Abstract
The potential value of parasites as ecosystem markers was tested by analyzing the metazoan assemblages of Urophycis brasiliensis caught in four locations distributed in three ecoregions of the Warm Temperate Southwestern Atlantic. A total of 5,001 metazoan parasites belonging to 33 species were found. The identified parasites varied across locations in terms of presence, prevalence, and abundance, and their multivariate analyses resulted in clear similarity patterns. No differences were observed between two locations of the same ecoregion, whereas an evident separation of samples was observed across ecoregions in support of the existing hypotheses regarding the ecoregional division of the southwestern Atlantic. We proposed that parasite assemblages, which are composed of several metazoan phyla, are potentially useful as ecosystem indicators. This suggestion is derived from the combined evidence of the evolutionary history and biogeography of multiple lineages, which is expected to be more efficient in capturing recurrent patterns in overall biodiversity than individual lineages. Furthermore, as many parasites have complex life cycles, their distribution patterns are dependent not only on environmental conditions but also on the distribution and population density of all hosts involved in their life cycles, adding further sources of distributional variability that act synergistically to define robust geographical patterns. The selection of long-lived parasites and their comparative analysis provided evidence supporting the existence of three different stocks in the four sampled areas. The best parasite tags were those with low specificity in fish hosts, constituting promising biological tags for the stock discrimination of other fish species in the region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among the applied aspects of ichthyoparasitology, the use of parasites as biological tags has been established as a valuable methodology for stock identification and the determination of connectivity between populations of marine fish (Cadrin et al. 2005; Timi and MacKenzie 2014). Parasite tags are applied to discriminate stocks of individual valuable host species across various locations of their distributional range. These types of studies have been successfully applied to several host species in the southwestern Atlantic, mainly to fishes from northern and central Argentine waters (Cantatore and Timi 2014), whose populations display significant differences in composition as a consequence of the contrasting oceanographic characteristics between these regions and their effect on parasite distribution (Timi 2007). However, the potential use of parasite tags remains unexplored in most regions of the vast South American Atlantic waters, particularly along the northern coast.
Indeed, with the exception of comparisons of several fish species between the regions of Rio de Janeiro, Brazil and northern Argentine waters (Timi et al. 2005, 2010; Braicovich et al. 2012), only one study using parasites to discriminate fish stocks of Micropogonias furnieri from five locations along the Brazilian coast, located between Rio de Janeiro and Rio Grande do Sul near the boundary with Uruguay, has been published (Luque et al. 2010). This region is part of the large ecosystem defined as the Brazilian Shelves by Miloslavich et al. (2011); it is a very large region that is hydrologically and topographically complex with contrasting dominant ecosystems of unique features, including mangroves, coral reefs, dunes, sand banks, sandy beaches, rocky shores, lagoons, estuaries, and salt marshes. This multiplicity of environments offers an excellent opportunity to use parasites not only as biological indicators of stock structure for an enormous variety of fish species but also as markers of the regions or waters they inhabit; consequently, they can also be used as ecosystem indicators (Cantatore and Timi 2014).
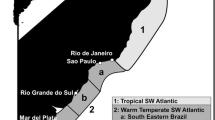
The distribution patterns and endemisms of southwestern Atlantic biota have been utilized to describe a series of biogeographical units (realms, provinces, and ecosystems) with different degrees of geographic resolution (Spalding et al. 2007; Miloslavich et al. 2011; Briggs and Bowen 2012). Specifically, the coastal and shelf region between Rio de Janeiro and the northern Argentine Sea shows a continuous distribution of warm temperate fauna and has historically been characterized as the Argentinean Province. It was recently renamed as the Temperate Western South Atlantic Province (Menni et al. 2010) or the Warm Temperate Southwestern Atlantic (Spalding et al. 2007). Its limits with neighboring provinces, as well as its subdivision in districts, are variably defined according to the group of organisms studied. For example, the region of Cabo Frio (22° 54′ S) has traditionally been proposed as the limit of this province with the northern Brazilian Province and western Atlantic Tropics based on the distribution of different zoological groups, such as mollusks, decapod crustaceans, cartilaginous fishes, etc. (Briggs 1995; Boschi 2000; Spalding et al. 2007; Menni et al. 2010). However, based on evidence obtained from mangrove gastropods and reef fishes, Floeter and Soares-Gomes (1999), Floeter and Gasparini (2000), and Floeter et al. (2001, 2008) concluded that this limit should be extended further south to Santa Catarina State. Similarly, the boundary between the two main districts of the Argentine Province (or Temperate Western South Atlantic Province), namely the southern Brazilian and Bonaerensean, has been variably located near the mouth of the Rio de la Plata, at 34° S or fluctuating between 30° S and 32° S, in front of the State of Rio Grande do Sul (Balech and Ehrlich 2008; Menni et al. 2010).
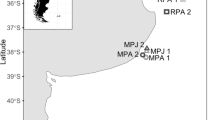
The aim of this study is to test the potential value of parasites as ecosystem markers (Cantatore and Timi 2014) by analyzing the metazoan assemblages of the Brazilian codling Urophycis brasiliensis (Kaup, 1858), a common inhabitant of the coastal waters of the southwestern Atlantic between the regions of Rio de Janeiro, Brazil and the northern Argentine Sea (Cousseau and Perrotta 2004). The parasite fauna of this species is relatively well known in the extremes of its distribution (Szidat 1961; Ivanov et al. 1997; Suriano and Labriola 1999; Martorelli et al. 2000; Alves et al. 2003, 2004; Lanfranchi et al. 2004; Menoret and Ivanov 2009); however, only Alves et al. (2004) have focused on the quantitative aspects of parasite assemblages. Conversely, information on parasites of U. brasiliensis in the central region of its distribution is scarce (Pereira et al. 1996). The data were analyzed following the geographical frame proposed by Spalding et al. (2007), which divides the southwestern Atlantic coasts in three biogeographical provinces (Tropical Southwestern Atlantic, Warm Temperate Southwestern Atlantic, and Magellanic), which in turn are subdivided in ecoregions (Fig. 1).
Study area, sampling localities, and biogeographical provinces and ecoregions adapted from Spalding et al. (2007)
U. brasiliensis is exploited by artisanal and industrial fisheries along its broad distributional range, particularly in Uruguay (Acuña et al. 2000, 2007; Acuña and Verocai 2001); however, no specific assessment of stocks, a prerequisite for control and management plans, has been performed for this resource to date. In the zoogeographical context of parasite distribution, we also analyzed the potential use of parasites as tags for the stock discrimination of U. brasiliensis, focusing on a subset of long-lived parasite species to provide information that could be applied to the implementation of management strategies for U. brasiliensis fisheries.
Materials and methods
Fish samples and parasite inventories
Fish samples were taken from catches made by commercial trawlers operating at four locations in the southwestern Atlantic, three from Brazil and one from Argentina (Table 1). Following Spalding et al. (2007), samples were assigned to three ecoregions belonging to the Warm Temperate Southwestern Atlantic (Table 1). A total of 222 specimens of U. brasiliensis were collected and examined for parasites following standard procedures. Fish were either kept fresh or deep frozen at −18 °C until examination. After thawing, specimens were measured for total length (cm). Body surface, gills, branchial and body cavities, viscera (stomach, intestine, liver, gall bladder, spleen, heart, gonads, and mesenteries), gas bladder, kidneys, and musculature were examined with the aid of a stereoscopic microscope.
Fish length was compared between samples using a one-way ANOVA (Zar 1999). The prevalence and mean abundance were calculated for each parasite species in each sample following Bush et al. (1997). Unparasitized fish were excluded from subsequent analyses at the infracommunity level.
Parasites as biological indicators of ecoregions
Given the variability of the samples in relation to the season of capture and particularly fish length, we expected that a considerable proportion of the observed differences in parasite abundance would be explained by these factors. Consequently, most analyses were conducted using presence/absence data to diminish the effect of season and host length on parasite loads, allowing the location effects to be more evident. However, given that samples from Rio de Janeiro (RJ) and Santa Catarina (SC) were caught almost simultaneously, we consequently expected that their levels of similarity were not affected by the seasonality of parasite loads. The differences in community structure between both samples were tested using a one-way permutational multivariate analysis of the variance (PERMANOVA, Anderson et al. 2008) on the abundance values for all the identified parasite species, introducing host length as a covariable (ANCOVA model). The structures of parasite infracommunities between samples (1 × 2 factorial design, “location” as fixed factor) were compared, and the main effects were tested after 9,999 permutations. Following Anderson et al. (2008), a permutation of residuals under a reduced model was used as the method of permutation. A sequential sum of squares (type I SS) was applied because host length was introduced as a covariable and the samples were unbalanced (different numbers of fish examined by sample). The Jaccard index was used as the similarity measure; this index uses binary presence/absence data (Magurran 1988) to compare species composition between samples. These procedures were repeated using the Bray-Curtis index as a similarity measure. As the Bray-Curtis coefficient is undefined when two samples contain no individuals (e.g., uninfected fish) (Clarke et al. 2006), only fish harboring at least one parasite species were included in the analyses.
Nonmetric multidimensional scaling (MDS) (Clarke and Gorley 2006) was performed to visualize the geographic patterns in the composition of parasite assemblages across the four locations. MDS was conducted using the Jaccard similarity index (presence/absence data) at the level of both the infracommunities and component communities (sensu Bush et al. 1997) in each location. In all cases, the fit of the MDS ordinations was quantified by a value of stress. To determine a possible differential effect of multivariate dispersion of infracommunities between groups, differences in dispersion were calculated using the routine PERMDISP. Dispersions were measured as distances to the centroids, and each term in the analysis was tested using 9,999 permutations (Anderson et al. 2008).
As a more quantitative approach to the analysis of parasite distribution, MDS was also conducted at the component community level using a Bray-Curtis similarity index on prevalence data. We expected a less pronounced effect of season and host length on parasite prevalence than on abundance. In addition, the Bray-Curtis index, although it includes quantitative information, emphasizes compositional dissimilarity over quantitative values in comparison with other dissimilarity and distance measures that are routinely applied in the multivariate analyses of communities (Anderson 2006; Anderson et al. 2006, 2011). A hierarchical agglomerative clustering was applied to the component communities using group-average linking, and resemblance levels were overlaid on the MDS plot (Clarke and Gorley 2006).
In the MDS performed at the component community level, a fifth sample was included (sample code RJ2004) for comparative purposes. The data were obtained from a previous study by Alves et al. (2004) on 75 specimens of U. brasiliensis caught in a broad area of the region of the State of Rio de Janeiro, Brazil (21–23° S, 41–45° W) during a period of 1 year. The re-examination of preserved materials in the authors’ collection confirmed that the materials identified as Raphidascaris sp. by Alves et al. (2004) were actually members of Hysterothylacium sp. These authors also discriminated two species of larval Nybelinia sp.; as it was not possible to identify the plerocercoids found in the present study as belonging to one of these species, this group of parasites was excluded from the analyses.
Parasites as biological tags for stock discrimination
The potential use of parasites as biological tags for the discrimination of host stocks between sampling regions was evaluated on values of parasite abundance. The most important criterion for an effective parasite marker is its long residence time in the fish to avoid the effect of short-term temporal variability on parasite abundance (Lester 1990; Lester and MacKenzie 2009). Following these guidelines, only long-lived parasites were retained for the analyses. These parasites were the metacestodes Grillotia carvajalregorum, Callitetrarhynchus gracilis, and Tentaculariidae gen. sp.; the larval anisakids Contracaecum sp., Hysterothylacium sp., and Terranova sp.; and the juvenile acanthocephalans Corynosoma australe, Corynosoma cetaceum, and Bolbosoma turbinella. However, long-lived parasites tend to accumulate as hosts grow, thus producing ontogenetic changes in the structure of parasite communities; fish length should be taken into account as a potential confounding variable in the interpretation of spatial patterns and stock structure (Cantatore and Timi 2014). Therefore, for this set of analyses, fish length was introduced as a covariable to mitigate its effect on parasite abundance and achieve consistent conclusions. The selection of fish parasitized only by long-lived parasites reduced the sample size from the Brazilian locations, particularly from RJ and SC; consequently, these samples were combined as SB (Southeastern Brazil, following Spalding et al. 2007), given that no differences between the regions were observed in previous analyses.
PERMANOVA analyses (1 × 3 factorial design, “location” as fixed factor) were performed as in the previous section. When differences were detected by PERMANOVA, pairwise comparisons were used to determine which samples differed.
Because PERMANOVA is sensitive to differences in multivariate dispersion between groups (sensu homogeneity of variances, which can inflate type 1 error even when centroids have identical locations), the same models were tested for differences in dispersion using the routine PERMDISP (Anderson et al. 2008). Dispersions were measured as distance to the centroid, and each term in the analysis was tested using 9,999 permutations.
Differences between infracommunities among samples were examined in more detail using canonical analysis of principal coordinates (CAP) (Anderson and Willis 2003; Anderson et al. 2008). The potential for overparameterization was prevented by choosing the number of PCO axes (m) that maximized a leave-one-out allocation success to groups (Anderson and Robinson 2003). CAP analyses were based on abundance data using a Bray-Curtis dissimilarity coefficient and repeated on the qualitative composition of samples using Jaccard coefficients. To test for significant differences between infracommunities among the samples, a permutation “trace” test (sum of squared canonical eigenvalues) was applied; the P was obtained after 9,999 permutations. Multivariate analyses were implemented in PERMANOVA+ for PRIMER package (Anderson et al. 2008).
Results
General results
Mean host body lengths were significantly different between samples (F 3, 218 = 471.66; P < 0.01), with all pairs of samples differing significantly in fish length (all P < 0.01). A high proportion of hosts were parasitized in all samples (Table 2), although eight individuals of U. brasiliensis were uninfected, five in the sample from RJ, one in SC, and two in Rio Grande do Sul (RS); the remaining individuals were infected by at least 1 of 33 parasite species (Table 3). A total of 5,001 metazoan parasites were recorded in the entire sample, with an uneven distribution across locations based on both abundance and composition (Table 3). The highest parasite loads and total species richness were observed in Buenos Aires (BA), and the species richness values were the same as those for RS; BA also displayed the richest infracommunities (Table 2).
Only two species, namely Hysterothylacium sp. and the plerocercoids of Tentaculariidae, both in the larval stage, were present in all locations; the highest number of shared species was observed for BA and RS with 12 parasites, although RJ also shared a high proportion of species with the other two Brazilian locations (ten and nine with SC and RS, respectively). Many long-lived species (those not affected by short-term or seasonal variations) occurred with a high prevalence in several locations while being absent in others (i.e., G. carvajalregorum in BA and B. turbinella in RJ and SC; both were absent in RS).
Parasites as biological indicators of ecoregions
The results of PERMANOVA analyses on both presence/absence and abundance data (Table 4) showed a strong effect of host length on the response variables, although no interaction was observed between the host length and location. After taking into account the variations among samples due to host length, no significant variability was detected among the parasite assemblages.
The multidimensional scaling of infracommunities based on the presence/absence data was calculated after omitting three fish considered as outliers (two from RJ, harboring only Cainocreadium oscitans, and one from SC, with monospecific infection by Acanthochondria triangularis). The MDS revealed an apparent pattern of separation between several of the locations (Fig. 2), and the stress level (0.14) indicated that community structure was substantially different from random. The fish from BA were clearly separated from the rest and showed a more homogeneous distribution in the bidimensional space. The fish from RS occupied an intermediate (central) position in the biplot, whereas those from SC and RJ were distributed toward the right side and were indistinguishable from each other. All the samples from the Brazilian locations showed a broad dispersion, reflecting a higher heterogeneity in the composition of samples.
Nonmetric two-dimensional ordination plot using Jaccard similarity-based presence/absence data of metazoan parasite species across 211 individual Urophycis brasiliensis from four samples from the southwest Atlantic: Cabo Frio, Rio de Janeiro, Brazil (white squares); Florianopolis, Santa Catarina, Brazil (black triangles); Rio Grande, Rio Grande do Sul, Brazil (white inverted triangles); and Mar del Plata, Buenos Aires, Argentina (circles)
The overall differences in multivariate dispersions (Table 3) of parasite infracommunities in terms of their deviations from centroids were significant (F 3, 210 = 36.97, P (perm) < 0.01). The pairwise comparisons showed that the sample from BA was significantly less dispersed than its Brazilian counterparts (all P (perm) < 0.01), which in turn did not differ from each other (all P (perm) > 0.01).
The MDS and cluster analyses based on the Jaccard index as a resemblance measure and applied to the component communities revealed an apparent pattern of separation between samples following a latitudinal pattern (Fig. 3a), and the stress level (0.0) indicated a community composition substantially different from random. The fish from RJ were clearly associated with those from SC and showed the highest values of similarity; both samples clustered together at lower similarity values with those from RJ2004. However, a high level of compositional similarity was evident for samples from RS and BA. Similarly, in the MDS on prevalence (Fig. 3b) with the same stress level (0.0), the samples from RJ and SC were the most closely related, with the difference that fish RS were more similar to RJ and SC than to BA. RJ2004 again showed a low level of similarity to the other Brazilian samples.
Nonmetric two-dimensional ordination plot and cluster analyses of parasite component communities of Urophycis brasiliensis from four regions in the southwest Atlantic. a Using Jaccard (presence/absence) similarity and b using Bray-Curtis similarity based on untransformed parasite prevalence. Results of a hierarchical agglomerative clustering are shown as dendrograms and overlaid on the MDS plot with similarity levels represented by a gray scale. BA Buenos Aires, RJ Rio de Janeiro, RS Rio Grande, SC Santa Catarina
Parasites as biological tags for stock discrimination
After selecting hosts with one or more long-lived parasite species, 64 unparasitized fish were excluded from the analyses, including 27 from RJ, 17 from SC, and 20 from RS. The results of PERMANOVA analyses on both presence/absence and abundance data (Table 5) showed a strong effect of host length on the response variables and therefore on the parasite community structure. The interaction of host length with the samples showed that the nature of the relationship between the covariate and the multivariate response did not differ within different levels of the factor. Furthermore, taking into account the variations among samples due to host length, significant variability was detected among the parasite assemblages. The pairwise tests showed that there were significant differences in all pairs of samples (all P (perm) < 0.01).
A proportion of these differences can be attributed to differences in the multivariate dispersions of parasite infracommunities in terms of their deviations from centroids because the PERMDISP results were significant for both the Jaccard and Bray-Curtis indices (F 2, 147 = 23.05 and F 2, 147 = 28.85; both P (perm) < 0.01). Indeed, the pairwise test showed significant differences in the multivariate dispersions between all pairs of samples for the Bray-Curtis index (all P (perm) < 0.01) but only for those involving fish from BA for the Jaccard index (both P (perm) < 0.01), with fish from both northern locations displaying similar values of average deviations from centroids (P (perm) > 0.05).
The CAP analysis based on binary data showed significant differences among the samples (tr = 1.28; P = 0.0001). The selected orthonormal PCO axes (m = 6) described 97.46 % of the variation in the data cloud, with a high percentage of correct allocations (88 %). The two first canonical axes resulting from the CAP analysis clearly separated samples from BA, but those from Brazilian locations were not clearly distinguishable (Fig. 4a). However, the strength of the association between the multivariate data cloud and the hypothesis of group differences was indicated by the large size of their canonical correlations (δ 1 = 0.92 and δ 2 = 0.66). By superimposing vectors corresponding to the Spearman correlations of individual species with the CAP axes (restricted to those species with lengths >0.30), we observed that C. australe, C. cetaceum, and G. carvajalregorum were indicators of samples from BA, whereas the abundances of Hysterothylacium sp. and B. turbinella were mainly associated with fish from RS and SB, respectively. Similar results were obtained after CAP analysis based on the abundance data, which also showed significant differences among the samples (tr = 1.42; P = 0.0001). The selected orthonormal PCO axes (m = 10) described 97.28 % of the variation in the data cloud, with the same percentage of correct allocations (88 %). The two first canonical axes resulting from CAP analysis clearly separated the three samples (Fig. 4b) with high values of canonical correlations (δ 1 = 0.93 and δ 2 = 0.75). The same set of indicator species was identified after superimposing vectors corresponding to the Spearman correlations of individual species with the CAP axes (restricted to those species having lengths >0.30).
Canonical analysis of principal coordinates (CAP) biplot based on a presence/absence data and Jaccard dissimilarities and b parasite untransformed abundance and Bray-Curtis dissimilarities of parasites in samples of Urophycis brasiliensis from three ecoregions in the southwest Atlantic according to Spalding et al. (2007): Southeastern Atlantic = Rio de Janeiro + Santa Catarina, Brazil (squares); Rio Grande, Rio Grande do Sul, Brazil (triangles); and Mar del Plata Buenos Aires, Argentina (circles). Vector overlay are Spearman correlations of parasite species with the CAP axes (restricted to those having r > 0.3): Bt, Bolbosoma turbinella; Ca, Corynosoma australe; Cc, Corynosoma cetaceum; Gc, Grillotia carvajalregorum; Hs, Hysterothylacium sp.
The cross-validation of the results showed that in both sets of analyses, high percentages of correctly allocated fish occurred in all locations (Table 6). The same percentage of correctly classified hosts was observed for SB with both indices; however, fishes from RS showed higher percentages when discriminant analysis was based on assemblage composition. By contrast, a high proportion of fish from BA was allocated to this location when the analysis was based on abundance.
Discussion
The parasite fauna of specimens of U. brasiliensis examined in the present study was represented by 33 metazoan species; 11 of these species were new host records, which when added to previous records, resulted in a richness of 40 species of parasites in the entire distributional range of U. brasiliensis. This value may be even larger given that some parasites were identified at the species complex level, i.e., Scolex sp. or Contracaecum sp. (Chambers et al. 2000; Mattiucci and Nascetti 2008). It is notable that most of the previously cited species that were not found in the present study were recorded in the region of Rio de Janeiro (Alves et al. 2004), whereas almost all the species known in U. brasiliensis from Argentine waters were recorded. This could indicate that the predictability of the composition of parasite assemblages is greater at higher latitudes, which is in agreement with the observed results when comparing multivariate dispersions across samples.
The parasites varied in terms of presence, prevalence, and abundance across locations, with a very low proportion (two species) shared by all the samples. The number of shared species was extended to five when considering the three ecoregions, with all the species displaying a clearly higher prevalence in BA, particularly when compared with the northernmost locations. The remaining parasites were unevenly distributed among the samples, with seven species found only in BA. Notwithstanding, these patterns are not determined by the distribution of parasites in all cases. In fact, some species found only in the Argentine samples, such as Diclidophoroides maccallumi and Ascarophis marina, were previously recorded in U. brasiliensis from RJ (Alves et al. 2004). By contrast, Ectenurus virgula, which was not found in BA in the present study, was reported by Szidat (1961) in U. brasiliensis from this region. These species are short-lived parasites, and their occurrence could depend on seasonal variations that are potentially associated with seasonal changes in diet (Acuña et al. 2007) or reproductive migrations (Acuña et al. 2000). Seasonality or short-term temporal variability can also explain the absence of Lecithocladium cristatum in Brazilian samples and Lecithochirium microstomum, Parahemiurus merus, and Aponurus laguncula in BA, given that these digeneans have been recorded in other host species from their respective regions, although not in U. brasiliensis (Timi et al. 1999; Braicovich et al. 2009). Similar results have been observed for long-lived parasites, which are not affected by seasonality, such as G. carvajalregorum and Callitetrarhynchus gracilis in Brazilian waters in hosts other than U. brasiliensis (Menoret and Ivanov 2009; Braicovich et al. 2012; Fonseca et al. 2012) and B. turbinella in other fish species from BA (Braicovich and Timi 2010; Braicovich et al. 2012). Because all these species are transmitted trophically, the absence of such parasites in U. brasiliensis from regions where they have previously been reported could be explained by the differential composition of host diet, prey availability, or prey selectivity across host distribution. Although U. brasiliensis feeds at similar trophic levels along the southwestern Atlantic coasts, taxonomical differences in prey composition (crustaceans, fishes, and mollusks) have been observed among regions in Brazil, Uruguay, and Argentina (Acuña et al. 2007). However, although these species appear to be distributed throughout the biogeographical province, they display variability in terms of abundance and prevalence across regions (see below). In addition, a possible effect of differences in host length on diet composition across samples cannot be disregarded because this host undergoes ontogenetic changes in its feeding ecology and switches from invertebrates to primarily fish as it grows (Mora and Pintos 1980; Goldstein 1986; Acuña et al. 2007).
The differential occurrence of other groups of parasites likely appears to be a consequence of their own distribution patterns, either directly due to the effect of environmental conditions or indirectly due to the distribution of their intermediate hosts. The cucullanids found in U. brasiliensis represent a clear example, as Cucullanus cirratus was only found in the Brazilian samples and was replaced by Cucullanus bonaerensis in BA. C. cirratus is a typical parasite of gadiform fishes, including Urophycis blennoides (Brünnich), and it has only been reported in European waters (Berland 1970; Moravec 1994), whereas its previous identification in the sciaenid Micropogonias undulatus (Linnaeus, 1766) from Brazil by Vicente and Santos (1973) was considered erroneous (Lanfranchi et al. 2004). However, the presence of C. bonaerensis in U. brasiliensis from BA constituted an accidental infection in an unsuitable host (Lanfranchi et al. 2004), with its definitive host later identified as the flounder Xystreuris rasile (Jordan, 1891) (Alarcos and Timi 2012).
Similarly C. cetaceum, Bucephalus margaritae, Prosorhynchus australis, Ellytrophalloides oatesi, Derogenes varicus, and Hysterothylacium aduncum were only found in BA and have never been cited in Brazilian hosts (Kohn et al. 2007; Santos et al. 2008; Luque et al. 2011). These results are expected for the latter three species, which are characteristic of the cold waters of the Magellanic zoogeographical province (Cantatore and Timi 2014). Conversely, several other species have never been recorded in Argentina, namely Procamallanus halitrophus, C. oscitans, Pseudolepidapedon brasiliensis, and the host-specific Capillaria gracilis, Pseudempleurosoma sp., and A. triangularis.
Independent of the different processes that yield the geographic distribution of individual parasite species, the combination of such patterns in multivariate analyses resulted in clear similarity patterns. In fact, no differences were observed between both locations in the ecoregion of southeastern Brazil (RJ and SC), whereas the evident separation of samples was observed in the MDS plots across ecoregions, particularly for the component communities given the higher values of multivariate dispersions of infracommunities in all the Brazilian samples. These results are in agreement with a previous study aimed to discriminate stocks of the whitemouth croaker M. furnieri (Luque et al. 2010); the authors considered the samples of croaker from SC and RJ as a single population but different from those of RS. In the study by Luque et al. (2010), two additional samples from northern latitudes (states of Ceará and Bahia) were identified as representatives of a single different population, which was not surprising as these Brazilian coasts belong to a different biogeographical province that is separated from the Warm Temperate Southwestern Atlantic by the Cabo Frio region (Briggs 1995; Boschi 2000; Spalding et al. 2007; Menni et al. 2010). The observed separation of the sample RJ2004 is explained by the existence of this boundary between provinces. In fact, Alves et al. (2004) analyzed samples gathered over a year in a region comprising the northern and southern coastal zones of this limit (21–23° S, 41–45° W). The resulting sample was composed of a mixture of hosts from both biogeographical provinces. Despite these results, RJ2004 was more similar in species composition to the northern samples than to RS and BA.
Different results were found in the MDS analysis based on parasite prevalence, where fish from RS were more closely related to other Brazilian samples than to BA. This result was found using a more quantitative analysis and reflects the transitional condition of the RS region, which has been considered as the boundary between the two main districts of the Temperate Western South Atlantic Province (Balech and Ehrlich 2008). In fact, the compositional similarity of RS in relation to BA is because most (12 out of 19) of its species are shared with BA; however, when prevalence is taken into account, the samples from BA exhibited a high number of exclusive species with an extremely high prevalence, as well as a higher prevalence for most species shared with RS. By contrast, the most prevalent species in RS, namely C. cirratus, was found at similar levels in the other Brazilian samples. Because the prevalence data were not transformed, the results of the MDS analysis were heavily influenced by the most prevalent species.
Similar to the prevalence, the species richness of the component communities was higher in the southern samples. We observed clearer latitudinal patterns for the mean total abundance and infracommunity species richness, with both increasing southward. However, these patterns must be reviewed cautiously due to the low number of locations sampled along the latitudinal gradient and because the latitudinal gradient in diversity can be disrupted by variations in species richness due to other positional (i.e., longitude, depth) and environmental variables, particularly in shallow water systems (Gaston 2000). In fact research on the latitudinal patterns in marine parasites has yielded opposite conclusions depending on the taxonomic groups of both the hosts and parasites, as well as on the spatial scale investigated (Rohde and Heap 1998; Rohde 1999; Studer et al. 2013; Kamiya et al. 2014).
However, at the individual species level, previous studies in the southwest Atlantic have demonstrated that several parasite species display marked latitudinal gradients of prevalence or abundance, including several of the parasites found in U. brasiliensis, with these clines being evident when analyzed across biogeographical provinces (Timi 2003, 2007; Cantatore and Timi 2014). These parasites have a low specificity and infect all available hosts; therefore, their distribution can be traced beyond the boundaries of the endemic areas of fish hosts. For example, G. carvajalregorum and C. australe decreased in prevalence and abundance from northern Argentine waters toward the southern waters of the Patagonian Shelf (Cantatore and Timi 2014); however, their occurrence also decreased toward lower latitudes in U. brasiliensis and the other host species, such as Cynoscion guatucupa, Pinguipes brasilianus, M. furnieri, and Trachurus lathami (Timi et al. 2005, 2010; Luque et al. 2010; Braicovich et al. 2012), indicating that their endemic region is the northern Argentine and the Uruguayan seas. By contrast, species such as B. turbinella, which is present in several of these host species, displays higher loads in the northern regions, whereas H. aduncum, for example, showed the opposite pattern.
The variation across regions in the occurrence of the parasites with restricted distributions, driven by singular suites of oceanographic and biological features in each zone, resulted in similarity patterns that are supported by existing hypotheses about the distribution of ecoregions in the southwestern Atlantic (Spalding et al. 2007). Indeed, excluding the sample from 2004, the results of clustering the ecoregions were the same as those obtained in a biogeographical study on mollusk species with the same ecoregional perspective (Fortes and Absalão 2011), indicating the potential of parasites as suitable tools for research on marine biogeography.
Parasites are ubiquitous components of fish communities, and their assemblages in fish hosts are composed by several phyla that generally include platyhelminthes (monogeneans, trematodes, cestodes), nematodes, acanthocephalans, and crustaceans, among the metazoans. We propose here that the potential usefulness of parasite assemblages as ecosystem indicators (Cantatore and Timi 2014) is derived from the combined evidence of the evolutionary history and biogeography of multiple lineages, which is expected to be more efficient in capturing the recurrent patterns in overall biodiversity rather than individual lineages. Furthermore, as many parasites have complex life cycles including both invertebrate and vertebrate hosts, their distribution patterns are not only dependent on environmental conditions but also on the distribution and population density of all hosts involved in their life cycles (Timi 2007), adding consequently other sources of distributional variability that act synergistically to define robust geographical patterns. Similarly, the ecoregional classification of coastal systems of Spalding et al. (2007), which is mirrored by parasites of U. brasiliensis in the southwestern Atlantic, is based on composite studies that combined multiple divergent taxa and oceanographic drivers in the derivation of its boundaries.
The usefulness of parasites as ecoregional markers is enhanced by clines in the prevalence of broadly distributed species when this descriptor is included in the analyses. Indeed, characteristics of the regional parasite faunas are demonstrated in these more quantitative analyses, such as the dominance of a suite of long-lived larval parasites in BA, a feature common to all fish species so far investigated in the region (Cantatore and Timi 2014). Due to their persistence in hosts, these species are more preferable than transient species as biological tags for stock discrimination (Lester and MacKenzie 2009) and, consequently, were selected for the analyses in this study. Indeed, analyses restricted to long-lived parasites that incorporated abundance data provided evidence supporting the existence of three different stocks in the four sampled areas. Several of the parasite species that contributed the most to the separation of the samples agreed with those identified as markers of the northern Argentine Sea (Cantatore and Timi 2014), namely G. carvajalregorum, C. australe, and C. cetaceum, whereas Hysterothylacium sp. and B. turbinella were associated with hosts from RS and SB, respectively. Due to their low specificity, these species constitute promissory biological tags for stock discrimination of other fish species along the Brazilian coast.
The identification of three stocks in the regions is a not surprising result, given the output of previous analyses and the singularity of the biogeographic forcing agents that define each ecoregion. However, more extensive studies in both time and space are desirable to accurately assess the stock composition of U. brasiliensis along its geographical range, particularly at lower geographical scales because variations in reproductive parameters have been observed in nearby locations of Uruguay (Acuña et al. 2000). The usefulness of parasites at these smaller scales needs to be tested, as stock assessment is the first step in the development of sustainable fisheries.
In addition, parasite tags are promissory for studies on population structure at higher spatial scales including samples from other biogeographical provinces, such as the Brazilian Province and north to Cabo Frio, where parasite communities apparently have a different structure (Alves et al. 2004). Samples should also be included from southern regions such as the Magellanic Province, where U. brasiliensis has been recently recorded together with a group of other species typical of more temperate northern waters (Bovcon et al. 2011). In these large-scale studies, parasites that are broadly distributed and exhibit low specificity, with geographical ranges beyond those of the individual host species and marked latitudinal gradients of abundance, will likely be helpful to identify the geographical patterns of host population structure.
References
Acuña A, Verocai J (2001) Importancia de la pesquería artesanal y biología de la brótola, Urophycis brasiliensis (Kaup, 1858) (Phycidae, Gadiformes) en la costa uruguaya. Invest Mar 29:47–58
Acuña A, Viana F, Vizziano D, Danulat E (2000) Reproductive cycle of female Brazilian codling, Urophycis brasiliensis (Kaup 1858), caught off the Uruguayan coast. J Appl Ichthyol 16:48–55
Acuña A, Sellanes J, Rodríguez L, Burone L (2007) Feeding ecology of Urophycis brasiliensis on the Uruguayan coast of the Río de la Plata estuary. J Appl Ichthyol 23:231–239. doi:10.1111/j.1439-0426.2007.00855.x
Alarcos AJ, Timi JT (2012) Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae): similar ecological filters driving toward repeatable assemblages. Parasitol Res 110:2155–2166. doi:10.1007/s00436-011-2741-5
Alves DR, Luque JL, Paraguassu AR (2003) Acanthochondria triangularis sp. nov. (Copepoda, Poecilostomatoida, Chondracanthidae) parasitic on Urophycis brasiliensis and U. mystaceus (Osteichthyes, Phycidae) from the Southern Brazilian coastal zone. Acta Parasitol 48:9–23
Alves D, Paraguassu AR, Luque JL (2004) Metazoários parasitos da abrótea, Urophycis brasiliensis (Kaup, 1858), (Osteichthyes: Phycidae) do litoral do estado do Rio de Janeiro, Brasil. Rev Bras Parasitol Vet 13:49–55
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253. doi:10.1111/j.1541-0420.2005.00440.x
Anderson MJ, Robinson J (2003) Generalized discriminant analysis based on distances. Aust N Z J Stat 45:301–318. doi:10.1111/1467-842X.00285
Anderson MJ, Willis TJ (2003) Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84:511–525
Anderson MJ, Ellingsen KE, McArdle BH (2006) Multivariate dispersion as a measure of beta diversity. Ecol Lett 9:683–693. doi:10.1111/j.1461-0248.2006.00926.x
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth
Anderson MJ, Crist TO, Chase JM, Vellend M, Inouye BD, Freestone AL, Sanders NJ, Cornell HV, Comita LS, Davies KF, Harrison SP, Kraft NJB, Stegen JC, Swenson NG (2011) Navigating the multiple meanings of b diversity: a roadmap for the practicing ecologist. Ecol Lett 14:19–28. doi:10.1111/j.1461-0248.2010.01552.x
Balech E, Ehrlich MD (2008) Esquema biogeográfico del Mar Argentino. Rev Invest Desarr Pesq 19:45–75
Berland B (1970) On the morphology of the head in four species of the Cucullanidae (Nematoda). Sarsia 43:15–64
Boschi EE (2000) Species of decapod crustaceans and their distribution in the American marine zoogeographic provinces. Rev Invest Desarr Pesq 13:1–136
Bovcon ND, Cochia PD, Góngora ME, Gosztonyi AE (2011) New records of warm-temperate water fishes in central Patagonian coastal waters (Southwestern South Atlantic Ocean). J Appl Ichthyol 27:832–839. doi:10.1111/j.1439-0426.2010.01594.x
Braicovich PE, Timi JT (2010) Seasonal stability in parasite assemblages of the Brazilian flathead, Percophis brasiliensis (Perciformes: Percophidae): predictable tools for stock identification. Folia Parasitol 57:206–212
Braicovich PE, Etchegoin JE, Timi JT (2009) Digenetic trematodes of the Brazilian flathead, Percophis brasiliensis Quoy et Gaimard, 1894 (Percophidae, Perciformes), from Argentinean and Uruguayan waters. Acta Parasitol 54:368–373
Braicovich PE, Luque JL, Timi JT (2012) Geographical patterns of parasite infracommunities in the rough scad, Trachurus lathami Nichols off southwestern Atlantic Ocean. J Parasitol 98:768–777. doi:10.1645/GE-2950.1
Briggs JC (1995) Global biogeography. Elsevier, Amsterdam, 472p
Briggs JC, Bowen BW (2012) A realignment of marine biogeographic provinces with particular reference to fish distributions. J Biogeogr 39:12–30. doi:10.1111/j.1365-2699.2011.02613.x
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Cadrin SX, Friedland KD, Waldman JR (2005) Stock identification methods: applications in fishery science. Elsevier, Amsterdam
Cantatore DMP, Timi JT (2014) Marine parasites as biological tags in South American Atlantic waters, current status and perspectives. Parasitology (in press)
Chambers CB, Cribb TH, Malcolm JK (2000) Tetraphyllidean metacestodes of teleosts of the Great Barrier Reef, and the use of in vitro cultivation to identify them. Folia Parasitol 47:285–292
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E Ltd., Plymouth, 190 p
Clarke KR, Somerfield PJ, Chapman MG (2006) On resemblance measures for ecological studies, including taxonomic dissimilarities and a zero-adjusted Bray–Curtis coefficient for denuded assemblages. J Exp Mar Biol Ecol 330:55–80. doi:10.1016/j.jembe.2005.12.017
Cousseau MB, Perrotta RG (2004) Peces marinos de Argentina. Biología, distribución, pesca. Publicaciones especiales INIDEP, Mar del Plata, Argentina 167 p
Floeter SR, Gasparini JL (2000) The southwestern Atlantic reef fish fauna: composition and zoogeographic patterns. J Fish Biol 56:1099–1114
Floeter SR, Soares-Gomes A (1999) Biogeographic and species richness patterns of Gastropoda on the Southwestern Atlantic. Rev Bras Biol 59:567–575
Floeter SR, Guimarães RZP, Rocha LA, Ferreira CEL, Rangel CA, Gasparini JL (2001) Geographic variation in reef-fish assemblages along the Brazilian coast. J Biogeogr 10:423–431
Floeter SR, Rocha LA, Robertson DR, Joyeaux JC, Smith-Vaniz WF, Edwards AJ, Barreiros JP, Ferreira CEL, Gasparini JL, Brito A, Falcón JM, Bowen BW, Bernardi G (2008) Atlantic reef fish biogeography and evolution. J Biogeogr 35:22–47
Fonseca MCG, São Clemente SC, Felizardo NN, Corrêa Gomes D, Knoff M (2012) Trypanorhyncha cestodes of hygienic-sanitary importance infecting flounders Paralichthys patagonicus Jordan, 1889 and Xystreurys rasile (Jordan, 1891) of the Neotropical region, Brazil. Parasitol Res 111:865–874. doi:10.1007/s00436-012-2912-z
Fortes RR, Absalão RS (2011) Biogeography and connectivity between western South American and Antarctic marine molluscs. Oecol Aust 15:111–123. doi:10.4257/oeco.2011.1501.09
Gaston KJ (2000) Global patterns in biodiversity. Nature 405:220–227
Goldstein H (1986) Morphological characteristics of the digestive system and alimentary habits of the squirrel hake (Urophycis brasiliensis) (Pisces, Gadidae). Pub Com Téc Mix Fr Mar 1:351–368
Ivanov VA, Navone GT, Martorelli SR (1997) Ascarophis marina n. comb. (Nematoda: Cystidicolidae) from the fishes Parona signata (Carangidae) and Urophycis brasiliensis (Gadidae) in the southwestern Atlantic. J Parasitol 83:917–921
Kamiya T, O’Dwyer K, Nakagawa S, Poulin R (2014) What determines species richness of parasitic organisms? A meta-analysis across animal, plant and fungal hosts. Biol Rev 89:123–134. doi:10.1111/brv.12046
Kohn A, Fernandes BMM, Cohen SC (2007) South American trematodes parasites of fishes. Editorial Imprinta, Rio de Janeiro, 318 p
Lanfranchi AL, Timi JT, Sardella NH (2004) Cucullanus bonaerensis n. sp. (Nematoda: Cucullanidae) parasitizing Urophycis brasiliensis (Pisces: Phycidae) from Argentinean waters. J Parasitol 90:808–812
Lester RJG (1990) Reappraisal of the use of parasites for fish stock identification. Aust J Mar Fresh Res 41:855–864. doi:10.1071/MF9900855
Lester RJG, MacKenzie K (2009) The use and abuse of parasites as stock markers for fish. Fish Res 97:1–2. doi:10.1016/j.fishres.2008.12.016
Luque JL, Cordeiro AS, Oliva ME (2010) Metazoan parasites as biological tags for stock discrimination of white mouth croaker Micropogonias furnieri from south-western Atlantic Ocean waters. J Fish Biol 76:591–600. doi:10.1111/j.1095-8649.2009.02515.x
Luque JL, Aguiar JC, Vieira FM, Gibson DI, Santos CP (2011) Checklist of Nematoda associated with the fishes of Brazil. Zootaxa 3082:1–88
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton
Martorelli SR, Navone GT, Ivanov V (2000) Proposed life cycle of Ascarophis marina (Nematoda: Cystidicolidae) in Argentine waters. J Parasitol 86:1047–1050
Mattiucci S, Nascetti G (2008) Advances and trends in the molecular systematics of anisakid nematodes, with implications for their evolutionary ecology and host-parasite co-evolutionary processes. Adv Parasitol 66:47–148
Menni RC, Jaureguizar AJ, Stehmann MFW, Lucifora LO (2010) Marine biodiversity at the community level: zoogeography of sharks, skates, rays and chimaeras in the southwestern Atlantic. Biodivers Conserv 19:775–796. doi:10.1007/s10531-009-9734-z
Menoret A, Ivanov AV (2009) New name for Progrillotia dollfusi Carvajal et Rego, 1983 (Cestoda: Trypanorhyncha): description of adults from Squatina guggenheim (Chondrichthyes: Squatiniformes) off the coast of Argentina. Folia Parasitol 56:284–294
Miloslavich P, Klein E, Díaz JM, Hernandez CE, Bigatti G, Campos L, Artigas F, Castillo J, Penchaszadeh P, Neill P, Carranza A, Retana M, Díaz de Astarloa JM, Lewis M, Yorio P, Piriz M, Rodriguez G, Yoneshigue-Valentin Y, Gamboa L, Martín A (2011) Marine biodiversity in the Atlantic and Pacific Coasts of South America: knowledge and gaps. PLoS ONE 6:e14631. doi:10.1371/journal.pone.0014631
Mora O, Pintos W (1980) Espectro alimentario de Urophycis brasiliensis (Kaup, 1858) (Pisces, Gadidae). Bol Inst Ocean 29:239–243
Moravec F (1994) Parasitic nematodes of freshwater fishes of Europe. Academia, Praha, 473 p
Pereira JJ, Robaldo RB, Souto-Raiter VMM (1996) Um possível ciclo de vida Bucephalus varicus Manter, 1940 (Trematoda: Bucephalidae) no Rio Grande do Sul. Com Mus Ciênc Tec, PUC RS 9:31–36
Rohde K (1999) Latitudinal gradients in species diversity and Rapoport’s rule revisited: a review of recent work and what can parasites teach us about the causes of the gradients? Ecography 22:593–613. doi:10.1111/j.1600-0587.1999.tb00509.x
Rohde K, Heap M (1998) Latitudinal differences in species and community richness and in community structure of metazoan endo- and ectoparasites of marine teleost fish. Int J Parasitol 28:461–474
Santos CP, Gibson DI, Tavares LER, Luque JL (2008) Checklist of Acanthocephala associated with the fishes of Brazil. Zootaxa 1938:1–22
Spalding MD, Fox HE, Allen GR, Davidson N, Ferdaña ZA, Finlayson M, Halpern NS, Jorge MA, Lombana A, Lourie SA, Martin KD, McManus E, Molnar J, Recchia CA, Robertson J (2007) Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience 57:773–583. doi:10.1641/B570707
Studer A, Widmann M, Poulin R, Krkošek M (2013) Large scale patterns of trematode parasitism in a bivalve host: no evidence for a latitudinal gradient in infection levels. Mar Ecol Progr Ser 491:125–135. doi:10.3354/meps10483
Suriano DM, Labriola JB (1999) Diclidophoroides maccallumi Price, 1943 and Neoheterobothrium paralichthyis sp. n. (Monogenea: Diclidophoridae), parasites of fishes (Gadiformes and Pleuronectiformes) from the southwestern Atlantic Ocean. Acta Parasitol 44:160–164
Szidat L (1961) Versuch einer Zoogeographie des Süd-Atlantik mit Hilfe von Leitparasiten der Meeresfische. Parasitol Schriften 13:1–98
Timi JT (2003) Parasites of Argentine anchovy in the south-west Atlantic: latitudinal patterns and their use for discrimination of host populations. J Fish Biol 63:90–107. doi:10.1046/j.1095-8649.2003.00131.x
Timi JT (2007) Parasites as biological tags for stock discrimination in marine fish from South American Atlantic waters. J Helminthol 81:107–111. doi:10.1017/S0022149X07726561
Timi JT, MacKenzie K (2014) Parasites in fisheries and mariculture. Parasitology (in press)
Timi JT, Martorelli SR, Sardella NH (1999) Digenetic trematodes parasitic on Engraulis anchoita (Pisces: Engraulidae) from Argentina and Uruguay. Folia Parasitol 46:132–138
Timi JT, Luque JL, Sardella NH (2005) Parasites of Cynoscion guatucupa along South American Atlantic coasts: evidence for stock discrimination. J Fish Biol 67:1603–1618. doi:10.1111/j.1095-8649.2005.00867.x
Timi JT, Lanfranchi AL, Luque JL (2010) Similarity in parasite communities of the teleost fish Pinguipes brasilianus in the southwestern Atlantic: infracommunities as a tool to detect geographical patterns. Int J Parasitol 40:243–254. doi:10.1016/j.ijpara.2009.07.006
Vicente JJ, Santos E (1973) Alguns helmintos de peixe do litoral norte fluminense. Mem Inst Oswaldo Cruz 71:95–113
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Upper Saddle River
Acknowledgments
Financial support to Aldenice Pereira and José Luis Luque was provided by research fellowships from Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brazil (CNPq). Camila Pantoja was supported by an M. Sc. fellowship from Coordenação de Aperfeiçoamento do Pessoal do Ensino Superior (CAPES). Financial support to Juan T. Timi was provided by grants from CONICET (PIP # 112-201101-00036) and ANPCYT (PICT 2012 # 02094).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pereira, A.N., Pantoja, C., Luque, J.L. et al. Parasites of Urophycis brasiliensis (Gadiformes: Phycidae) as indicators of marine ecoregions in coastal areas of the South American Atlantic. Parasitol Res 113, 4281–4292 (2014). https://doi.org/10.1007/s00436-014-4106-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4106-3