Abstract
The cardiovascular nematode Angiostrongylus vasorum is spreading in the fox and dog populations of northern Europe. A. vasorum can result in severe clinical manifestations in dogs; therefore, specific diagnosis is crucial for assessing its prevalence. In the present study, faecal samples from foxes and domestic dogs were tested by a new polymerase chain reaction (PCR) targeting the second internal transcribed region of the ribosomal DNA (ITS2) of A. vasorum. Initial isolation of faecal larvae by sieving facilitated the processing of larger sample volumes and allowed for the recovery of dead larvae from frozen samples. The sieve-PCR method enabled the identification of a single larva per 2 g of faecal sample and did not amplify DNA of a range of canine helminths, thus presenting a non-invasive tool for wildlife surveillance and for confirmative diagnosis in dogs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiostrongylus vasorum is a metastrongylid nematode residing in the cardiopulmonary arteries of canines and can have fatal consequences. The first-stage larvae (L1) hatch and penetrate into the lung alveoli and then migrate up to the bronchi, where they are coughed up, swallowed and passed into the environment via the faeces of the host. Snails and slugs become infected by foraging on the faecal deposits and thereby serve as intermediate hosts (Bolt et al. 1993). Over the past decade, the prevalence of A. vasorum in wildlife has increased in several European countries (Morgan et al. 2005; Van Doorn et al. 2009; Helm et al. 2010), the most important definitive hosts being domestic dogs and foxes (Koch and Willesen 2009), whilst the modes of transmission to the final host are still unclear (Morgan et al. 2005).
The reference method for detection of patent Angiostrongylus infection is the Baermann technique (Cury et al. 2001; Barcante et al. 2003; Traversa and Guglielmini 2008; Koch and Willesen 2009), which relies on active larval migration from the faecal matter into the fluid, followed by morphological identification of the larvae. However, based on the intermittent shedding of L1 (Oliveira et al. 2006), subclinical cases in dogs with low worm burdens are often missed and the diagnostic sensitivity of this test for A. vasorum has not been thoroughly evaluated in foxes (Traversa and Guglielmini 2008; Koch and Willesen 2009). Furthermore, in studies conducted in areas where the zoonotic tapeworm Echinococcus multilocularis could potentially be found, faecal samples are routinely frozen at −80°C to kill the larvae and thereby excluding the use of the Baermann technique for the detection of A. vasorum larvae.
Thoracic radiographs, high-resolution computerised tomography scanning and magnetic resonance imaging have been used for assessing pathological changes in natural and experimental angiostrongylosis in dogs (Cury et al. 2001; Koch and Willesen 2009). Although not commercially available, an ELISA detecting circulating A. vasorum antigens in serum samples (Verzberger-Epshtein et al. 2008) was able to identify 92% of 24 naturally infected dogs, as compared to the Baermann technique. Caldeira et al. (2003) differentiated worms and larvae of Angiostrongylus costaricensis, Angiostrongylus cantonensis, Angiostrongylus vasorum and Aelurostrongylus abstrusus by a polymerase chain reaction (PCR) with universal primers targeting the mtDNA coxI gene and the ITS2 region followed by RFLP, which produced cleaved DNA fragments of A. vasorum of unexpected sizes. Denk et al. (2009) also used universal primers to confirm A. vasorum infection in a dog after sequencing of ITS2 region. The specificity of a PCR test, based on universal primers, could alternatively be assured by carrying out expensive sequencing of all amplified PCR products. Recently, Helm et al. (2009) designed a PCR to detect A. vasorum in clinical cases based on sequences of ITS2 region of A. vasorum, A. cantonensis, A. costaricensis and A. abstrusus. The PCR specifically amplified DNA of A. vasorum, confirmed by sequencing, although specificity testing with DNA of other common canine nematodes was not done. Such a specificity test was carried out during the development of a real-time (RT) PCR which also targeted the ITS2 region of A. vasorum (Jefferies et al. 2009). No amplification was found for Crenosoma vulpis, Eucoleus aerophilus (Capillaria aerophila), Dirofilaria imitis, Toxocara canis or Strongyloides ratti. The developed RT PCR was evaluated on both blood and faecal samples, but as compared to Baermann isolation, the test had a reduced sensitivity for the faecal samples. Under field conditions, fresh blood samples may be difficult to obtain from hunted foxes and virtually impossible from road kills.
Thus, the present study aims to (1) refine DNA isolation techniques from faecal samples, (2) develop specific primers for detecting A. vasorum DNA, (3) assess the specificity of the primers with DNA of a range of canid helminths and (4) evaluate the test on faeces from domestic dogs without previous diagnosis and from wild foxes.
Materials and methods
Reference parasite material
Reference material, consisting of a range of carnivore helminths, was provided from collaborators in Switzerland, Norway or Denmark (Table 1). Samples were kept either frozen at −20°C in distilled water or in 70% alcohol until processed for DNA extraction.
Frozen faecal samples from foxes
Thirty-two Danish foxes were examined (Table 2). The carcasses were collected from Jutland (N = 10), an area of low prevalence rate of A. vasorum in foxes (1.4%; Saeed et al. 2006), and from North East Zealand (n = 22), an area where the infection is endemic in foxes (48%; Saeed et al. 2006). Prior to necropsy, all foxes were frozen at −80°C for at least 5 days in order to kill any E. multilocularis eggs, if present. Hearts were opened with scissors and lung tissue was dissected using a metallic needle to free the adult A. vasorum worms. The dissected lung tissue was then sieved and worms were identified under a stereo microscope (×10; Hendrix and Robinson 2006). Faeces were collected as rectal samples and any dead larvae were isolated using a modified sieving method (Davidson et al. 2009). Briefly, 2 g of faecal material was mixed with 8 ml sugar–salt flotation fluid (specific gravity 1.2) and vortexed. The suspension was then centrifuged at 1,600 × g for 10 min, and the supernatant was poured through a 21-µm mesh sieve. The material retained in the sieve was washed with distilled water and poured into a 3.5-ml petri dish for microscopic examination. The sieved material containing the first-stage larvae (L1) of A. vasorum was then collected for DNA extraction.
Fresh faecal samples from hunting dogs
From October to November 2007, 181 faecal samples from hunting dogs were collected. The samples were collected from the southern area of Jutland (n = 94) and North East Zealand (n = 87). The dogs did not exhibit any clinical signs and had not received anthelmintic treatment for at least 2 months prior to sampling. Fresh faecal samples (4 g) were obtained for each dog, on three consecutive days, and were examined by the Baermann method (Roepstorff and Nansen 1998). All observed larvae were examined morphologically under a dissecting microscope (×40) and thereafter collected with a glass pipette and stored frozen, at −20°C, in a 200-μl tube containing 5 μl distilled water or PBS until use.
DNA isolation from adult worms and larvae
DNA was isolated from adult worms (Table 1) using a commercial kit according to the manufacturer’s instructions (Qiamp DNA mini kit®, Qiagen, Hilden, Germany), with a final elution volume of 200 µl. Extraction of DNA from Baermanized larval suspension was done by adding 2 μl Tris-HCl (final concentration of 10 mM, pH 8.3) and incubated at 90°C for 10 min, followed by proteinase K digestion by adding 3 µl (final concentration of proteinase K 10 μg/μl). The suspension was heated at 56°C for 1 h and the enzyme was then denatured at 90°C for 10 min. The samples were cooled to 4°C before storage at −20°C. For sieved samples, DNA extraction was carried out on all the sieved material using a commercial kit designed for copro-DNA extraction (PSP® spin stool DNA kit, Invitek, Berlin, Germany), with a final elution volume of 200 µl.
Design and in silico validation of primers
Primers were designed from a partial sequence of A. vasorum rDNA second internal transcribed spacer (ITS2) retrieved from GenBank, using online software (primer 3: http://fokker.wi.mit.edu/primer3/input.htm). The designed left primer (AV5): CGA TGA CGG TAG CAA TGA CA and right primer (AV4): TTT GCG TGG TTC TTTACG TG give an amplicon size of 218 bp. The specificity of the primers was assessed by in silico alignment (http://www.ncbi.nlm.nih.gov/BLAST/). DNA from a wide range of helminths (Table 1) were evaluated for cross reactivity.
PCR cycling conditions
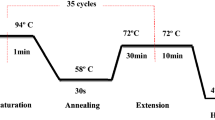
Template DNA (4 μl) were amplified by PCR using 100 pmol of each primer. The reaction mixture (50 μl) consisted of PCR buffer (10 mM Tris-HCl, pH 8.4, 50 mM KCl, 1.5 mM MgCl2, 0.5% Tween 20) supplemented with 0.2 mM of each dNTP, 2.5 U of hot start Taq DNA polymerase (VWR, Haasrode, Belgium) and 1 μl of bovine serum albumin (1 μg/μl). PCR cycling conditions started with a pre-heating step at 95°C for 5 min. Each cycle consisted of the three steps: heating at 95°C for 45 s, then annealing at 58°C for 45 s and an elongation step of 72°C for 45 s. The 35 cycles were terminated with a final elongation step of 72°C for 10 min. Amplicons were detected on 1.5% agarose gels stained with Envision DNA dye® (N313-1 ml, Amersco, Solon, USA).
Sequencing
Automated DNA sequencing was carried out by a private company (MWG, Martinsried, Germany). The amplicons were directly purified (DNA and gel band purification kit®, Amersham Biosciences, UK), and the sequences obtained were compared with GenBank entries.
Statistics
Prevalences were calculated, with the 95% confidence intervals (CI) based upon a binomial distribution, using the SAS statistical package (version 9.1®; SAS institute Inc. Cary, NC, USA).
Results
Primer sensitivity and specificity
The primer pair AV4 and AV5 produced a distinct 218-bp band even with DNA from a single L1 larva, and DNA from other helminths was not amplified. The specificity of the AV4 and AV5 primers were also tested in silico to exclude matching to sequences of hosts and other parasite of carnivores, including closely related Angiostrongylus species (data not shown).
Detection in foxes
Adult A. vasorum were recovered from 22 of the 32 foxes examined by PM (Table 2). L1 larvae were morphologically identified in the faeces of 17 of the 22 infected foxes, with larval counts ranging from one to 15 larvae per gram of faeces. PCR detected DNA of A. vasorum in all 17 cases with sieved larvae, in addition to one case that did not exhibit L1 in the sieved fraction, but in which adult A. vasorum worms were detected during the PM examination. The PCR analysis produced negative results on sieved faeces from ten foxes originating from an area of low prevalence rate for A. vasorum. None of these had foxes had A. vasorum infection according to the PM examination but did harbour other helminths, including C. vulpis and E. aerophilus. The sequencing of the PCR products, from all the positive samples, confirmed the amplification of A. vasorum.
Detection in hunting dogs
Larvae of A. vasorum were isolated from faeces of four of the 181 hunting dogs using the Baermann method. Three dogs shed larvae on only one of the three sampling days whilst the fourth dog excreted L1 on two consecutive days. Amongst the dogs from Zealand, three were infected (3.5% (9.8–0.7%, 95% CI)) whereas one of the dogs from Jutland was infected (1.1% (5.8–0.03%, 95% CI)). The positive dogs excreted larvae in low numbers: one, two, eight and 13 larvae per 4 g of faecal material. Although one sample from Zealand was lost, larvae were collected by needle aspiration and DNA was isolated from the three remaining larval suspensions. All three samples were successfully amplified using the AV4 and AV5 primers giving the expected 218-bp product for A. vasorum.
Discussion
Larval isolation techniques
The efficacy of sieving for the recovery of larvae from frozen faecal samples, where active migration of live larvae is not possible, suggests that the method is suitable for the processing of both fresh and frozen samples. For more detailed parasitological studies, the sieved fraction can allow for initial morphological differentiation of both parasitic and free living soil nematodes. Applied in larger epidemiological studies, the initial screening of faecal samples for presence of larvae, irrespective of sample storage, followed by a simple confirmatory PCR, may present a cost-effective and specific two-step diagnosis. In areas where A. vasorum is not endemic, a precise morphological diagnosis may be hampered by the inexperience of the investigator, and a simple confirmatory PCR may have value as a confirmative diagnostic tool in wildlife, in domestic dogs or in exotic animals (Patterson-Kane et al. 2009). Finally, this PCR may potentially be used for larval identification in intermediate hosts (Jefferies et al. 2009); however, this remains to be assessed.
Intermittent larval excretion, prepatent and low-grade infections are a fundamental obstacle to all methods based on faecal analysis (Bolt et al. 1993; Oliveira et al. 2006; Verzberger-Epshtein et al. 2008), which may explain why no A. vasorum larvae were detected in five foxes although adult worms were recovered at PM. The intermittent excretion necessitates the examination of samples from three consecutive days, as suggested by Willesen et al. (2004).
It is noteworthy that the four infected dogs were all Labrador retrievers. However, of the 181 dogs examined, representing 19 different breeds, Labradors were overrepresented. In total, 57 out of 181 dogs included in the study were Labrador Retrievers. Given that Labrador retrievers are renowned known for their scavenging behaviour, recovering L1 of A. vasorum might be indicative of subclinical infections but could, however, also be attributed to coprophagia. Nonetheless, the recovery of L1 A. vasorum larvae in dog faeces does seem to indicate the presence of the infection in the investigated area. One of the four infected dogs detected in this study came from the area of Jutland, which makes it the first record of isolating L1 of A. vasorum from dogs from that area after the previous records in an otter (Madsen et al. 1999) and in foxes (Saeed et al. 2006) from the same area. However, the infected dog has been visiting the area of West Zealand, an endemic area for A. vasorum, 5 to 6 weeks prior faecal sampling; thus, the infection could be originated from the endemic area. Uncontrolled dog transportation from endemic areas may facilitate the transmission and expansion of the distribution of the infection to and non-endemic areas (Helm et al. 2010). One of the prominent risk factors for the establishment of Angiostrongylus infection is the use of hunting dogs without giving them prophylactic anthelmintic treatment (Conboy 2004). Prophylactic anthelmintic treatment is not allowed in Denmark, Therefore, it is recommend that dogs used for hunting and those living in endemic areas should be examined on a regular basis for the presence of A. vasorum infection.
DNA extraction technique
Although the present addition of flotation fluids to frozen faecal samples, followed by sieving, may deform frozen larvae and prevent morphological identification (Traversa and Guglielmini 2008), DNA in the larval fragments may, in such cases, still produce positive PCR results. The major obstacle facing copro-PCR tests is the presence of inhibitory substances (Al-Sabi et al. 2007), and several steps can be done to eliminate the presence of such substances from the extracted DNA. Copro-DNA extraction kits are designed originally for processing approximately 0.2 g of faeces. This limited sample quantity can result in false-negative results due to low larval intensities (Jefferies et al. 2009). Thus, the advantage of using the sieving method is being able to process bigger sample volumes (2 g), with the added advantage of microscopic examination of the sieved fraction for the detection and quantification of a wide spectrum of parasites (Al-Sabi et al. 2007; Mathis et al. 1996).
PCR performance
The PCR test proved to be specific for A. vasorum, as apparent from the absence of DNA amplification of other tested canid helminths. As the PCR was able to amplify DNA from single larvae, the sensitivity of PCR is determined by the efficacy of the method used for isolation of larvae. Although the PCR successfully amplified all the L1 identified morphologically, the labour-intensive PM examination proved to be the most sensitive diagnostic method and remained the method of choice for more broad parasitological studies on foxes. For epidemiological studies, specifically aimed at A. vasorum, either on fox or dog scats or from faecal samples from road kills, larval isolation by sieving, followed by the PCR, may be a more feasible and faster way to acquire an overview of the presence of infection in an area.
Conclusions
The two-step diagnostic strategy presented here allows for the uniform recovery of A. vasorum from faecal samples, and subsequent simple amplification of DNA, down to a single larva without cross reaction with DNA of other relevant carnivore helminths, confirms the diagnosis.
References
Al-Sabi MNS, Kapel CMO, Deplazes P, Mathis A (2007) Comparative copro-diagnosis of Echinococcus multilocularis in experimentally infected foxes. Parasitol Res 101:731–736
Barcante JMP, Barcante TA, Dias SRC, Vieira LQ, Lima WS, Negrao-Correa D (2003) A method to obtain axenic Angiostrongylus vasorum first-stage larvae from dog feces. Parasitol Res 89:89–93
Bolt G, Monrad J, Frandsen F, Henriksen P, Dietz HH (1993) The common frog (Rana-temporaria) as a potential paratenic and intermediate host for Angiostrongylus vasorum. Parasitol Res 79:428–430
Caldeira RL, Carvalho OS, Mendonca CLFG, Graeff-Teixeira C, Silva MCF, Ben R, Maurer R, Lima WS, Lenzi HL (2003) Molecular differentiation of Angiostrongylus costaricensis, A. cantonensis, and A. vasorum by polymerase chain reaction-restriction fragment length polymorphism. Mem Inst Oswaldo Cruz 98:1039–1043
Conboy G (2004) Natural infections of Crenosoma vulpis and Angiostrongylus vasorum in dogs in Atlantic Canada and their treatment with milbemycin oxime. Vet Rec 155:16–18
Cury MC, Lima WS, Guimaraes MP, Muzzi RAL, Bregunci GC (2001) Radiology, eletrocardiography and echocardiography for the diagnosis of Angiostrongylus vasorum (Baillet, 1866) infection in dogs. Rev Med Vet 152:153–156
Davidson RK, Oines O, Madslien K, Mathis A (2009) Echinococcus multilocularis adaptation of a worm egg isolation procedure coupled with a multiplex PCR assay to carry out large-scale screening of red foxes (Vulpes vulpes) in Norway. Parasitol Res 104:509–514
Denk D, Matiasek K, Just FT, Hermanns W, Baiker K, Herbach N, Steinberg T, Fischer A (2009) Disseminated angiostrongylosis with fatal cerebral hemorrhages in two dogs in Germany: a clinical case study. Vet Parasitol 160:100–108
van Doorn DCK, van de Sandea AH, Nijssea ER, Eyskera M, Ploegera HW (2009) Autochthonous Angiostrongylus vasorum infection in dogs in The Netherlands. Vet Parasitol 162:163–166
Helm J, Gilleard JS, Jackson M, Redman E, Bell E (2009) A case of canine Angiostrongylus vasorum in Scotland confirmed by PCR and sequence analysis. J Small Anim Pract 50:255–259
Helm JR, Morgan ER, Jackson MW, Wotton P, Bell R (2010) Canine angiostrongylosis: an emerging disease in Europe. J Vet Emerg Crit Care 20:98–109
Hendrix CM, Robinson E (2006) Diagnostic parasitology for veterinary technicians. Elsevier Science, Amsterdam
Jefferies R, Morgan ER, Shaw SE (2009) A SYBR green real-time PCR assay for the detection of the nematode Angiostrongylus vasorum in definitive and intermediate hosts. Vet Parasitol 116:112–118
Koch J, Willesen JL (2009) Canine pulmonary angiostrongylosis: an update. Vet J 179:348–359
Madsen AB, Dietz HH, Henriksen P, Clausen B (1999) Survey of Danish free living otters Lutra lutra—a consecutive collection and necroscopy of dead bodies. IUCN Otter Spec Group Bull 16:65–76
Mathis A, Deplazes P, Eckert J (1996) An improved test system for PCR-based specific detection of Echinococcus multilocularis eggs. J Helminthol 70:219–222
Morgan ER, Shaw SE, Brennan SF, De Waal TD, Jones BR, Mulcahy G (2005) Angiostrongylus vasorum: a real heartbreaker. Trends Parasitol 21:49–51
Oliveira SD, Barcante JMP, Barcante TA, Dias SRC, Lima WS (2006) Larval output of infected and re-infected dogs with Angiostrongylus vasorum (Baillet, 1866) Kamensky, 1905. Vet Parasitol 141:101–106
Patterson-Kane JC, Gibbons LM, Jefferies R, Morgan ER, Redrobe SR (2009) Pneumonia due to Angiostrongylus vasorum infection in a red panda (Ailurus fulgens fulgens). J Vet Diagn Invest 21:270–273
Roepstorff A, Nansen P (1998) The epidemiology, diagnosis and control of helminth parasites of swine. Food and Agriculture Organization of the United Nations, Rome
Saeed I, Maddox-Hyttel C, Monrad J, Kapel CMO (2006) Helminths of red foxes (Vulpes vulpes) in Denmark. Vet Parasitol 139:168–179
Traversa D, Guglielmini C (2008) Feline aelurostrongylosis and canine angiostrongylosis: a challenging diagnosis for two emerging verminous pneumonia infections. Vet Parasitol 157:163–174
Verzberger-Epshtein I, Markham RJF, Sheppard JA, Stryhn H, Whitney H, Conboy GA (2008) Serologic detection of Angiostrongylus vasorum infection in dogs. Vet Parasitol 151:53–60
Willesen JL, Jensen AT, Kristensen AT, Kjelgaard-Hansen M, Jessen R, Koch J (2004) Tidlig diagnostik af Angiostrongylus vasorum (fransk hjerteorm) og Crenosoma vulpis (rævens lungeorm) hos hunde er mulig ved hjælp af modificeret Baermann test. Dan Vet Tidsskrift 87:6–10
Acknowledgements
We would like to acknowledge Prof. Jørgen Koch for providing materials and Charlotte Fischer for her help in the lab.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Sabi, M.N.S., Deplazes, P., Webster, P. et al. PCR detection of Angiostrongylus vasorum in faecal samples of dogs and foxes. Parasitol Res 107, 135–140 (2010). https://doi.org/10.1007/s00436-010-1847-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-010-1847-5