Abstract
The results of a study of the composition, ecology, and formation of parasite communities in the Acadian redfish Sebastes fasciatus Storer, 1856 are considered in the present article. The geographical features of the parasite community composition in this host fish species indicate the existence of six Acadian redfish groups with different degrees of isolation in the Northwest Atlantic. The most isolated groups inhabit the Gulf of St. Lawrence and the Flemish Cap waters. The results of a comparative analysis of genetic data and some morphological characters of S. fasciatus available in published literature are consistent with the findings of the present study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The Acadian redfish Sebastes fasciatus, like other North Atlantic species of the genus Sebastes, is a demersal/pelagic species with internal fertilization and ovovivipary. It is usually found to live in sympatry with the beaked redfish S. mentella Travin, 1951 and the golden redfish S. norvegicus Ascanius, 1772. The external similarity of these closely related fish complicates their species identification [9, 21, 49, 59]. The challenge of distinguishing between them is also explained by their interspecific hybridization [29, 48, 50, 60], which significantly restricts the use of morphological methods for species identification and even exacerbates the necessity to clarify the species structure. Furthermore, until the 1970s, most of the available data referred to the combined species S. marinus which included the above-mentioned redfish species [8].
Ecological and population studies on the Acadian redfish that inhabit waters off the Canadian coast and in the Flemish Cap Bank are limited to attempts to differentiate individuals of this species from beaked redfish and their hybrids, assess the ratios of their numbers in various areas, and also identify migrations of S. fasciatus, its breeding grounds, and local groups [9, 25, 48, 49, 56, 57, 59]. It was assumed that up to nine local groups of this species exist [21, 55].
A study of 13 microsatellite loci and some of morphological characters showed that the Acadian redfish group in the Gulf of St. Lawrence not only differs from groups of this fish in other areas, but is also heterogeneous within the gulf [58]. Data of microsatellite and morphometric analyses [60], as well as the results of a study of single-nucleotide polymorphisms (SNPs) using genotype-by-sequencing methods [31] indicated the existence of a significantly isolated Acadian redfish population in the Gulf of St. Lawrence that inhabit the waters of Bonne Bay (western coast of Newfoundland). A weakly pronounced structuring of the species was observed within a large region between three vast areas: the Gulf of St. Lawrence with the Laurentian Channel, where the rate of interspecific hybridization with beaked redfish is high (up to 15%), the Gulf of Maine, and the Grand Banks of Newfoundland (GBN) [48].
Results of genetic studies showed a probability of migration of Acadian redfish individuals towards the Gulf of St. Lawrence from adjacent waters and suggested the independence of the group of this species in the Gulf of Maine. The population structure of the Acadian redfish on a smaller spatial scale, as revealed using genetic (Gulf of St. Lawrence + Laurentian Channel) and morphometric (Gulf of Maine) analyses, requires further study [60]. No dedicated genetic studies have been carried out for S. fasciatus that inhabit the Flemish Cap waters.
Such uncertainties increase the relevance of parasitological studies of the Acadian redfish, because the use of parasites as natural tags to investigate the population biology, migrations, and phylogeny of fish is a generally accepted approach [15, 18, 43, 44, 61]. The combined ecological and parasitological method implies study of not only the species composition, but also the conditions for the formation of parasite communities in a host, which is not considered in publications on parasites of this redfish species [32, 37, 46, 52].
Attempts have been made to distinguish between groups of redfish of the genus Sebastes off the Canadian coast on the basis of discrete pattern of their infection by the copepods Sphyrion lumpi, Chondracanthus nodosus, and Peniculus clavatus, by larval nematodes Anisakis sp. and Contracaecum sp., and also by the cestode Eubothrium sp. [28, 36, 53]. However, the lack of approaches to reliable differentiation of the species of the genus Sebastes by the time of these publications and the limited number of species (one to six) of redfish parasites used in the occurrence analysis are weaknesses of these works.
The present study was aimed at elucidating the ecological and geographical pattern of the compositions of the parasite communities in the Acadian redfish from the northwestern Atlantic Ocean and use it to identify the population biology characteristics of this host.
MATERIALS AND METHODS
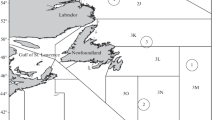
Acadian redfish samples were collected from bottom trawl tows made at depths of 240–410 m in April–June 1986–1987 and 1990 in the Flemish Cap (Division 3M), and in two southern (Divisions 3N and 3O) and two northern (Divisions 3L and 3K) sectors of GBN (Fig. 1). The boundaries of the Divisions were established by the Northwest Atlantic Fisheries Organization (NAFO) [47]. Complete parasitological dissection [13, 16] and species identification of the found parasites were carried out for 110 specimens of Acadian redfish at ages of 4–20 yr with a TL body length of 15 to 38 cm (90 specimens from GBN and 20 specimens from the Flemish Cap waters). Blood parasites were not studied. The specimens were attributed to the Acadian redfish on the basis of Methodological Guidelines… [22]; the ages of the fish were estimated using the scale reading technique [26].
The areas where parasite communities of the Acadian redfish Sebastes fasciatus were studied (the authors of the present article conducted studies in areas 1–5; data for areas 6–9 were published earlier): (1) NAFO Division 3K; (2) northeastern GBN; (3) Flemish Cap Bank; (4 and 5) southeastern and southwestern GBN; (6) Gulf of St. Lawrence; (7) St. Pierre Bank; (8) Scotian shelf; (9) Southern Labrador. The digit-letter designations in parentheses are the codes of divisions in accordance with the map of the Northwest Atlantic Fisheries Organization (NAFO) [47].
The main parameters used to characterize the degree of parasitic infection were the prevalence of infection (PI), which is the proportion of fish with parasites of certain species (the percentage of the number of examined fish), and the abundance index (AI), which is the number of parasites of this species per one examined fish [34]. The significance of differences (p) in PI by parasites was assessed by the chi-square (χ2) test with a significance level of p < 0.05 [12]. To assess the degree of infection of fish by the copepod Sphyrion lumpi, live individuals of the parasite and traces of its activity (remnants of cephalothorax) were recorded [7]. A dominant species showing the highest values of infection parameters and found in fish from all or most of the areas were considered as species forming the “core” of parasite communities. The taxonomic composition of parasite communities is consistent with recent data [62].
The ecological analysis of parasite community composition was based on the method of vertical zoning of ecological groups, as proposed by Andriyashev [2] and Gaevskaya [14]. The affiliation of parasite species to zoogeographical and ecological complexes was determined on the basis of the literature data [17, 35, 42] and the results of our own research.
When assessing the degree of isolation of the compared Acadian redfish groups, we used its inverse relationship with the degree of similarity (L0) of parasite community compositions [1] estimated by the Sørensen–Czekanowski similarity coefficient [10] and also by the method of principal component analysis [19]. When studying the pattern of the geographical structure of Acadian redfish parasite communities, we used our own data obtained during the survey of the Flemish Cup waters (NAFO Division 3M) and GBN (NAFO Divisions 3K, 3L, 3N, and 3O), and also the published literature data for the Gulf of St. Lawrence [46] because information about most taxa of parasites found in this host is available only for these areas. A “form of population rank” was defined as a group of individuals of the same species with evident reproduction, but with its constancy and duration not confirmed [27]. Groups were defined as aggregations of Acadian redfish with a certain degree of isolation leading to a decrease in gene flow.
RESULTS
As a result of examination of Acadian redfish from five areas, we identified parasites of 17 species belonging to six classes (Table 1): Myxozoa (4 species), Cestoda (2 species), Trematoda (5 species), Chromadorea (2 species), Palaeacanthocephala (1 species), and Hexanauplia (3 species). Three Myxozoa species: Myxidium obliquelineolatum, Leptotheca adeli, and Pseudoalataspora sebastei, found in fish from all areas that we surveyed (with PI values ranging from 5 to 65%) were recorded from this host for the first time. Derogenes varicus and Podocotyle reflexa (Trematoda), with their PI values in the northeast of GBN reaching 40 and 80%, respectively, and also larval forms of the chromadorids Anisakis simplex (PI from 20 to 75%) and Hysterothylacium aduncum (PI from 20 to 90%) occurred everywhere. The copepods Chondracanthus nodosus and Peniculus clavatus, the myxosporidian Leptotheca adeli, and larvae of the cestode Scolex pleuronectis were identified in Acadian redfish from four areas. Other parasites were found in this host from one to three areas.
The parasite community composition in Acadian redfish varied from 10 species in the north of the study region (Fig. 1, area 1) to 15 species in the south (Fig. 1, area 4). The results indicate pronounced geographical differences both in the parasite community composition in this host and in the degree of infection by parasites of many common species (see Table 1). The identified species of parasites belong to mesobenthic (7 species), polyzonal (6 species), and mesopelagic (4 species) ecological complexes with the dominance (11 species) of the Arctic/Boreal zoogeographical complex, of which 5 species are Arctic/amphi-Boreal.
DISCUSSION
An analysis of the results and literature data [32, 37, 46, 52] has shown that the parasite communities in Acadian redfish over the area covering most of its range in the Northwest Atlantic comprise a total of 29 species belonging to eight classes (see Table 1): Microsporea (1 species), Conoidasida (1 species), Myxozoa (6 species), Cestoda (3 species), Trematoda (10 species), Chromadorea (3 species), Palaeacanthocephala (1 species), and Hexanauplia (4 species).
Some parasites such as Gonocerca sp., Steganoderma formosum, and Lafystius morhuanus are untypical for Acadian redfish, because they were found only in 0.3–2.0% of the fish, as a rule, only from one of the considered areas or, like Ichthyophonus hoferi, a very long time ago (in the 1950s). The presence of Myxidium sphaericum and Ceratomyxa macrospora (Myxozoa), previously reported [37] for areas 1–5 (Fig. 1), is questioned, since we did not find these species in a few hundred examined individuals of the genus Sebastes there [3, 5, 6]. Therefore, the actual number of species that determine the overall appearance of the Acadian redfish parasite communities is no greater than 20. Among them, six species (P. sebastei, S. pleuronectis, D. varicus, P. reflexa, A. simplex, and H. aduncum) constitute the “core” of the parasite communities of this host (Table 1) and six species (the myxosporidians L. adeli, M. obliquelineolatum, P. sebastei, and the copepods C. nodosus, P. clavatus, and S. lumpi) are specific to the genus Sebastes from the North Atlantic seas [4, 5].
The presence of dominant and rarer species of parasites that infect Acadian redfish feeding on benthic planktonic invertebrates (Calanoida, Hyperiidea, Euphausiacea, and Sagittoidea), which are used as intermediate hosts by many helminths in their life cycles [38–40, 51, 54], indicates that ecological and trophic factors are the key ones responsible for the formation of parasites communities in this host that are characteristic of planktivorous fish.
The overall appearance of Acadian redfish parasite communities is formed by species of four ecological complexes (see Table 1), where the polyzonal (eight species) and mesobenthic (seven species) ones are represented most widely (Fig. 2a). The zoogeographical complexes are dominated by species of the Arctic/Boreal complex (65%), 15% of species belong to the Boreal complex, and 20% are cosmopolitan species (Fig. 2b).
The highest diversity (20 species) was observed in the parasite community of Acadian redfish that inhabit the Gulf of St. Lawrence due to the rich composition of helminths (70% of the total number of parasites); in other areas, there were 10–15 species, of which the proportion of helminths made up 46–60%. The results of a comparative analysis of the S. fasciatus parasite communities from the pairs of adjacent areas (except for areas 5 and 4 in Fig. 1) revealed differences in their composition and significant differences in the degree of infection by parasites of many common species (see Table 1).
A geographical analysis of the compositions of Acadian redfish parasite communities (Fig. 3) showed a maximum degree of similarity (L0 = 94%) between areas 4 and 5, as well as areas 1 and 2 (L0 = 86%). The greatest difference in the composition of parasite communities in this host was characteristic of area 6 (Gulf of St. Lawrence) (L0 = 48–63%) and area 3 (Flemish Cap Bank) (L0 = 79%).
A dendrogram of the similarity (L0) between the parasite community compositions in Acadian redfish from six areas. Designation of areas as in Fig. 1.
The results of a cluster analysis also indicated significant differences in the parasite community composition between Acadian redfish from the Gulf of St. Lawrence and, to a lesser extent, from the Flemish Cap waters (Fig. 4), because six parasite species (Eimeriida gen. sp., Brachyphallus crenatus, Gonocerca sp., Opecoelidae gen sp., Pseudoterranova decipiens l., and Lafystius morhuanus) were recorded only from the Gulf of St. Lawrence [46], while the indicator parasites Leptotheca adeli (Table 2), Scolex pleuronectis pl., and Lecithophyllum botryophoron, previously found in this host in most areas, did not occur in the Flemish Cap Bank [32, 46].
The geographical structure of the composition of parasite communities in Acadian redfish from six areas, as inferred from the cluster analysis (Jaccard Index). The numerals indicate bootstrap analysis values. Designation of areas as in Figs. 1 and 3.
The high degree of similarity in the pattern of geographical structure between the Acadian redfish parasite communities inferred by the two methods (see Figs. 3 and 4) is the evidence of the correctness of our results and the validity of such estimates.
It is known that out of the three redfish species of the genus Sebastes that sympatrically inhabit waters of the Canada’s continental shelf, mainly Acadian redfish, dominating in number (56–99% of individuals), extrude pre-larvae (81–90%) in April–July [9, 30, 41, 45, 57]. Larvae and pelagic juveniles of Acadian redfish are assumed to not be subject to long-range transfer with currents, but rather enter quasi-stationary gyres in the Canadian shelf zone [20, 21].
Judging by the clearly settled lifestyle of the Acadian redfish, its population characteristics, and the results of ecological and parasitological analysis, it can be assumed that six groups of this species exist with varying degrees of isolation, defined as forms of a population rank, in Canadian shelf waters. The most significant isolation of the group of this redfish species in the Gulf of St. Lawrence (Fig. 1, area 6) is explained by the specific oceanographic conditions of this largely enclosed area. The results of a study of microsatellite loci and some morphological characters of fish have shown that this group is not homogeneous even within the gulf [31, 60].
Literature data [32, 52] on 13 species of helminths that parasitize Acadian redfish in waters of the Scotian shelf (area 8) and St. Pierre Bank (Fig. 1 area 7) indicate a similarity of the compositions of their parasite communities (Table 1). It is likely that these areas are inhabited by a single Acadian redfish group. Significant differences in values of infection parameters were recorded only for three helminth species common to these areas (Podocotyle reflexa, Anisakis simplex l., and Hysterothylacium aduncum l.) and typical of this host and other fish species. The isolation of the Acadian redfish group of these areas from the group that inhabit the adjacent Gulf of St. Lawrence is evidenced by the absence of Hexanauplia [32] and the significant differences (p < 0.05) in the prevalence of infection by such species as Scolex pleuronectis pl., Derogenes varicus, Lecithophyllum botryophoron, Lecithaster gibbosus, H. aduncum l., and Pseudoterranova decipiens l. [46].
Data obtained through genetic studies [58, 60] show a probability of limited migrations of Acadian redfish into the Gulf of St. Lawrence from the adjacent waters of the shelf of Newfoundland, Nova Scotia, and Labrador. These migrations are facilitated by the deep current that flows into the bay, bringing the mixed waters of the Labrador Current and the Scotian shelf in the southeast and the waters of the Labrador Current in the northeast [23]. Results of a microsatellite analysis have shown independence of the group of this species living in the Gulf of Maine [48, 60].
The presence of the helminths Grillotia sp. pl., Anomalotrema koiae, Hemiurus levinseni, and L. gibbosus in Acadian redfish only from the waters of the St. Pierre Bank and the Scotian shelf, as well as the significant differences (p < 0.05) in the prevalence of infection by S. pleuronectis pl., D. varicus, L. botryophoron, A. simplex l., and H. aduncum l. common to this host, are evidence for the isolation of its group in areas 8 and 7 (Fig. 1) from the group in the southern part of GBN (see Table 1).
The significant isolation of the Acadian redfish groups in the southern (areas 4 and 5) and northeastern parts of GBN (area 2) is indicated by a significantly lower (p < 0.05) prevalence of infection by all four Myxozoa species, by the helminths S. pleuronectis pl., D. varicus, P. reflexa, and H. aduncum l. with a significantly higher occurrence rate of L. botryophoron and larval A. simplex, and also by the absence of the trematodes A. koiae and L. gibbosus and the presence of only the cestode Bothriocephalus scorpii here, and by traces of infection by the copepod S. lumpi. The isolation of the Acadian redfish groups in the southern and northeastern parts of GBN is caused by the quasi-stationary gyres over these areas and the shallow depths (40–80 m) of the central part of GBN. The genetic heterogeneity of Acadian redfish samples from different GBN areas is also confirmed by the results of comprehensive studies of genetic and morphological features [31, 60].
The Flemish Cap Bank is separated from the neighboring GBN by the deep-water Flemish Pass, while the closed anticyclonic current flowing over it causes pelagic juvenile fish to drift mainly towards the center of the bank [11, 24, 33]. These factors act as a relative barrier preventing mutual migrations of fish between these areas. This is confirmed by the absence of the myxosporidian L. adeli (indicator parasite), the helminths S. pleuronectis pl., L. botryophoron, and L. gibbosus (that are characteristic of this host in the GBN areas) from the parasite community of Acadian redfish in the Flemish Cap Bank, and also by a significantly lower occurrence rate of the indicator parasites Myxidium incurvatum, M. obliquelineolatum, P. sebastei, and C. nodosus and a significantly higher prevalence of infection by the helminths B. scorpii, A. simplex l., and H. aduncum l.
Areas 1 (NAFO Division 3K) and 9 (Southern Labrador) are probably occupied by a group of Acadian redfish largely isolated from aggregations in the northeastern GBN (area 2). This is confirmed by significant differences (p < 0.05) in prevalence of infection by common parasite species: three Myxozoa species (indicator parasites), and also by the helminths S. pleuronectis pl., D. varicus, and P. reflexa (Tables 1, 2). The low degree of similarity (L0 = 87%) between the parasite community compositions of this host is explained by the absence of some species of Trematoda (A. koiae, H. levinseni, L. botryophoron, and L. gibbosus) and Hexanauplia (C. nodosus, P. clavatus) from one of these areas and their presence in the other.
Thus, the results of our analysis of parasitological, population, and oceanographic data indicate the existence of six groups of Acadian redfish and their breeding grounds in the Atlantic shelf of Canada, which can be classified as forms of a population rank (in the interpretation by Yablokov [27]). The most isolated groups are confined to the Gulf of St. Lawrence and the Flemish Cap Bank. Limited migrations of Acadian redfish are possible to the former of them from the adjacent waters of the Scotian shelf and the St. Pierre Bank inhabited by a single group of this species, as evidenced by the results of parasitological studies. The southern GBN is also home to a single Acadian redfish group. Its isolation from the groups of this species in both the northeastern part of GBN and its northernmost part (NAFO Division 3K and Southern Labrador) is caused by the specific oceanographic regime, the shallow depths of the central GBN, and also by the relatively settled lifestyle of Acadian redfish. The isolation of the Acadian redfish group in the Flemish Cap waters from the above-mentioned groups is due to the closed anticyclonic current and the deep-water Flemish Pass separating it from GBN.
The results of this intraspecific differentiation of the Acadian redfish, as inferred from the analysis of geographical patterns of compositions of its parasites communities, are consistent with data of a comprehensive analysis of the genetic and morphometric characteristics of this species available in the literature. The information we obtained is highly relevant for clarifying the status of the identified groups and the probable boundaries between them, and also for regulating the Acadian redfish fishery.
REFERENCES
Andreev, V.L. and Reshetnikov, Yu.S., Analysis of composition of the freshwater fish fauna in the northeastern part of the Soviet Union based on the methods of set theory, Zool. Zh., 1978, vol. 57, no. 2, pp. 165–174.
Andriyashev, A.P., On some issues of the vertical zoning of marine benthic fauna, in Biologicheskiye resursy Mirovogo okeana (Biological Resources of the World Ocean), Moscow: Nauka, 1979, pp. 117–138.
Bakay, Yu.I., Ecological and parasitological characteristics of the golden redfish Sebastes marinus (Scorpaeniformes), Vestn. Murm. Gos. Tekh. Univ., 2012, vol. 15, no. 4, pp. 706–715.
Bakay, Yu.I., Features of formation of the parasite fauna in North Atlantic redfishes of the genus Sebastes (Scorpaenidae), in Sovremennye problemy teoreticheskoi i morskoi parazitologii: Sbornik statei (Current Problems in Theoretical and Marine Parasitology: Collected Papers), Sevastopol: Bondarenko N.Yu., 2016, pp. 208–211.
Bakay, Yu.I., Ecological and population features of beaked redfish Sebastes mentella (Sebastidae) in the Northwest Atlantic based on the analysis of its parasite fauna, J. Ichthyol., 2020, vol. 60, no. 3, pp. 475–484.
Bakay, Yu.I. and Grudnev, M.A., Fauna of myxosporidians (Myxozoa: Myxosporea) in the scorpionfish genus Sebastes from North Atlantic, Parazitologiya, 2009, vol. 43, no. 4, pp. 317–329.
Bakay, Yu.I. and Karasev, A.B., Diagnostika i registratsiya ektoporazhenii morskikh okunei (metodicheskoye rukovodstvo) (Diagnostics and Registration of Ectolesions in Redfishes: A Methodological Guide), Murmansk: PINRO, 1995.
Barsukov, V.V. and Zakharov, G.P., Morphological and biological features of Acadian redfish, Tr. Polyarn. Nauchno–Issled. Inst. Morsk. Rybn. Khoz. Okeanogr., 1972, vol. 28, pp. 143–173.
Barsukov, V.V., Oganin, I.A., and Pavlov, A.I., Morphological and ecological differences of Sebastes fasciatus and S. mentella in the Newfoundland shelf and the Flemish Cap Bank, Vopr. Ikhtiol., 1990, vol. 30, no. 5, pp. 791–803.
Bailey, N.T.J., The Mathematical Approach in Biology and Medicine, London: Wiley, 1967.
Borovkov, V.A., Korsakov, A.L., and Vaskov, A.A., The role of water circulation in dynamics of strength of redfish and cod year-classes in the Flemish Cap Bank, Vopr. Promysl. Okeanol., 2005, vol. 2, pp. 243–252.
Breev, K.A., Application of mathematical methods in parasitology, Izv. Gos. Gos. Nauchno-Issled. Inst. Ozern. Rechn. Rybn. Khoz., 1976, vol. 105, pp. 109–126.
Bykhovskaya-Pavlovskaya, I.E., Parazity ryb. Rukovodstvo po izucheniyu (Fish Parasites: A Study Guide), Leningrad: Nauka, 1985.
Gaevskaya, A.V., Fish parasites in the Northeast Atlantic: Fauna, ecology, and patterns of formation, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Leningrad: Zool. Inst., Akad. Nauk SSSR, 1984.
Gaevskaya, A.V. and Kovaleva, A.A., Parasitological method in population studies of fishes from the Atlantic Ocean and its seas, in Biologicheskiye resursy Atlanticheskogo okeana (Biological Resources of the Atlantic Ocean), Moscow: Nauka, 1986, pp. 329–338.
Donets, Z.S. and Shul’man, S.S., On the methods to study Myxosporidia (Protozoa, Cnidosporidia), Parazitologiya, 1973, vol. 7, no. 2, pp. 191–192.
Zubchenko, A.V., Vertical zoning and patterns of parasite fauna formation in deep-sea fishes from the open part of the North Atlantic, in Parazitologicheskiye issledovaniya ryb Severnogo basseina (Parasitological Studies of Fish of the North Basin), Murmansk: PINRO, 1993, pp. 39–60.
Konovalov, S.M., Differentsiatsiya lokal’nykh stad nerki Oncorhynchus nerka (Walbaum) (Differentiation of Local Stocks of the Sockeye Salmon Oncorhynchus nerka Walbaum), Leningrad: Nauka, 1971.
Korosov, A.V. and Gorbach, V.V., Komp’yuternaya obrabotka biologicheskikh dannykh: Metodicheskoye posobiye (Computer Processing of Biological Data: A Methodological Guide), Petrozavodsk: Petrozavodsk. Gos. Univ., 2010.
Litvinenko, N.I., Migrations of redfishes of the genus Sebastes, Scorpaenidae and their association with currents in the North Atlantic, in Aktual’nye problemy obshchestvennykh, estestvennykh i tekhnnicheskikh nauk: Tezisy dokladov (Current Problems in Social, Natural, and Technical Sciences, Abstr. Conf.), Perm, 1981, pp. 49–50.
Litvinenko, N.I., Redfishes (genus Sebastes) of the North Atlantic: Their morphology, ecology, distribution, dispersal, and evolution, Extended Abstract of Cand. Sci. (Biol.) Dissertation, Leningrad: Leningr. Gos. Univ., 1985.
Metodicheskiye ukazaniya po opredeleniyu vidov morskikh okunei severnoi chasti Atlanticheskogo okeana i prilezhashchikh morei (Methodological Guidelines for Identification of Redfish Species from the North Atlantic Ocean and Adjacent Seas), Kaliningrad: AtlantNIRO, 1984.
Okeanograficheskaya entsiklopediya (Oceanographic Encyclopedia), Leningrad: Gidrometeoizdat, 1974.
Serebryakov, V.P., On the study of ichthyoplankton in the Newfoundland and Labrador regions, in Sovetskiye rybokhozyaistvennye issledovaniya v severo-zapadnoi chasti Atlanticheskogo okeana (Soviet Fisheries Research in the Northwestern Atlantic Ocean), Moscow: VNIRO–PINRO, 1962, pp. 227–233.
Sorokin, V.P., Genus of redfishes, in Promyslovye biologicheskiye resursy Severnoi Atlantiki i prilegayushchikh morei Severnogo Ledovitogo okeana (Commercial Biological Resources of the North Atlantic and Adjacent Seas of the Arctic Ocean), Murmansk: PINRO, 1977, part 2, pp. 58–90.
Chugunova, N.I., Rukovodstvo po izucheniyu vozrasta i rosta ryb (Metodicheskoe posobie po ikhtiologii) (A Guide to the Study of Fish Age and Growth: A Textbook of Methods in Ichthyology), Moscow: Akad. Nauk SSSR, 1959.
Yablokov, A.V., Populyatsionnaya biologiya (Population Biology), Moscow: Vysshaya Shkola, 1987.
Yanulov, K.P., On the groups of the beaked redfish (Sebastes mentella) in the Labrador–Newfoundland region, in Sovetskiye rybokhozyaistvennye issledovaniya v severo-zapadnoi chasti Atlanticheskogo okeana (Soviet Fisheries Research in the Northwestern Atlantic Ocean), Moscow: VNIRO–PINRO, 1962, pp. 285–296.
Artamonova, V., Makhrov, A., Karabanov, D., et al., Hybridization of beaked redfish (Sebastes mentella) with small redfish (S. viviparus) and diversification of redfish (Actinopterygii: Scorpaeniformes) in the Irminger Sea, J. Nat. Hist., 2013, vol. 47, nos. 25–28, pp. 1791–1801.
Bainbridge, V. and Cooper, G., Populations of Sebastes larvae in the North Atlantic, Int. Comm. Northwest Atl. Fish. Res. Bull., 1971, no. 8, pp. 27–35.
Benestan, L.M., Rougemont, Q., Senay, C., et al., Population genomics and history of speciation reveal fishery management gaps in two related redfish species (Sebastes mentella and Sebastes fasciatus), Evol. Appl., 2021, vol. 14, no. 2, pp. 588–606.
Bourgeois, C.E. and Ni, I.-H., Metazoan parasites of Northwest Atlantic redfishes (Sebastes spp.), Can. J. Zool., 1984, vol. 62, pp. 1879–1885.
Borovkov, V.A. and Kudlo, B.P., Results of USSR oceanographic observations on Flemish Cap, 1977–78, in International Commission for the Northwest Atlantic Fisheries: Selected Papers no. 6, Dartmouth, Canada: ICNAF, 1980, pp. 47–52.
Bush, A.O., Lafferty, K.D., Lotz, J.M, and Shostak, A.W., Parasitology meets ecology on its own terms: Margolis et al. revisited, J. Parasitol., 1997, vol. 83, no. 4, pp. 575–583.
Hemmingsen, W. and McKenzie, K., The parasite fauna of the Atlantic cod, Gadus morhua L., Adv. Mar. Biol., 2001, vol. 40, pp. 1–80.
Kabata, Z., Parasites as biological tags, in International Commission for the Northwest Atlantic Fisheries: Special Publication no. 4, Dartmouth, Canada: ICNAF, 1963, pp. 31–37.
Khan, R.A., Bowering, W.R., Bourgeois, C., et al., Myxosporean parasites of marine fish from the continental shelf off Newfoundland and Labrador, Can. J. Zool., 1986, vol. 64, pp. 2218–2286.
Køie, M., On the morphology and life-history of Derogenes varicus (Müller, 1784) Looss, 1901 (Trematoda, Hemiuridae), Z. Parasitenkd., 1979, vol. 59, pp. 67–78.
Køie, M., On the morphology and life-history of Podocotyle reflexa (Creplin, 1825) Odhner, 1905, and a comparison of its developmental stages with those of P. atomon (Rudolphi, 1802) Odhner, 1905 (Trematoda, Opecoelidae), Ophelia, 1981, vol. 20, no. 1, pp. 17–43.
Køie, M., Aspects of the life cycle and morphology of Hysterothylacium aduncum (Rudolphi, 1802) (Nematoda, Ascaridoidea, Anisakidae), Can. J. Zool., 1993, vol. 71, pp. 1289–1296.
Lambert, Y., Dutil, J.-D., and Sévigny, J.-M., Variability in reproductive characteristics and larvae production of redfish (Sebastes fasciatus) in the Gulf of St. Lawrence, in Redfish Multidisciplinary Research Zonal Program (1995–1998): Final Report, Gascon, D., Ed., Can. Tech. Rep. Fish. Aquat. Sci., 2003, no. 2462, pp. 99–118.
Lile, N.K., Halvorsen, O., and Hemmingsen, W., Zoogeographical classification of the macroparasite faunas of four flatfish species from the northeastern Atlantic, Polar Biol., 1994, vol. 14, pp. 137–141.
MacKenzie, K. and Abaunza, P., Chapter 11 – Parasites as biological tags, in Stock Identification Methods: Applications of Fisheries Science, Cadrin, S.X., Friedland, K.D., and Waldman, J.R., Eds., New York: Academic, 2005, pp. 211–226.
MacKenzie, K. and Hemmingsen, W., Parasites as biological tags in marine fisheries research: European Atlantic waters, Parasitology, 2015, vol. 142, no. 1, pp. 54–67.
Melnikov, S.P., Intraspecies structure of beaked redfish Sebastes mentella of the Atlantic and Arctic oceans, J. Ichthyol., 2016, vol. 56, no. 1, pp. 52–71.
Moran, J.D.W., Arthur, J.R., and Burt, M.D.B., Parasites of sharp-beaked redfishes (Sebastes fasciatus and S. mentella) collected from the Gulf of St. Lawrence, Canada, Can. J. Fish. Aquat. Sci., 1996, vol. 53, pp. 1821–1826.
NAFO Regulatory Area. https://www.nafo.int/Portals/0/Images/maps/RegulatoryAreaMap.png. Accessed November 20, 2020.
Roques, S., Sévigny, J.-M., and Bernatchez, L., Evidence for broadscale introgressive hybridization between two redfish (genus Sebastes) in the North-west Atlantic: a rare marine example, Mol. Ecol., 2001, vol. 10, pp. 149–165.
Saborido-Rey, F., The genus Sebastes Cuvier, 1829 (Scorpaenidae) in the North Atlantic: Species and population identification using morphometric techniques; Growth and reproduction of the Flemish Cap populations, PhD Thesis, Madrid: Univ. Autónoma, 1994.
Saha, A., Johansen, T., Hedeholm, R., et al., Geographic extent of introgression in Sebastes mentella and its effect on genetic population structure, Evol. Appl., 2017, vol. 10, no. 1, pp. 77–90.
Scott, J.S., Trematode populations in the Atlantic argentine, Argentina silus, and there use as biological indicators, J. Fish. Res. Board Can., 1969, vol. 26, pp. 879–891.
Scott, J.S., Helminth parasites of redfish (Sebastes fasciatus) from the Scotian Shelf, Bay of Fundy, and eastern Gulf of Maine, Can. J. Zool., 1988, vol. 66, no. 3, pp. 617–621.
Sindermann, C.J., Parasitological tags for redfish of the western North Atlantic, in International Commission for the Northwest Atlantic Fisheries: Special Publication no. 3, Dartmouth, Canada: ICNAF, 1961, pp. 111–117.
Smith, J.W., Larval Anisakis simplex (Rudolphi, 1809, det. Krabbe, 1878) and larval Hysterothylacium sp. (Nematoda: Ascaridoidea) in euphausiids (Crustacea: Malacostraca) in the North-East Atlantic and northern North Sea, J. Helminthol., 1983, vol. 57, pp. 167–177.
St-Pierre, J.-F. and de Lafontaine, Y., Fecundity and reproduction characteristics of beaked redfish (Sebastes fasciatus and S. mentella) in the Gulf of St. Lawrence, Can. Tech. Rep. Fish. Aquat. Sci., 1995, no. 2059, pp. 1–32.
Templeman, W., Redfish distribution off Baffin Island, Northern Labrador, and in Ungava Bay in August–September 1959, in International Commission for the Northwest Atlantic Fisheries: Special Publication no. 3, Ottawa: Fisheries and Oceans Canada, pp. 157–162.
Templeman, W., Incidence of subcaudal melanophores in pre-extrusion larvae of redfish species in the Newfoundland-Labrador area, J. Northwest Atl. Fish. Sci., 1980, vol. 1, pp. 7–19.
Valentin, A., Structure des populations de Sébaste de L’Atlantique du nord-ouest duns un contexte de gestion des stocks et d’évolution, Thèse de Doctorat, Quebec: Univ. Quebec, 2006.
Valentin, A., Sévigny, J.-M., and Chanut, J.-P., Geometric morphometrics reveals body shape differences between sympatric redfish Sebastes mentella, S. fasciatus and their hybrids in the Gulf of St Lawrence, J. Fish Biol., 2002, vol. 60, pp. 857–875.
Valentin, A., Penin, X., Chanut, J.-P., et al., Combining microsatellites and geometric morphometrics for the study of redfish (Sebastes spp.) population structure in the Northwest Atlantic, Fish. Res., 2014, vol. 154, pp. 102–119.
Williams, H.H., MacKenzie, K., and McCarthy, A.M., Parasites as biological indicators of the population biology, migrations, diet and phylogenetics of fish, Rev. Fish Biol. Fish., 1992, vol. 2, pp. 144–176.
WoRMS Editorial Board, World Register of Marine Species. http://www.marinespecies.org. Accessed September 2019.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflict of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Translated by E. Shvetsov
Rights and permissions
About this article
Cite this article
Bakay, Y.I., Rolskii, A.Y. The Ecological and Population Characteristics of the Acadian Redfish Sebastes fasciatus Storer, 1856 (Scorpaeniformes: Sebastidae) Inferred from Analysis of its Parasite Community Composition. Russ J Mar Biol 48, 10–18 (2022). https://doi.org/10.1134/S1063074022010023
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1063074022010023