P < 0.01, Table 1).
By age, G. duodenalis infection was first detected in 1-week-old calves. Infection rates increased rapidly afterwards, reached near 60% by 3 weeks of age and maintained at high levels until weaning (Fig. 2). The difference infection rates between 1-week-old calves and older calves was significant (P < 0.01).
G. duodenalis genotypes and subtypes
Of the 492 G. duodenalis-positive specimens, 448 were positive in bg PCR, 300 in gdh PCR, and 239 in tpi PCR. Three genotypes were found among the 492 genotyped specimens, including assemblages E (482), A (5), and B (1), with four specimens having both assemblages A and E (Table 1).
Sequence analysis at each locus revealed a high genetic diversity of assemblage E. At the bg locus, assemblage E sequences had one to three single nucleotide substitutions (SNPs) at 11 positions, compared with reference sequence DQ116624. Altogether, 12 subtypes of assemblage E were observed, including eight subtypes having sequences identical to those in GenBank: E1 (KT922247), E2 (KP635113), E3 (KP635114), E5 (EU189361), E6 (EU726982), E8 (KR075939), E9 (KT369775), and E11 (DQ116622), which were from cattle and sheep (Di Giovanni et al. 2006; Qi et al. 2015; Ruiz et al. 2008; Wang et al. 2016a; Wegayehu et al. 2016; Ye et al. 2015). The remaining four represented novel subtypes E4, E7, E10, and E12 (KY432834-KY432837). Double peaks were observed in the sequence trace files from 47 assemblage E specimens at nucleotide positions 117, 222, 300, and 467. The sequence variations among all subtypes at the bg locus are summarized in Table S1.
Using KF843923 and DQ157270 as the references, 13 and 17 subtypes of assemblage E were identified at the gdh and tpi loci, respectively, including six novel subtypes (KY432838–KY432843) at the gdh locus and eight novel subtypes (KY432845–KY432851) at the tpi locus (Tables S2 and S3). Double peaks were identified in sequence trace files from 14 assemblage E at the gdh locus. No double peaks were present in trace files at the tpi locus. The dominant subtypes E11 and E3 at the tpi locus were common subtypes in other bovine studies in the USA and China (Feng et al. 2008; Liu et al. 2015; Wang et al. 2016b).
Among the nine specimens positive for assemblage A, two sub-assemblages (AI and AII) were present. Within sub-assemblage AI, subtype A1 was found in one, one, and four specimens at the bg, gdh, and tpi loci, respectively. It was identical to sequence AB469365 at the bg locus, AB159795 at the gdh locus and L02120 at the tpi locus. Likewise, within sub-assemblage AII, A2 was found in three and two specimens at the bg and tpi loci, respectively, with sequences identical to AY072723 at the bg locus and U57897 at the tpi locus. An A2-like subtype (KY432844) with two SNPs at position 281 and 288 compared with EF507674 was detected in one specimen at the gdh locus. The assemblage B sequence detected at the tpi locus was identical to reference sequence KF679746 from monkeys in China (Karim et al. 2014).
Distribution of G. duodenalis assemblages and subtypes
Assemblage E was the dominant G. duodenalis genotype (98%, 482/492) on all five farms. At the bg locus, the dominant subtype E3 was detected on all five farms, with frequency ranging from 33% (farm 4) to 65% (farm 5). This was followed by E2 (20%), which was mainly observed on farms 1, 2, and 4; E8 (20–30%) on farms 3, 4, and 5; E5 (10%) on farms 1 and 4; and E11 (10%) on farms 2 and 3 (Fig. 3a). Longitudinally, these common subtypes were mostly found on these farms at each of the sampling occasions. Thus, E3 was the most common subtype on all farms at all sampling occasions. Farm 3, however, had E11 as the second most common subtype at the first and fifth samplings but E8 at the second, third, and fourth samplings (Table 2).
Distribution of subtypes (as percentage of the subtype among subtypes identified at each genetic locus) in Giardia duodenalis assemblages A, B, and E on five farms at the bg, gdh, and tpi loci. a Distribution of 15 assemblage E and A subtypes at the bg locus. b Distribution of 16 assemblage E and A subtypes at the gdh locus. c Distribution of 19 assemblage E and A subtypes and assemblage B at the tpi locus
Similar distributions of assemblage E subtypes were observed at the other two genetic loci. At the gdh locus, E3 was the dominant subtype on four of the five farms (farms 2, 3, 4, and 5), with frequency ranging from 40% (farms 3 and 5) to 60% (farms 2 and 4). This was followed by E1, with a frequency of 30% on these farms. However, E1 (50%) was the most common subtype on farm 1, although E3 (20%) was also relatively common on the farm (Fig. 3b). At the tpi locus, the dominant subtype E11 was common on all five farms, with frequency ranging from 29% (farm 1) to 68% (farm 4). This was followed by E3 with frequencies of 16% (farm 4) to 50% (farm 3). However, E3 was detected in only a few specimens on farm 5, and E3, instead of E11, was the most common assemblage E subtype on farm 2 (Fig. 3c).
Assemblage A was detected on farms 1, 3, and 4, either alone or in concurrence with assemblage E. Among them, subtype A2 of sub-assemblage AII (n = 2) and mixed infection of A2 and E (n = 1) were detected on farm 1. Subtype A1 of sub-assemblage AI (n = 1), A2 (n = 2), and mixed infection of A1 and E (n = 1) were detected on farm 3. Concurrence of A1 and E was detected in two specimens from farm 4. The only detection of assemblage B was in a specimen from farm 3 (Table 1).
There was no noticeable difference in the distribution of G. duodenalis genotypes and assemblage E subtypes by age. Assemblages A was detected in a small number of specimens (≤3 specimens) from most age groups except for 1, 5, and 6 weeks in age. The only detection of assemblage B was in a specimen from a 1-week-old calf.
Distribution of G. duodenalis MLGs
Altogether, 131 specimens were successfully subtyped at all three genetic loci, forming 58 assemblage E MLGs and one assemblage A MLGs (Table 3). None of these MLGs were reported previously. Because of the presence of double peaks at the bg and gdh loci, data from additional 24 specimens were excluded in the MLG data analysis.
Of the 58 assemblage E MLGs, seven (MLG-E1–E7) were detected in more than five specimens on multiple farms. The most common ones included MLG-E1 (n = 13), MLG-E2 (n = 9), MLG-E3 (n = 8), MLG-E4 and MLG-E5 (n = 7 each), and MLG-E6 and MLG-E7 (n = 6 each). Among them, MLG-E3 was identified on all five farms, MLG-E1, 2, 4, 6, and 7 each on three farms, and MLG-E5 on two farms. MLG-E1 and MLG-E5 (in six specimens each) were common MLGs on farm 5, while MLG-E2 (n = 5) and MLG-E4 (n = 5) were common on farm 3. The remaining assemblage E MLGs (MLG-E8–E58) were each detected in one to four specimens on different farms (Table 3). Farm-unique assemblage E MLGs included MLG-E8 (n = 4), MLG-E10 (n = 3), MLG-E13, MLG-E14, MLG-E16, and MLG (20) (n = 2 each) on farm 3. Because of the small sample size, it is impossible to compare the distribution of MLGs among samplings. However, MLG-E2 and MLG-E4 were observed on farm 3 at most sampling occasions (Table 4).
In eBURST analysis of the 58 assemblage E MLGs, two clusters and five singletons were observed. Cluster 1 included 51 assemblage E MLGs, with MLG-E3 as the primary founder and other common MLGs, such as MLG-E1, MLG-E4, and MLG-E6, as subgroup founders. Cluster 2 was formed by two assemblage E MLGs from farms 3 and 5. The five singletons were from farms 2, 3, and 5 (Fig. 4).
Single-locus variant eBURST networks for Giardia duodenalis assemblage E. Each MLG is represented by a dot. The blue dot is the primary founder, and the yellow dots are subgroup founders. The size of dots is propotional to the sample size of the MLGs. The single-locus variants are connected by lines
Only one assemblage A specimen from a 3-week-old calf on farm 1 was successfully typed at all three loci, forming a novel sub-assemblage AII-like MLG.
Discussion
A high occurrence of G. duodenalis infection is apparently present in pre-weaned dairy calves in suburbs of Shanghai, China, despite the presence of only a limited number of farms in the metropolitan area. The 60.1% (492/818) infection rate obtained in this study is higher than those found in dairy calves at a similar age (<2 months old) in many studies worldwide. The infection rates of G. duodenalis in pre-weaned cattle reported in recent studies are 9.7–20.0% in northwestern China (Huang et al. 2014; Qi et al. 2016; Zhang et al. 2016), 17.6% in central China (Wang et al. 2014), and 13.3–16.1% in northeast China (Liu et al. 2012, 2015). Elsewhere in developing countries, they are 9.9–34.5% in Ethiopia (Kakandelwa et al. 2016; Wegayehu et al. 2016) and ∼15% in Brazil (Silva et al. 2012). Prior to the present studies, high occurrence of G. duodenalis infection in pre-weaned dairy calves have been usually reported in industralized nations, such as Belgium (48%), Western Australia (52.3%), USA (40%), and Canada (89%) (Becher et al. 2004; Dixon et al. 2011; Fayer et al. 2000; Geurden et al. 2008; Trout et al. 2004). The high density of succeptable animals on large dairy farms is probably responsible for the high endemicity of giardiasis on these farms. In the present study, however, only farm 5 is a large dairy operation by modern standard. Other factors such as hygine and animal management can affect the transmission of G. duodenalis. In the present study, pre-weaned calves were kept in groups instead of individual hutches or stalls, which might promote the transmission of G. duodenalis infection among susceptable animals.
Accompanying the high transmission of G. duodenalis is a high genetic heterogeneity of the pathogen within a small geographic area. In this study, three G. duodenalis assemblages were detected on the five study farms in two neighboring districts in suburban Shanghai, although assemblage E was the dominant genotype. A number of studies also reported assemblage E as the dominant G. duodenalis in cattle in China (Huang et al. 2014; Li et al. 2016; Zhang et al. 2016) and elsewhere around the world (Asher et al. 2016; Cardona et al. 2015; Dixon et al. 2011; Santin et al. 2012; Uehlinger et al. 2011). In contrast, the two zoonotic assemblages A and B were only occasionally detected in pre-weaned dairy calves in this study. The assemblage A subtypes belong to subtype A1 of sub-assemblage AI (in four specimens) and subtype A2 of sub-assemblage AII (in five specimens) at individual loci characterized. Both subtypes are well-known human pathogens (Feng and Xiao 2011). As they have been seen in most studies at low frequencies, G. duodenalis infection in pre-weaned dairy calves does not appear to present a major public health thread. Previously, assemblages A and B were seen at higher frequencies in a few studies in China, New Zealand, Italy, and Canada (Coklin et al. 2007; Lalle et al. 2005; Liu et al. 2015; Wang et al. 2014; Winkworth et al. 2008). Reasons for the occurrence of these zoonotic G. duodenalis genotypes should be examined in future studies.
The high genetic heterogeneity of G. duodenalis in this study is mostly reflected by the high subtype diversity of assemblage E. All three genetic loci in this study showed high sequence polymorphism, yielding 12, 13, and 17 subtypes at the bg, gdh, and tpi loci, respectively. The combination of these sequence variations at the three genetic loci has led to the detection of 58 MLGs within assemblage E. Reasons for the high subtype diversity of assemblage E are not clear, but intra-assemblage genetic recombination was suggested to be a contributing factor (Aguiar et al. 2016; Caccio and Sprong 2010). With such a high infection rate of G. duodenalis on the study farms and the concurrence of multiple subtypes within some animals (as reflected by the presence of double peaks in trace files from DNA sequencing), it is expected that genetic recombination may occur within assemblage E. In the eBURST analysis of sequence data from this study, common MLGs are either the primary founder or subgroup founders, with other MLGs mostly derivatives of them, supporting the role of genetic recombination in the generation of high sequence diversity. Population genetic studies with sequence data from more loci are needed to confirm this conclusion.
Despite the presence of a high G. duodenalis subtype diversity on study farms, there appear to be some exchanges of parasite populations among farms. All dominant subtypes at individual loci, such as E3, E2, and E8 at the bg locus, E3 and E1 at the gdh locus, and E11 and E3 at the tpi locus, are present on all study farms. Similarly, the seven common assemblage E MLGs (MLG-E1–E7) were found on multiple farms. Because of the differences in amplification efficiency among loci and the presence of mixed assemblage E subtypes in some specimens, it became difficult to follow the spread of MLGs between farms and on the same farms longitudinally. In previous studies in China, assemblage E MLGs in dairy calves appear to be geographically isolated (Qi et al. 2016; Wang et al. 2014, 2016b; Zhang et al. 2016). These studies, however, were conducted in distant areas, which might have been responsible for the observed differences in the distribution of major assemblage E MLGs among studies. In this present study, all study farms are located within two neighboring districts of Shanghai and farms 1, 2, and 3 are located next to each other. Inter-farm transmission of some G. duodenalis subtypes is expected within such a confined geographic area.
In conclusion, a very high prevalence of G. duodenalis is present in pre-weaned dairy calves in suburbs of Shanghai, China, despite the less-intensive nature of dairy farming in this area. This is accompanied by a high genetic heterogeneity of assemblage E, the dominant G. duodenalis genotype in cattle worldwide. Some inter-farm transmission of assemblage E appears to be possible based on subtyping and MLG analysis, at least in a small geographic area. More extensive characterizations of assemblage E specimens are needed to understanding the epidemiological implications of the high subtype diversity of G. duodenalis.
References
Aguiar JM, Silva SO, dos Santos VA, Taniwaki SA, Oliveira TMFD, Ferreira HL, Keid LB, Gregori F, Soares RM (2016) Evidence of heterozygosity and recombinant alleles in single cysts of Giardia duodenalis. Rev Bras Parasitol 25:187–195
Asher AJ, Hose G, Power ML (2016) Giardiasis in NSW: identification of Giardia duodenalis assemblages contributing to human and cattle cases, and an epidemiological assessment of sporadic human giardiasis. Infect Genet Evol 44:157–161
Becher KA, Robertson ID, Fraser DM, Palmer DG, Thompson RC (2004) Molecular epidemiology of Giardia and Cryptosporidium infections in dairy calves originating from three sources in Western Australia. Vet Parasitol 123:1–9
Caccio SM, Sprong H (2010) Giardia duodenalis: genetic recombination and its implications for taxonomy and molecular epidemiology. Exp Parasitol 124:107–112
Caccio SM, Beck R, Lalle M, Marinculic A, Pozio E (2008) Multilocus genotyping of Giardia duodenalis reveals striking differences between assemblages A and B. Int J Parasitol 38:1523–1531
Cardona GA, de Lucio A, Ballo B, Cano L, de Fuentes I, Carmena D (2015) Unexpected finding of feline-specific Giardia duodenalis assemblage F and Cryptosporidium felis in asymptomatic adult cattle in Northern Spain. Vet Parasitol 209:258–263
Coklin T, Farber J, Parrington L, Dixon B (2007) Prevalence and molecular characterization of Giardia duodenalis and Cryptosporidium spp. in dairy cattle in Ontario, Canada. Vet Parasitol 150:297–305
Di Giovanni GD, Betancourt WQ, Hernandez J, Assadian NW, Margez JPF, Lopez EJ (2006) Investigation of potential zooanthroponotic transmission of cryptosporidiosis and giardiasis through agricultural use of reclaimed wastewater. Int J Environ Heath Res 16:405–418
Dixon B, Parrington L, Cook A, Pintar K, Pollari F, Kelton D, Farber J (2011) The potential for zoonotic transmission of Giardia duodenalis and Cryptosporidium spp. from beef and dairy cattle in Ontario, Canada. Vet Parasitol 175:20–26
Fayer R, Trout JM, Graczyk TK, Lewis EJ (2000) Prevalence of Cryptosporidium, Giardia and Eimeria infections in post-weaned and adult cattle on three Maryland farms. Vet Parasitol 9:103–112
Feng Y, Xiao L (2011) Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin Microbiol Rev 24:110–140
Feng Y, Ortega Y, Cama V, Terrell J, Xiao L (2008) High intragenotypic diversity of Giardia duodenalis in dairy cattle on three farms. Parasitol Res 103:87–92
Geurden T, Geldhof P, Levecke B, Martens C, Berkvens D, Casaert S, Vercruysse J, Claerebout E (2008) Mixed Giardia duodenalis assemblage a and E infections in calves. Int J Parasitol 38:259–264
Huang J, Yue D, Qi M, Wang R, Zhao J, Li JQ, Shi K, Wang M, Zhang L (2014) Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in dairy cattle in Ningxia, northwestern China. BMC Vet Res 10:292
Kakandelwa C, Siwila J, Nalubamba KS, Muma JB, Phiri IGK (2016) Prevalence of Giardia in dairy cattle in Lusaka and Chilanga districts, Zambia. Vet Parasitol 215:114–116
Karim MR, Zhang S, Jian F, Li J, Zhou C, Zhang L, Sun M, Yang G, Zou F et al (2014) Multilocus typing of Cryptosporidium spp. and Giardia duodenalis from non-human primates in China. Int J Parasitol 44:1039–1047
Lalle M, Jimenez-Cardosa E, Caccio SM, Pozio E (2005) Genotyping of Giardia duodenalis from humans and dogs from Mexico using a beta-giardin nested polymerase chain reaction assay. J Parasitol 91:203–205
Lebbad M, Mattsson JG, Christensson B, Ljungstrom B, Backhans A, Andersson JO, Svard SG (2010) From mouse to moose: multilocus genotyping of Giardia isolates from various animal species. Vet Parasitol 168:231–239
Lebbad M, Petersson I, Karlsson L, Botero-Kleiven S, Andersson JO, Svenungsson B, Svard SG (2011) Multilocus genotyping of human Giardia isolates suggests limited zoonotic transmission and association between assemblage B and flatulence in children. PLoS Negl Trop Dis 5(8):e1262
Li F, Wang H, Zhang Z, Li J, Wang C, Zhao J, Hu S, Wang R, Zhang L et al (2016) Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in dairy cattle in Beijing, China. Vet Parasitol 228:69–69
Liu A, Zhang X, Zhang L, Wang R, Li X, Shu J, Zhang X, Shen Y, Zhang W et al (2012) Occurrence of bovine giardiasis and endemic genetic characterization of Giardia duodenalis isolates in Heilongjiang Province, in the northeast of China. Parasitol Res 111:655–661
Liu G, Su Y, Zhou M, Zhao J, Zhang T, Ahmad W, Lu H, Jiang N, Chen Q et al (2015) Prevalence and molecular characterization of Giardia duodenalis isolates from dairy cattle in northeast China. Exp Parasitol 154:20–24
Qi M, Cai J, Wang R, Li J, Jian F, Huang J, Zhou H, Zhang L (2015) Molecular characterization of Cryptosporidium spp. and Giardia duodenalis from yaks in the central western region of China. BMC Microbiol 15:1–7
Qi M, Wang H, Jing B, Wang R, Jian F, Ning C, Zhang L (2016) Prevalence and multilocus genotyping of Giardia duodenalis in dairy calves in Xinjiang, northwestern China. Parasit Vectors 9:546
Ruiz A, Foronda P, Gonzalez JF, Guedes A, Abreu-Acosta N, Molina JM, Valladares B (2008) Occurrence and genotype characterization of Giardia duodenalis in goat kids from the Canary Islands, Spain. Vet Parasitol 154:137–141
Ryan U, Caccio SM (2013) Zoonotic potential of Giardia. Int J Parasitol 43:943–956
Santin M, Dargatz D, Fayer R (2012) Prevalence of Giardia duodenalis assemblages in weaned cattle on cow-calf operations in the United States. Vet Parasitol 183:231–236
Scalia LAM, Fava NMN, Soares RM, Limongi JE, da Cunha MJR, Pena IF, Kalapothakis E, Cury MC (2016) Multilocus genotyping of Giardia duodenalis in Brazilian children. Trans R Soc Trop Med Hyg 110:343–349
Silva FMPE, Lopes RS, Araujo JP (2012) Genetic characterisation of Giardia duodenalis in dairy cattle in Brazil. Folia Parasitol 59:15–20
Solarczyk P, Majewska AC, Moskwa B, Cabaj W, Dabert M, Nowosad P (2012) Multilocus genotyping of Giardia duodenalis isolates from red deer (Cervus elaphus) and roe deer (Capreolus capreolus) from Poland. Folia Parasitol 59:237–240
Thompson RCA, Ash A (2016) Molecular epidemiology of Giardia and Cryptosporidium infections. Infect Genet Evol 40:315–323
Trout JM, Santin M, Greiner E, Fayer R (2004) Prevalence of Giardia duodenalis genotypes in pre-weaned dairy calves. Vet Parasitol 124:179–186
Uehlinger FD, Greenwood SJ, O’Handley R, McClure JT, Coklin T, Dixon BR, de Boer M, Zwiers H, Barkema HW (2011) Prevalence and genotypes of Giardia duodenalis in dairy and beef cattle in farms around Charlottetown, Prince Edward Island, Canada. Can Vet J 52:967–972
Wang H, Zhao G, Chen G, Jian F, Zhang S, Feng C, Wang R, Zhu J, Dong H et al (2014) Multilocus genotyping of Giardia duodenalis in dairy cattle in Henan, China. PLoS One 9(6):e100453
Wang H, Qi M, Zhang K, Li JQ, Huang J, Ning C, Zhang L (2016a) Prevalence and genotyping of Giardia duodenalis isolated from sheep in Henan Province, central China. Infect Genet Evol 39:330–335
Wang X, Wang R, Ren G, Yu Z, Zhang L, Zhang S, Lu H, Peng X, Zhao G (2016b) Multilocus genotyping of Giardia duodenalis and Enterocytozoon bieneusi in dairy and native beef (Qinchuan) calves in Shaanxi province, northwestern China. Parasitol Res 115:1355–1361
Wegayehu T, Karim MR, Erko B, Zhang L, Tilahun G (2016) Multilocus genotyping of Giardia duodenalis isolates from calves in Oromia special zone, Central Ethiopia. Infect Genet Evol 43:281–288
Winkworth CL, Learmonth JJ, Matthaei CD, Townsend CR (2008) Molecular characterization of Giardia isolates from calves and humans in a region in which dairy farming has recently intensified. Appl Environ Microbiol 74:5100–5105
Ye J, Xiao L, Wang Y, Guo Y, Roellig DM, Feng Y (2015) Dominance of Giardia duodenalis assemblage A and Enterocytozoon bieneusi genotype BEB6 in sheep in Inner Mongolia, China. Vet Parasitol 210:235–239
Zhang X, Tan Q, Zhao G, Ma J, Zheng W, Ni X, Zhao Q, Zhou D, Zhu X (2016) Prevalence, risk factors and multilocus genotyping of Giardia intestinalis in dairy cattle, Northwest China. J Eukaryot Microbiol 63:498–504
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31630078, 31425025, and 31602042), the China Postdoctoral Science Foundation (2016M601531), and the Fundamental Research Funds for the Central Universities, China (WB1714039).
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Ethic Committee of the East China University of Science and Technology (Permit No. 2015015 approved on January 3, 2015). The dairy calves were handled in accordance with the Animal Ethics Procedures and Guidelines of the People’s Republic of China. Permissions were obtained from the management of dairy farms before specimen collections.
Rights and permissions
About this article
Cite this article
Wang, X., Cai, M., Jiang, W. et al. High genetic diversity of Giardia duodenalis assemblage E in pre-weaned dairy calves in Shanghai, China, revealed by multilocus genotyping. Parasitol Res 116, 2101–2110 (2017). https://doi.org/10.1007/s00436-017-5509-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-017-5509-8