Abstract
Echinococcus multilocularis, causing alveolar echinococcosis in humans, is a highly pathogenic emerging zoonotic disease in central Europe. The gold standard for the identification of this parasite in the main host, the red fox, namely identification of the adult parasite in the intestine at necropsy, is very laborious. Copro-enzyme-linked immunosorbent assay (ELISA) with confirmatory polymerase chain reaction (PCR) has been suggested as an acceptable alternative, but no commercial copro-ELISA tests are currently available and an in-house test is therefore required. Published methods for taeniid egg isolation and a multiplex PCR assay for simultaneous identification of E. multilocularis, E. granulosus and other cestodes were adapted to be carried out on pooled faecal samples from red foxes in Norway. None of the 483 fox faecal samples screened were PCR-positive for E. multilocularis, indicating an apparent prevalence of between 0% and 1.5%. The advantages and disadvantages of using the adapted method are discussed as well as the results pertaining to taeniid and non-taeniid cestodes as identified by multiplex PCR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alveolar echinococcosis, caused by Echinococcus multilocularis, is a highly pathogenic zoonotic disease emerging in many countries in central Europe (Eckert and Deplazes 2004; Schweiger et al. 2007). The tapeworm E. multilocularis is found in the small intestine of canids, in particular red foxes (Vulpes vulpes; Eckert and Deplazes 2004). Domestic dogs and cats can also be infected and are of concern when assessing risk for human infection (Antolová et al. 2008), although experimental evidence suggests that patent infections rarely establish in cats (Kapel et al. 2006). Rodents act as the normal intermediate host. Human infection occurs when eggs of the tapeworm are ingested and the human becomes an aberrant intermediate host. Unless treated, infection in humans can be fatal (Eckert and Deplazes 1999). The opening of the borders in Europe and lifting of travel restrictions on pets between most EU countries could facilitate the spread of this parasite into regions previously free from this disease. Currently, there is no evidence to suggest that this infection has established itself on the Scandinavian peninsula. However, in 1999, foci of E. multilocularis infection were identified in Denmark (Copenhagen; Kapel and Saeed 2000) and on the Norwegian high arctic island of Svalbard (Henttonen et al. 2001). Extensive surveys of red fox populations in Finland and Sweden (Anon 2004, 2005) have not documented the presence of this parasite. In a recent assessment in Sweden (Vågsholm 2006), the risk of importing the disease, through the importation of infected dogs, was estimated as high without compulsory anti-parasitic treatment prior to entry, whereas the potential for spread to wildlife and establishment of a sylvatic cycle was considered to be moderate to high.
Screening of red fox and other wild canids is carried out in many countries, with and without endemic E. multilocularis, to monitor the occurrence, spread and prevalence of this parasite. Identification of adult cestodes in the small intestine at necropsy (sedimentation counting technique) has been considered the gold standard (Deplazes et al. 2003). However, this technique is exceedingly laborious, highly dependent on the experience of the examiner and could allow false negatives to occur, particularly in low-intensity infections. In non-endemic areas, large sample sizes are required for reliable surveillance to ensure that all regions of interest are surveyed. Necropsy of this high number of animals would be economically unrealistic, rendering other high-throughput methods more suitable. Many surveillance programmes use a screening enzyme-linked immunosorbent assay (ELISA) for detection of copro-antigens (Anon 2004, 2005; Deplazes et al. 2003). The specificities of these copro-ELISA methods range from 70% to 99% (Deplazes et al. 2003). Hence, in areas with a very low prevalence, a high number of false positives are to be expected, thereby reducing the value of this approach under these conditions. An alternative is to detect the presence of DNA from these parasites using polymerase chain reaction (PCR), and a number of different PCR assays are available to detect E. multilocularis DNA (Bretagne et al. 1993; Casulli et al. 2004; Dinkel et al. 1998; Trachsel et al. 2007). The DNA used can either be isolated directly from faecal material or, in order to minimise co-isolation of PCR-inhibitory substances, from segregated parasite eggs which microscopically cannot be differentiated from those of other taeniid species. PCR carried out on DNA from isolated eggs has been shown to be more sensitive during patent infections than a copro-ELISA (Al-Sabi et al. 2007). As DNA isolation with either approach can be regarded as laborious, an expedient diagnostic strategy has involved the use of copro-ELISA followed by PCR-based confirmation of positive samples. However, this approach is feasible only for laboratories which have homemade copro-ELISAs available, as there are no such commercial tests currently available.
A pilot surveillance programme was set up in 2006 to investigate if wild red foxes in Norway could harbour E. multilocularis infections. The aim of the study was to collect faecal samples from foxes and ascertain E. multilocularis DNA presence in these using a recently developed PCR assay. The current study describes a modification of the egg isolation technique (Mathis et al. 1996; Stefanic et al. 2004) and multiplex PCR (Trachsel et al. 2007) to carry out a large-scale screening of a wild fox population in which the prevalence for the disease is expected to be low in order to document absence or presence of E. multilocularis in the red fox population in Norway.
Materials and methods
Faecal samples were collected from foxes shot during the 2006–2007 licensed hunting season. The vast majority of foxes were hunted by placing out bait lures. Each hunter completed a standard form that included information on where, when, how and by whom the fox had been killed as well as age (juvenile, less than 1 year old or adult, greater than 1 year old) and sex of the animal. Faecal samples were either removed directly per rectum, or when this proved insufficient, the abdomen was opened and faeces expressed from the intestinal tract. Each sample was frozen upon arrival in the laboratory for a minimum of 3 days at −80°C. The package was then opened and the contents assigned a unique identity number and stored in a −20°C freezer until further analysis.
Egg isolation procedure
Worm eggs were isolated as described by Mathis et al. (1996) and Stefanic et al. (2004) with a few modifications: preparation included pooling of the samples and separate sequential sieving steps. The faecal samples were pooled in groups of three prior to analysis to enable faster analysis of more samples. Pooled samples were made from three random faecal isolates, with 1 g of faeces from each animal so that the amount per 15 ml falcon tube did not exceed 3.5 g in total. The minimum amount of faeces present to allow for pooling of the individual samples was set to 4 g prior to pooling. This meant that a further 3 g faeces was available for individual retesting should a pooled sample prove to be E. multilocularis-positive. If less than a total of 4 g were available from the individual foxes, the sample was run as a single sample. If less than 1 g of faeces was present in total, the sample was excluded from the study.
The faecal samples were defrosted overnight prior to egg isolation. Single samples weighed between 1 and 3 g. All remaining faeces were placed in storage at −20°C in case further analysis was required. Approximately 12 ml of distilled water was added to each sample prior to thorough mixing and centrifugation at 1600×g for 3 min. A similar volume of flotation fluid, zinc chloride (ZnCl2, specific gravity of 1.45) was then added and the samples were again thoroughly mixed prior to centrifugation at 400×g for 30 min. Egg isolation was carried out using sequential sieving of the supernatant. Sieving was carried out using modified 50 ml falcon tubes containing nylon mesh with either 44 μm or 21 μm mesh size. The supernatant was first sieved through the 44 μm mesh into a plastic cup and then through the 21 μm mesh into a reservoir. The 21 μm nylon mesh was then inverted and the contents carefully flushed into a 15 ml falcon tube. After sedimentation for 30 min, excess fluid was aspirated from the tube, ensuring that the final volume was approximately 0.5 ml. All steps were carried out using disposable plastic equipment to avoid possible cross-contamination between samples.
DNA extraction and amplification
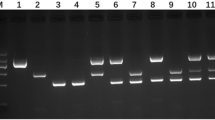
DNA isolation was performed on the sediments from each tube. Approximately 500 μL of the egg isolate were transferred to 1.5 ml Eppendorf tubes, and DNA isolation on the whole sample was performed according to Stefanic et al. (2004). DNA extracts were stored at −20°C. PCR conditions, reagents and primers used in the multiplex PCR were as according to Trachsel et al. (2007), yielding amplicons of 395 bp for E. multilocularis, 117 bp for E. granulosus and approximately 267 bp for Taenia spp. and non-taeniid cestodes, respectively. PCR products were visualised using 2% LE-agarose (USB) stained with ethidium bromide after being run in TAE 1× buffer for 60 min (110 V). One DNA-negative control and two positive controls (E. multilocularis and Taenia polyacantha) were included in all PCR runs. Nine of the non-E. multilocularis cestode products, generated in the screening, were sequenced using MEGABACE 1000 (GE-Healthcare, Bucks, UK) and DyeET terminator (GE-Healthcare) using ‘Cest-5’ and ‘Cest-5seq’ primers (Trachsel et al. 2007) after amplicons were purified using Nucleospin® Extract II from Macherey-Nagel (Düren, Germany). Chromatograms were manually edited and aligned using Vector Nti (Invitrogen), and BLAST searches (www.ncbi.nlm.nih.gov/BLAST) were also performed using this bioinformatic software. Additionally, a serial dilution experiment of the positive E. multilocularis control DNA solution was carried out to estimate the lowest detection level of DNA allowing amplification by PCR. The concentration of the DNA in the positive control was measured and found to be 14 μg/ml. The control sample was then analysed at the following dilutions: 1:1, 1:20, 1:200, 1:2 000 and 1:20 000.
Statistical analysis
Sex, age and geographic distribution of the foxes sampled were analysed using JMP 6.0 (SAS Institute). Chi-squared analysis of the age and sex distribution was carried out as well as contingency analysis of the geographic distribution of the samples compared to geographic distribution of hunted foxes during recent hunting seasons (2001–2005). The 95% confidence interval for E. multilocularis prevalence was calculated using FreeCalc version 2 (AusVet Animal Health Services). A significance level of 5% was selected for the statistical comparisons (p < 0.05).
Results
The multiplex PCR method was able to detect 14 pg (a 1:2 000 dilution of the positive E. multilocularis control) of E. multilocularis DNA after the dilution of stock DNA isolated from adult E. multilocularis worms. In total, 483 fox faecal samples were included in the current analysis. The age and sex of the animals from which faeces were included is shown (Table 1). Information on the sex of five animals and an age estimate of another five was not available. Significantly more samples came from male foxes (58.2%) than female (40.8%), and fewer faecal samples were examined from juveniles (39.8%) than adults (59.2%). The regional distribution of the foxes is shown (Table 2) as well as the number of foxes hunted in each region compared to the proportion sampled. The counties of Akershus, Oslo, Sogn og Fjordane, Sør Trøndelag and Nordland are overrepresented in the sampling compared to the proportion of foxes hunted from these regions in 2001–2005. The counties of Østfold, Oppland, Vest-Agder, Møre og Romsdal and Nord-Trøndelag are underrepresented in the sampling compared to the proportion of foxes that are normally hunted in these regions. All other counties were within acceptable boundaries to be representative of the proportion of annually hunted foxes.
In total, 153 samples pooled from three foxes were examined, five pooled samples from two foxes were examined and a total of 14 individual faecal samples were examined. All samples were PCR-negative for E. multilocularis. The prevalence (0/483) was therefore not significantly different from 0 with a 95% confidence interval of 0–1.5%, assuming a sensitivity of 50% and a specificity of 100% (Ziadinov et al. 2008) in an overall population with an estimated 70,000 individuals (Olav Hjeljord, UMB, Ås, personal communication). Five of the individual samples were PCR-positive for the 267 bp band indicating Taenia or other closely related cestode targets, whilst three of the pooled samples from two foxes and 58 pooled samples from three foxes were positive, representing between 102 and 294 positive fox samples. Sequence analysis of nine randomly selected 267 bp amplicons revealed two sequences with 99.6% identity (248 bp) to Mesocestoides lineatus (EF567417), one sequence with 97% identity (257 bp) to both Diphyllobothrium ditreum (AB031366) and Diphyllobothrium latum (AB269325), whereas the remaining samples, after alignments of high-quality read lengths that were between 162 and 240 bp, generated scores with highest identity score of approximately 88% with a 12S-sequence Genbank entry from a Mesocestoides spp. (DQ102755; five samples) or 81.4% identity (269 bp) to a Hymenolepis diminuta (AB031359) entry (one sample). One faint PCR band which was slightly larger than the E. multilocularis-specific product was also sequenced, revealing a non-specific amplification. BLAST search indicated close similarity to sequences from either a bacteriophage or Enterobacter spp.
Discussion
The survey did not identify E. multilocularis in any of the red fox samples investigated. Pre-patent infections would be difficult to detect with this method as it relies on the detection of DNA isolated from the eggs. The overall sensitivity of the approach was calculated to be 50% by employing Bayesian techniques (Ziadinov et al. 2008). The pre-patent period for E. multilocularis is approximately 28–30 days, and eggs are excreted in varying amounts for a further 40 to 60 days (Al-Sabi et al. 2007; Matsumoto and Yagi 2008). It is very unlikely that all the foxes tested, if infected, would only have been infected within the 30 days prior to the sampling. It is, however, possible that E. multilocularis could be present in low numbers (prevalence <1.5%) in the sylvatic population or only found in a restricted geographic area. Such an occurrence of E. multilocularis on a very small spatial scale has been described in valleys of the Swiss alps (Tanner et al. 2006). Given the wide geographic area covered in the sampling, our results indicate that E. multilocularis has yet to establish on mainland Norway. Prolonged surveillance with wide geographic coverage is, however, required to confirm the continued absence of this parasite from mainland Norway.
The samples were processed more rapidly than described by Mathis et al. (1996) and Stefanic et al. (2004) by omitting examination by light microscopy for taeniid eggs, which may also decrease the potential for errors in egg identification. The PCR results of the pooled samples indicate that the prevalence of Mesocestoides and other cestodes in the fox population sampled here lies somewhere between 21% and 61%. One third of the individual samples tested were PCR-positive, indicating that the level of infection may tend towards the lower end of this range. M. lineatus has previously been reported in red foxes in Norway (Vik 1966), so this finding is not unexpected. The prevalence of Mesocestoides spp. and other cestodes in the current study is, however, surprisingly high. This might partly reflect the detection of cestodes in passage through the vulpine gastrointestinal tract from prey animals or improved sensitivity with the molecular methods, given that the method was able to detect such small amounts of DNA. A comparative study carried out on Norwegian red fox faeces collected between 2002 and 2004, using classical egg flotation and McMaster slide for examination and counting, identified taeniid eggs in just 3.3% (9/271) of the samples (unpublished results National Veterinary Institute, Norway). Examination of Norwegian red foxes in the 1960s found 8% (42/543) infected with M. lineatus and 9% infected with Taenia spp. (Vik 1966). It seems unlikely that the underlying cestode prevalence, based on egg flotation techniques, could have increased tenfold from 3% in 2002–2004 to the current level of >20% by 2006–2007. It appears, therefore, that based on these data, the egg isolation and detection using PCR allows more sensitive detection of these worms compared to traditional techniques.
Detailed information on age, sex and geographic variations in population density for the red fox in Norway is lacking. There are no reliable red fox population estimates in Norway, although a rough estimate of 70,000 breeding animals has been given. Comparison of the sampling in this study to the hunting statistics for each county gives an indication of whether the sampling is representative or not, given that the exact population density in each region is unknown. The majority of regions were within acceptable limits to be representative. Overrepresentation of Akershus and Oslo counties in the current study, compared to the proportion of foxes hunted from these regions, is beneficial. These counties represent the region in and around Oslo with highest freight and tourist traffic (Statistics Norway www.ssb.no) and thus greatest risk of E. multilocularis exposure either through the illegal import of an infected dog or accidental import of an infected intermediate host with road, rail, air or sea freight.
It is, however, more difficult to assess if the age and sex biases seen are a true reflection of the situation in the general population. Although there is a significant difference in the proportion of samples from male foxes compared to females, this should not affect the reliability of the test, as sex bias has not been seen in the distribution of this parasite (Hofer et al. 2000). Age-related differences in the distribution of E. multilocularis are found, with juvenile foxes harbouring higher worm burdens than adults (Hofer et al. 2000). The age of the fox was estimated by the hunters themselves and no guidelines were given as to how to do this. Therefore, the ageing of foxes in the current study, as adult or juvenile, is only a rough indication, and it could be possible that a number of juvenile foxes were assessed as adults. This does not, however, influence the overall result here; future studies might consider introducing sampling bias to include more juveniles as well as including guidelines for age determination.
Based on this survey, there is no evidence that E. multilocularis is present in the Norwegian red fox population. The egg isolation method with pooled samples, followed by multiplex PCR, appear well suited to carrying out large-scale screenings of a wild fox population in areas with anticipated low E. multilocularis prevalence. As prevalence levels increase, however, it may be of more benefit to carry out a screening copro-ELISA prior to egg isolation or to avoid pooling of the samples. The estimation of taeniid and non-taeniid cestode prevalence in foxes based on the multiplex PCR was limited due to the pooling of the samples, but by allowing the presence of the 267 bp “taeniid” PCR product to function as an internal control, it was possible to confirm that DNA extraction was successful and, of equal importance, that PCR inhibitors if present did not prevent successful PCR in at least these samples. The PCR was able to detect both prey animal cestodes and vulpine cestodes. Therefore, PCR on individual samples, cloning of each product followed by sequencing of all the positives would be required to determine which cestode species were involved and prevalence figures adjusted accordingly.
References
Al-Sabi MNS, Kapel CMO, Deplazes P, Mathis A (2007) Comparative copro-diagnosis of Echinococcus multilocularis in experimentally infected foxes. Parasitol Res 101:731–736
Anon (2004) Finland trends and sources of zoonoses and zoonotic agents in humans, foodstuffs, animals and feedingstuffs, including information on foodborne outbreaks and antimicrobial resistance in zoonotic agents in 2004. Zoonoses Monitoring, pp 1–207
Anon (2005) Sweden trends and sources of zoonoses and zoonotic agents in humans, foodstuffs, animals and feedingstuffs, including information on foodborne outbreaks and antimicrobial resistance in zoonotic agents in 2005. Zoonoses Monitoring, pp 1–194
Antolová D, Reiterová K, Miterpáková M, Dinkel A, Dubinský P (2008) The first finding of Echinococcus multilocularis in dogs in Slovakia: an emerging risk for spreading of infection. Zoonoses Public Health. doi:10.1111/j.1863-2378.2008.01154.x
Bretagne S, Guillou JP, Morand M, Houin R (1993) Detection of Echinococcus multilocularis DNA in fox faeces using DNA amplification. Parasitology 106:193–199
Casulli A, La Rosa G, Manfredi MT, Di Cerbo AR, Dinkel A, Romig T, Deplazes P, Genchi C, Pozio E (2004) Copro-diagnosis of Echinococcus multilocularis by a nested PCR in red foxes (Vulpes vulpes) from northern Italy. Parassitologia 46:419–420
Deplazes P, Dinkel A, Mathis A (2003) Molecular tools for studies on the transmission biology of Echinococcus multilocularis. Parasitology 127:S53–S61
Dinkel A, von Nickisch-Rosenegk M, Bilger B, Merli M, Lucius R, Romig T (1998) Detection of Echinococcus multilocularis in the definitive host: coprodiagnosis by PCR as an alternative to necropsy. J Clin Microbiol 36:1871–1876
Eckert J, Deplazes P (1999) Alveolar Echinococcosis in humans: the current situation in Central Europe and the need for countermeasures. Parasitol Today 15:315–319
Eckert J, Deplazes P (2004) Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 17:107–135
Henttonen H, Fuglei E, Gower CN, Haukisalmi V, IMS RA, Niemimaa J, Yoccoz NG (2001) Echinococcus multilocularis on Svalbard: introduction of an intermediate host has enabled the local life-cycle. Parasitology 123:547–552
Hofer S, Gloor S, Müller U, Mathis A, Hegglin D, Deplazes P (2000) High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zürich, Switzerland. Parasitology 120:135–142
Kapel CMO, Saeed I (2000) Echinococcus multilocularis—en ny zoonotisk parasit i Danmark. Dansk Vet Tidsskr 83:14–16
Kapel CMO, Torgerson PR, Thompson RCA, Deplazes P (2006) Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int J Parasitol 36:79–86
Mathis A, Deplazes P, Eckert J (1996) An improved test system for PCR-based specific detection of Echinococcus multilocularis eggs. J Helminthol 70:219–222
Matsumoto J, Yagi K (2008) Experimental studies on Echinococcus multilocularis in Japan, focusing on biohazardous stages of the parasite. Exp Parasitol 119:534–541
Schweiger A, Ammann RW, Candinas D, Clavien PA, Eckert J, Gottstein B, Halkic N, Meullhaupt B, Prinz BM, Reichen J, Tarr PE, Torgerson PR, Deplazes P (2007) Human alveolar echinococcosis after fox population increase, Switzerland. Emerg Infect Dis 13:878–882
Stefanic S, Shaikenov BS, Deplazes P, Dinkel A, Torgerson PR, Mathis A (2004) Polymerase chain reaction for detection of patent infections of Echinococcus granulosus (“sheep strain”) in naturally infected dogs. Parasitol Res 92:347–351
Tanner F, Hegglin D, Thoma R, Brosi G, Deplazes P (2006) Echinococcus multilocularis in Grisons: distribution in foxes and presence of potential intermediate hosts. Schweiz Arch Tierheilkd 148:501–510
Trachsel D, Deplazes P, Mathis A (2007) Identification of taeniid eggs in the faeces from carnivores based on multiplex PCR using targets in mitochondrial DNA. Parasitology 134:911–920
Vågsholm, I (2006) An assessment of the risk that EM is introduced with dogs entering Sweden from other EU countries without and with antihelmintic treatments. Report S 751 89. National Veterinary Institute, Uppsala, Sweden
Vik R (1966) Platyhelminths in wild fox in Norway. International Congress of Parasitology. Proceedings of the First International Congress of Parasitology 1; 481
Ziadinov I, Mathis A, Trachsel D, Rysmukhambetova A, Abdyjaparov TA, Kuttubaev OT, Deplazes P, Torgerson PR (2008) Canine echinococcosis in Kyrgyzstan: using prevalence data adjusted for measurement error to develop transmission dynamics models. Int J Parasitol 38:1179–1190
Acknowledgements
We would like to thank all the hunters who participated in the study as well as Nina Tvedt and Marthe Opland, Section for Wildlife Diseases, for their help with the logistics, and Inger Sofie Hamnes and Kristin Henriksen, Section for Parasitology, for their help with laboratory work. This work was funded by the Norwegian Food Health Safety Authority.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Davidson, R.K., Øines, Ø., Madslien, K. et al. Echinococcus multilocularis—adaptation of a worm egg isolation procedure coupled with a multiplex PCR assay to carry out large-scale screening of red foxes (Vulpes vulpes) in Norway. Parasitol Res 104, 509–514 (2009). https://doi.org/10.1007/s00436-008-1222-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-008-1222-y