Abstract
To determine the growth pattern and in vitro susceptibility of Blastocystis hominis to metronidazole (MTZ), garlic, ginger, white cumin, and black pepper. Stool specimens were collected from 16 irritable bowel syndrome (IBS) and 10 controls between July–November 2010. Stool microscopy and culture for B. hominis was performed. Drug susceptibility assays was done using 0.01 and 0.1 mg/ml of MTZ, garlic, ginger, white cumin, and black pepper. Effect was assessed on B. hominis culture after 48 h. Stool DNA was extracted using stool DNA extraction kit (Qiagen) and polymerase chain reaction (PCR) done using subtype-specific sequence-tagged-site primers. B. hominis genotype 3 and coinfection of 1 and 3 tended to grow well in culture compared to isolated type 1 infection. Exposed to MTZ at a concentration of 0.01 mg/ml, 38% (6/16) B. hominis from IBS did not grow in culture compared to 100% (10/10) of B. hominis from control (p = 0.001). When they were exposed to MTZ at 0.1 mg/ml, 56% (9/16) B. hominis from IBS did not grow in cultures compared to 100% (10/10) from control (p = 0.01). Forty-four percent (7/16) B. hominis from IBS did not grow in culture compared to 100% (10/10) B. hominis from control when exposed to garlic at a concentration of 0.01 mg/ml (p = 0.003) and following exposure to garlic at 0.1 mg/ml, 38% (6/16) B. hominis from IBS did not grow in cultures compared to 100% (10/10) from control (p = 0.001). B. hominis isolates from IBS had a cell count of 6,625 at a MTZ concentration of 0.01 mg/ml that reduced to 1,250 as MTZ concentration was increased to 0.1 mg/ml (p = 0.08). B. hominis from IBS with a mean cell count of 3 × 105 at baseline decreased to 1 × 104 when exposed to garlic at 0.01 mg/ml (p < 0.001) and to 1 × 103 (p < 0.001) when garlic was 0.1 mg/ml. B. hominis from IBS cell count decreased to 1 × 105 when exposed to white cumin at 0.01 mg/ml (p = 0.01) and to 1 × 105 (p < 0.001) when white cumin was 0.1 mg/ml. Exposed to black pepper at 0.1 mg/ml, cell count of B. hominis from IBS decreased to 1 × 105 (p = 0.01). B. hominis from IBS decreased to 1.3 × 105 exposed to ginger at 0.01 mg/ml (p = 0.001). B. hominis isolates were mostly genotypes 3, type 1 and 3 coinfection, and non-typeable B. hominis isolates. B. hominis isolates from IBS mostly genotype 1 demonstrated an increased sensitivity to garlic at 0.01 mg/ml with a B. hominis cell count of 3,714 compared to 6,142 when exposed to 0.01 mg/ml of MTZ. However, this sensitivity did not increase as garlic concentration was increased to 0.1 mg/ml, for B. hominis cell count was 6,000 compared to 1,428 as MTZ was increased to 0.1 mg/ml.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Blastocystis hominis is one of the most common intestinal protozoa which is found in large intestine in humans. It appears to infect both immunocompetent and immunocompromised individuals. Although several reports have suggested that B. hominis could cause gastrointestinal disorders, the specific pathogenicity of this organism has not yet been defined. The clinical consequences of B. hominis infection are mainly diarrhea or abdominal pain with nonspecific gastrointestinal symptoms such as nausea, anorexia, vomiting, weight loss, lassitude, dizziness, and flatulence. It has been speculated that thick-walled cysts might be responsible for external transmission. The various mechanisms suggested for B. hominis-mediated gastrointestinal symptoms include adherence of B. hominis to the gut epithelium, triggering a lysis mechanism as shown for Entamoeba histolytica, Giardia lamblia, and existence of a diarrheagenic toxin.
Various antibiotics that include metronidazole, furazolidone, nitazoxanide, sulfamethoxazole/trimethoprim, etc. have been previously used for the treatment of diarrhea and enteritis associated with B. hominis as the sole identified pathogen in children and adults (Dunn and Boreham 1991). In a randomized, single blind study, Dinleyici et al. (2010) compared no treatment to the efficacy of Saccharomyces boulardii or metronidazole (MTZ) for the duration of diarrhea and the duration of colonization in children with gastrointestinal symptoms and positive stool examination for B. hominis. On day 15, clinical cure was observed in 77.7% in group A (n = 18); in 66.6% in group B (n = 15); and 40% in group C (n = 15) (p < 0.031, between groups A and C). At the end of the first month after inclusion, clinical cure rate was 94.4% in group A and 73.3% in group B (p = 0.11). Parasitological cure rate for B. hominis was very comparable between both groups (94.4% vs. 93.3%, p = 0.43) (Dinleyici et al. 2010). Metronidazole is an antimicrobial drug with high activity against Trichomonas vaginalis, E. histolytica, G. lamblia, etc. However, resistance to ciproxin and metronidazole was demonstrated previously compared to furazolidone (Yakoob et al. 2004).
Garlic (Allium sativum) contains a wide range of the thiosulfinates which are responsible for the antibacterial activity (Jonkers et al. 1999; Iimuro et al. 2002; Sivam et al. 1997). The antimicrobial activity of garlic has been attributed to the presence of thiosulfinates (e.g, allicin). The amino acid, allicin, is metabolized by allinase (a cysteine sulfoxide lyase) to allicin and other thiosulfinates (Block 1985). Allicin acts by totally inhibiting RNA synthesis and partially inhibiting DNA and protein synthesis (Feldberg et al. 1988). Bacterial susceptibility to garlic may also depend on structural differences of the bacterial strains. The cell wall of content of Gram-positive and -negative microorganisms contain a variable quantity of polysaccharides 15–60% and 0–20% lipid, (Carpenter 1968). The polysaccharide and lipid contents of the cell wall have an effect on the permeability of allicin and other garlic constituents; this may be responsible for the difference in susceptibility to garlic between Gram-negative e.g., Helicobacter pylori and Gram-positive e.g., Staphylococcus aureus (Cellini et al. 1996; Sivam et al. 1997).
Cumin (Cuminum cyminum) is a popular spice in Middle Eastern and South Asian cuisine. It is used for the treatment of dyspepsia, diarrhea, flatulence, and as an appetite stimulant in traditional medicine. Cumin seeds are also rich in calcium, magnesium, iron, zinc, and some of the vitamins B (http://www.vegetarian-nutrition.info/herbs/cumin.php). Both aqueous and ethanolic extracts of black pepper (Piper nigrum) contain an alkaloid piperine that has demonstrated antibacterial activity (Pundir and Jain 2010; Singh et al. 2005; Khajuria et al. 2002). Black pepper finds an extensive use in traditional antibacterial preparations. A number of piperidine and pyrrolidine alkamides are known to occur in P. nigrum (Parmar et al. 1997), the most important being piperine, known to possess a variety of biological properties like analgesic, antipyretic, antibacterial, etc. (Miyakado et al. 1979). Black pepper finds an extensive use in traditional antibacterial preparations.
An extensive genetic variability has been described recently in B. hominis isolates. In a previous study, we demonstrated the predominant genotype of B. hominis in IBS-D was type 1, while in healthy control, it was genotype 3 (Yakoob et al. 2010a). Infection with single genotype of B. hominis was present in 73% with IBS-D and in 27% in control group while with multiple genotypes in 25 (64%) in IBS-D and 14 (36%) in control group (p = 0.30), respectively (Yakoob et al. 2010b). In this study, we evaluated the in vitro effect of dietary herbs such as garlic, ginger, white cumin, and black pepper in two different concentrations on the parasite counts at 48 h using a reproducible method previously described (Yakoob et al. 2004). Metronidazole was used as a control for its known anti-B. hominis effect. These strains of B. hominis were isolated from the stool specimens obtained from irritable bowel syndrome (IBS) patients and healthy controls in our previous study (Yakoob et al. 2010a).
Materials and methods
This prospective study was conducted at the Aga Khan University Hospital, Karachi, Pakistan. Stool specimens were collected from 16 irritable bowel syndrome (IBS) patients attending the gastroenterology clinic and 10 (38%) healthy controls volunteered stool specimens between July and November 2010. These B. hominis were cultured from fecal samples of 16 (62%) with IBS, of these, 14 (54%) were males and 12 (46%) females. There was no history of antibiotic use in these patients. Stool microscopy and culture for B. hominis were done as described before (Zaman and Khan 1994).
Microscopy of fecal smear
Briefly, fecal sample microscopy was done as described before (Zaman and Khan 1994). Briefly, direct wet mount in which approximately 2 mg of feces was thoroughly emulsified on a glass slide in one drop of physiologic saline and covered with a cover slip. A similar preparation was made on another slide using Lugol’s iodine. These preparations were examined under both the low power (×10) and high dry (×40) objectives.
Culture of feces
Cultures were done, by inoculating approximately 50 mg of feces into 5 ml of Jones’ medium. For culturing B. hominis, Jones medium without starch was used, as it supports good growth of the parasite as described before (Zaman and Khan 1994). The cultures were incubated at 37°C and examined after 24, 48, 72, and 96 h. If no B. hominis were seen up to the end of this period, they were regarded as negative. The sediment was examined under both the low power (×10) and high dry (×40) objectives.
Plant extracts
Extracts were prepared of garlic, white cumin, black pepper, and ginger. Spices were measured, grounded, and soaked in double distilled water for about 48 h at room temperature to have a stock solution of 100 mg/mL. Solution was filtered through Grade 1 filter paper (Whatman, UK). Infusions were neutralized to pH 7.0. Extracts were stored in the dark at −20°C until use. Infusion of dried spices was used except for garlic which was used fresh. Herb extracts and MTZ stock solution of 1 mg/ml was prepared and added to media containing falcon tubes to give final concentration of 0.01 and 0.1 mg/ml.
Drug susceptibility assays
In vitro susceptibility assays were performed using a method previously described (Zaman and Zaki 1996). Briefly, in each media tube, 50 μl of cultures containing 200,000 B. hominis was added, which had been counted in a Neubauer chamber. These were incubated for 48 h at 37°C with different concentrations of dietary herbs and metronidazole each added as a solution in water. The calculated amount of metronidazole was first added to media containing tubes to achieve 0.01 and 0.1 mg/ml of the drug. Same method was used for the various plant extracts. The media tubes with B. hominis culture and without drug were used as controls. The effect of the drug was assessed after allowing the B. hominis to grow for 48 h. For this, 4 ml of the supernatant medium from each tube was carefully removed without disturbing the culture pellet at the bottom that contained the B. hominis. The sediment containing B. hominis was then gently agitated to obtain a uniform distribution and counts again made in a Neubauer chamber and the percentage increase or decrease in growth between the control and the test tubes was calculated for each strain.
Extraction of genomic DNA
Genomic DNA of B. hominis was extracted by using Stool DNA Extraction kit (Qiagen) according to the manufacturer’s protocol. Extracted DNA was stored at −20°C until PCR was carried out for B. hominis genotyping.
Genotyping by PCR with sequence-tagged-site primers
Seven kinds of subtype-specific sequence-tagged-site (STS) primers developed for typing the Blastocystis isolates were used as described previously (Abe et al. 2003a, b, c; Li et al. 2007a, b; Yan et al. 2006; Yoshikawa et al. 1998, 2000, 2003). Seven standardized subtype-specific STS primers were used, namely SB83 (351 bp) for subtype 1, SB340 (704 bp) for subtype 2, SB227 (526 bp) for subtype 3, SB337 (487 bp) for subtype 4, SB336 (317 bp) for subtype 5, SB332 (338 bp) for subtype 6, and SB155 (650 bp) for subtype 7 (Yoshikawa et al. 2004), according to a recent classification terminology (Stensvold et al. 2007). Typing of the Blastocystis isolates was conducted through PCR amplification on the basis of the presence or absence of the products within parallel control PCR amplification. The PCR conditions consisted of one cycle denaturing at 94°C for 3 min, 30 cycles including annealing at 59°C for 30 s, extending at 72°C for 60 s, denaturing at 94°C for 30 s, and additional cycle with a 5-min chain elongation at 72°C (PCR System 9700, Perkin Elmer, USA). The PCR products and molecular markers were electrophoresed in 2% agarose gel with Tris-acetate-EDTA electropheresis buffer. The size markers were 100 base-pair ladder (Promega, USA). The PCR amplification for each primer pair was repeated at least thrice. Bands were visualized by the imaging system (Gel Doc 2000, Gel Documentation System, Bio Rad, UK) after being stained with ethidium bromide.
Statistical analysis
Results are expressed as mean + standard deviation for continuous variables (e.g., age) and number (percentage) for categorical data (e.g., gender, stool culture, diarrhea etc.).
Univariate analysis was performed by using the independent sample t test, Pearson Chi-square test, and Fisher Exact test were also used whenever appropriate. A p value of <0.05 was considered as statistically significant. All p values were two sided. Statistical interpretation of data was performed by using the computerized software program SPSS version 16.0.
Result
Culture characteristics of B. hominis genotypes
B. hominis genotypes 3 and 1 and 3 tended to grow well in culture when present as a coinfection compared to isolated type 1 infection.
Metronidazole
B. hominis (38% (6/16)) isolated from IBS patients did not grow in culture compared to 100% (10/10) B. hominis from control when exposed to MTZ at a concentration of 0.01 mg/ml (p = 0.001). When they were exposed to MTZ at 0.1 mg/ml, 56% (9/16) B. hominis isolated from IBS patients did not grow in cultures compared to 100% (10/10) from control (p = 0.01) (Table 1).
Garlic
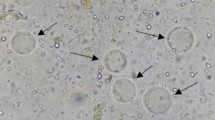
B. hominis isolates (44% (7/16)) from IBS patients did not grow in culture compared to 100% (10/10) B. hominis from control when exposed to garlic at a concentration of 0.01 mg/ml (p = 0.003). When they were exposed to garlic at 0.1 mg/ml, 38% (6/16) B. hominis isolated from IBS did not grow in cultures compared to 100% (10/10) from control (p = 0.001) (Table 1; Fig. 1).
Comparison of effect of herbs on B. hominis
B. hominis isolates from both IBS and control were sensitive to garlic, ginger, black pepper, and white cumin (Figs. 2, 3, 4, and 5). B. hominis isolates from IBS had a cell count of 6625 ± 66 at a MTZ concentration of 0.01 mg/ml that reduced to 1,250 ± 20 as MTZ concentration was increased to 0.1 mg/ml (p = 0.08). B. hominis from IBS with a mean cell count of 3 × 105 at baseline decreased to 1 × 104 when exposed to garlic at 0.01 mg/ml (p < 0.001) and to 1 × 103 (p < 0.001) when garlic was 0.1 mg/ml. B. hominis from IBS cell count decreased to 1 × 105 when exposed to white cumin at 0.01 mg/ml (p = 0.01) and to 1 × 105 (p < 0.001) when white cumin was 0.1 mg/ml. Exposed to black pepper at 0.1 mg/ml, cell count of B. hominis from IBS decreased to 1 × 105 (p = 0.01). B. hominis from IBS decreased to 1.3 × 105 exposed to ginger at 0.01 mg/ml (p = 0.001).
Correlation of B. hominis genotype and effect of herbs
B. hominis isolates from control exhibited equal sensitivity to both MTZ and garlic (Table 1). They were mostly type 3, type 1 and 3 coinfection, and non-typeable B. hominis isolates. B. hominis isolates from IBS mostly genotype 1 demonstrated an increased sensitivity to garlic at 0.01 mg/ml with a B. hominis cell count of 3,714 compared to 6,142 when exposed to 0.01 mg/ml of MTZ. However, this sensitivity did not increase as garlic concentration was increased to 0.1 mg/ml, for B. hominis cell count was 6,000 compared to 1,428 as MTZ was increased to 0.1 mg/ml.
Discussion
B. hominis has been associated with diseases in immunocompetent and immunocompromised subjects (Dunn and Boreham 1991; Jonkers et al. 1999). These patients are treated with MTZ when B. hominis is suspected of causing disease. Currently, metronidazole is the drug of choice for treating protozoal infection. Previously, an aqueous extract of Nigella sativa at concentrations of 100 and 500 μg/ml showed a potent lethal effect on both B. hominis isolates. There was no significant difference between the inhibitory effect of N. sativa and metronidazole on the B. hominis living cell count (El Wakil 2007).
This study demonstrated that clinical isolates of B. hominis sensitivity to garlic was proportional to MTZ in suppressing the growth of B. hominis. Garlic was equally effective in both tested concentrations. B. hominis isolates were not sensitive to ginger, black pepper, and cumin compared to garlic and MET. They were unable to suppress B. hominis growth whereas ginger appeared to be promoting the growth of B. hominis isolates at higher concentration. B. hominis genotype 3 from control was more sensitive to metronidazole compared to genotype 1, and this was also demonstrated when there was a coinfection of genotype 1 and 3. B. hominis genotypes 1 and 3 as coinfection and non-typeable genotypes flourished in in vitro culture, and their growth was sustained over time as compared to that of isolated genotypes 1 or 3.
In this study, the viable cell count method provided a reliable method to determine the activity of the herbs against clinical isolates of B. hominis (Singh et al. 2005). The ability of the B. hominis isolates to grow at a concentration of 0.1 mg/ml showed that these herbs had no effect on B. hominis isolates (Khajuria et al. 2002). However, it is possible that their active ingredients might demonstrate activity against B. hominis when obtained by another method. There are previous reports of B. hominis trophozoites and cysts demonstrating resistance to MTZ (Khajuria et al. 2002; Yakoob et al. 2010a, b; Zaman and Zaki 1996; Haresh et al. 1999; Germani et al. 1998). However, Dinleyici et al. (2010) demonstrated clinical efficacy of both MTZ and S. boulardii in symptomatic children with B. hominis infection presenting with abdominal pain, diarrhea, nausea-vomiting, and flatulence for more than 2 weeks. Both MTZ and S. boulardii had beneficial effects on symptoms and presence of parasites (Dinleyici et al. 2010). Similarly, Moghaddam et al. (2005) evaluated the effects of metronidazole and trimethoprim/sulfamethoxazole (TMP/SMX) on persons infected with B. hominis. A total of 104 non-immunocompromised subjects were monitored for 1 year after treatment. Of the 104 infected individuals with B. hominis infection, 28 had large numbers of B. hominis present in stool before treatment. Of these 28 severely infected individuals, 12 were treated with metronidazole/250–750 mg at a dose of 3×/day/10 days, and 4 of the 12 were eradicated. Nine individuals were treated with TMP/SMX 1 tab 3×/day/10 days, and 2 of the 9 were eradicated. For severe B. hominis infections, it appeared that metronidazole and TMP/SMX were effective in some individuals but not in all (Moghaddam et al. 2005). Possible mechanisms for apparent failure include extensive use of MTZ, TMP/SMX in the local community for various indications, inadequate dosage, patient noncompliance, and inactivation of the drug by the normal bacteria flora. Similar explanation might be offered for these herbs which are commonly used in cooking recipes. In this study, garlic was the most potent plant extracts against B. hominis. Other natural herbs that have been tried against B hominis include oregano, etc. (Force et al. 2000). Oil of Mediterranean oregano Origanum vulgare was orally administered to 14 adult patients whose stools tested positive for B. hominis. After 6 weeks of supplementation with 600 mg emulsified oil of oregano daily, there was complete disappearance of B. hominis in eight cases. Also, B. hominis scores declined in three additional cases. Gastrointestinal symptoms improved in 7 of the 11 patients who had tested positive for B. hominis (Force et al. 2000).
The strains of B. hominis used were isolated before experiment so they reflected their natural susceptibility. There are metronidazole-resistant B. hominis infections, and treatment of such cases by metronidazole might not provide eradication. However, whether garlic extract can be used in such cases needs to be looked at. If a protozoal cause of diarrhea is suspected, MTZ use should be reviewed if symptoms persist. In conclusion, this study has shown that B. hominis isolates vary in their degree of susceptibility to ginger, garlic, cumin, and black pepper common constituents of diet. However, further study is required to search for drugs that can be effective against B. hominis.
References
Abe N, Wu Z, Yoshikawa H (2003a) Molecular characterization of Blastocystis isolates from birds by PCR with diagnostic primers and restriction fragment length polymorphism analysis of the small subunit ribosomal RNA gene. Parasitol Res 89:393–396
Abe N, Wu Z, Yoshikawa H (2003b) Molecular characterization of Blastocystis isolates from primates. Vet Parasitol 113:321–325
Abe N, Wu Z, Yoshikawa H (2003c) Zoonotic genotypes of Blastocystis hominis detected in cattle and pigs by PCR with diagnostic primers and restriction fragment length polymorphism analysis of the small subunit ribosomal RNA gene. Parasitol Res 90:124–128
Block E (1985) The chemistry of garlic and onions. Sci Am 252:114–119
Carpenter PL (1968) Microbiology, 2nd edn. WB Saunders, Philadelphia, p 476
Cellini L, DiCampli E, Masuli M, DiBartolomeo S, Allocati N (1996) Inhibition of Helicobacter pylori by garlic extracts (Allium sativum). FEMS Immunol Med Microbiol 13:273–277
Dinleyici EC, Eren M, Dogan N, Reyhanioglu S, Yargic ZA, Vandenplas Y (2010) Clinical efficacy of Saccharomyces boulardii or metronidazole in symptomatic children with Blastocystis hominis infection. Parasitol Res (in press)
Dunn LA, Boreham PFL (1991) The in vitro activity of drugs against Blastocystis hominis. J Antimicrob Chemother 27:507–516
El Wakil SS (2007) Evaluation of the in vitro effect of Nigella sativa aqueous extract on Blastocystis hominis isolates. J Egypt Soc Parasitol 37:801–813
Feldberg RS, Chang SC, Kotik AN, Nadler M, Neuwirth Z, Sundstrom DC, Thompson NH (1988) In vitro mechanism of inhibition of bacterial growth by allicin. Antimicrob Agents Chemother 32:1763–1768
Force M, Sparks WS, Ronzio RA (2000) Inhibition of enteric parasites by emulsified oil of oregano in vivo. Phytother Res 14:213–214
Germani Y, Minssart P, Vohito M, Yassibanda S, Glaziou P, Hocquet D, Berthélémy P, Morvan J (1998) Etiologies of acute, persistent, and dysenteric diarrheas in adults in Bangui, Central African Republic, in relation to human immunodeficiency virus serostatus. Am J Trop Med Hyg 59:1008–1014
Haresh K, Suresh K, Khairul Anuar A, Saminathan S (1999) Isolate resistance of Blastocystis hominis to metronidazole. Trop Med and Inter Health 4: 274–277. http://www.vegetarian-nutrition.info/herbs/cumin.php
Iimuro M, Shibata H, Kawamori T, Matsumoto T, Arakawa T, Sugimura T, Wakabayashi K (2002) Suppressive effects of garlic extract on Helicobacter pylori-induced gastritis in Mongolian gerbils. Cancer Lett 187:61–68
Jonkers D, van den Broek E, van Dooren I, Thijs C, Dorant E, Hageman G, Stobberingh E (1999) Antibacterial effect of garlic and omeprazole on Helicobacter pylori. J Antimicrob Chemother 43:837–839
Khajuria A, Thusu N, Zutshi U (2002) Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultra structure and enzyme kinetics. Phytomedicine 9:224–231
Li LH, Zhou XN, Du ZW, Wang XZ, Wang LB, Jiang JY, Yoshikawa H, Steinmann P, Utzinger J, Wu Z, Chen JX, Chen SH, Zhang L (2007a) Molecular epidemiology of human Blastocystis hominis in a village in Yunnan province, China. Parasitol Int 56:281–286
Li LH, Zhang XP, Lv S, Zhang L, Yoshikawa H, Wu Z, Steinmann P, Utzinger J, Tong XM, Chen SH, Zhou XN (2007b) Cross-sectional surveys and subtype classification of human Blastocystis hominis isolates from four epidemiological settings in China. Parasitol Res 102:83–90
Miyakado M, Nakayam I, Yoshioka H, Nakatani N (1979) The Piperaceae amides I: structure of pipercide, a new insecticidal amide from Piper nigrum L. Agric Biol Chem 43:1609–1611
Moghaddam DD, Ghadirian E, Azami M (2005) Blastocystis hominis and the evaluation of efficacy of metronidazole and trimethoprim/sulfamethoxazole. Parasitol Res 96:273–275
Parmar VS, Jain SC, Bist KS, Jain R, Taneia P, Jha A, Tyagi OD, Prasad AK, Wegel J, Olsen CE, Boll PM (1997) Photochemistry of the genus Piper. Phytochemistry 46:597–673
Pundir RK, Jain P (2010) Comparative studies on the antimicrobial activity of black pepper (Piper nigrum) and turmeric (Curcuma longa) extracts. Int J Appl Biol Pharm Technol 1:492–494
Singh G, Marimuthu P, Murali HS, Bawa AS (2005) Antioxidative and antibacterial potentials of essential oils and extracts isolated from various spice materials. J Food Saf 25:130
Sivam GP, Lampe JW, Ulness B, Swanzy SR, Potter JD (1997) Helicobacter pylori-in vitro susceptibility to garlic (Allium sativum) extract. Nutr Cancer 27:118–121
Stensvold CR, Suresh GK, Tan KSW, Thompson RCA, Traub RJ, Viscogliosi E, Yoshikawa H, Clark CG (2007) Terminology for Blastocystis hominis subtypes—a consensus. Trends Parasitol 23:93–96
Yakoob J, Jafri W, Jafri N, Islam M, Asim Beg M (2004) In vitro susceptibility of Blastocystis hominis isolated from patients with irritable bowel syndrome. Br J Biomed Sci 61:75–77
Yakoob J, Jafri W, Beg MA, Abbas Z, Naz S, Islam M, Khan R (2010a) Irritable bowel syndrome: is it associated with genotypes of Blastocystis hominis. Parasitol Res 106:1033–1038
Yakoob J, Jafri W, Beg MA, Abbas Z, Naz S, Islam M, Khan R (2010b) Blastocystis hominis and Dientamoeba fragilis in patients fulfilling irritable bowel syndrome criteria. Parasitol Res 107:679–684
Yan Y, Su S, Ye J, Lai R, Liao H, Ye J, Li X, Luo X, Chen G (2006) Genetic variability of Blastocystis hominis isolates in China. Parasitol Res 99:597–601
Yoshikawa H, Nagano I, Wu Z, Yap EH, Singh M, Takahashi Y (1998) Genomic polymorphism among Blastocystis hominis strains and development of subtype-specific diagnostic primers. Mol Cell Probes 12:153–159
Yoshikawa H, Abe N, Iwasawa M, Kitano S, Nagano I, Wu Z, Takahashi Y (2000) Genomic analysis of Blastocystis hominis strains isolated from two long-term health care facilities. J Clin Microbiol 38:1324–1330
Yoshikawa H, Abe N, Wu Z (2003) Genomic polymorphism among Blastocystis hominis isolates and development of PCR-based identification of zoonotic isolates. J Eukaryot Microbiol 50:710–711
Yoshikawa H, Morimoto K, Wu Z, Singh M, Hashimoto T (2004) Problems in speciation in the genus Blastocystis hominis. Trends Parasitol 20:251–255
Zaman V, Khan K (1994) A comparison of direct microscopy with culture for the diagnosis of Blastocystis hominis. Southeast Asian J Trop Med Hyg Public Health 25:792–793
Zaman V, Zaki M (1996) Resistance of Blastocystis hominis cysts to metronidazole. Trop Med Int Health 5:677–678
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yakoob, J., Abbas, Z., Beg, M.A. et al. In vitro sensitivity of Blastocystis hominis to garlic, ginger, white cumin, and black pepper used in diet. Parasitol Res 109, 379–385 (2011). https://doi.org/10.1007/s00436-011-2265-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2265-z