Abstract
The complementary DNA (cDNA) plasmid libraries of adult worm, metacercariae and egg of Clonorchis sinensis (C. sinensis) were constructed for researches on genomics and proteomics of C. sinensis. The full-length cDNA sequence encoding tegumental protein 31.8 kDa (CsTP31.8) was identified from the adult cDNA library. The cDNA sequence has been submitted to the GeneBank Database with accession number ABK60086. This novel cDNA sequence contains 828 bp with a putative open reading frame of 275 amino acids. The deduced amino acid sequence shows identity to membrane-associate antigens or tegumental antigens of other species. There were conserved calcium-binding EF hand and dynein light chain type 1 in the sequence. CsTP31.8 transcripts were detected in cDNA libraries of adult worm and metacercariae but not in that of egg. Recombinant CsTP31.8 was expressed and purified from Escherichia coli BL21 (DE3). CsTP31.8 was immunolocalized at the tegument of adult C. sinensis by using antirecombinant CsTP31.8 sera.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human clonorchiasis, caused by the infection of Clonorchis sinensis (C. sinensis), is endemic in Southern China, Korea, Japan, and other Southeast Asian countries. Nearly 35 million people have been infected with C. sinensis globally, of whom 15 million are in China (Lun et al. 2005). People are infected with C. sinensis in endemic regions by consuming raw or undercooked freshwater fish and shrimp with metacercariae of C. sinensis. The infection rate is increasing in some provinces of China because of people’s unhealthy eating habit (Pan et al. 2000; Fang et al. 2000). Despite its threat to human health, little is known about the genomics and proteomics of C. sinensis. Many biological and biochemical characteristics of this worm have not yet been defined.
The outermost surface of intramammalian stages of platyhelminth parasite, the tegument, is generally viewed as the most susceptible target for vaccines and drugs because it is importance for host response and parasite survival (Van Hellemond et al. 2006). In the present study, we described sequence analysis, expression, and characterization of one tegumental protein of C. sinensis, tegumental protein 31.8 kDa (CsTP31.8). This basic study might be the cornerstone for further application of this protein as a molecule for diagnosis and vaccine of clonorchiasis.
Materials and methods
Preparation of C. sinensis adult worms sections
C. sinensis adult worms were harvested from biliary tracts of infected cats. The worms were fixed with formalin after twice washing gently with sodium chloride. The worms were embedded with paraffin wax and sliced into 5-μm sections for immunohistochemical localization.
Identification of complementary DNA sequence encoding CsTP31.8
The complementary DNA (cDNA) library of adult C. sinensis was constructed by the method of Switching Mechanism at 5′ end of the RNA Transcript (SMART). The sequences of 8,900 expressed sequence tags and 1,775 Unigenes were obtained as described previously (Song et al. 2004; Yang et al. 2005; Zheng et al. 2005). All Unigenes were screened, and full-length cDNA sequences were determined by BLASTx program at the National Center for Biotechnology Information (NCBI) web server http://www.ncbi.nlm.nih.gov/BLAST). Meanwhile, the identities of the deduced amino acid sequences of these cDNAs with proteins of other species were analyzed.
Bioinformatics analysis of CsTP31.8
The complete encoding sequence (CDS) of CsTP31.8 was determined by Open Reading Frame (ORF) Finder program at NCBI web server http://www.ncbi.nlm.nih.gov/projects/gorf/). The cDNA and deduced amino acid sequence of CsTP31.8 were analyzed by Vector NTI suite 8. The conserved domain and physico-chemical properties such as molecular weight (MW), isoionic point (pI), halflife and so on were predicted by tools provided by ExPASy Proteomics Server http://expasy.org/).
Transcript analysis of CsTP31.8 at life-stage of C. sinensis
Polymerase chain reaction (PCR) was employed to amplify the transcripts of CsTP31.8 from cDNA library of adult worm, metacercariae and egg, respectively, by using specific primers (forward primer: 5′-CGGGAATTCATGGATGCTTTTATCG-3′ and reverse primer: 5′-GGCTCGAGTCAATAATATGGTGTCTTG-3′). PCR products were analyzed on a 1% agarose gel by ethidium bromide staining. The specific PCR products amplified from each library were cloned into pGEM-T vector and sequenced.
Cloning, expression, and purification of CsTP31.8
There were recognition sequences (underlined in the sequences of primers) of EcoRI and XhoI in the PCR products amplified from cDNA library of adult worm by using the same primers mentioned above. The specific PCR product was purified and digested with EcoRI and XhoI and then inserted into pGEX-4T-1 vector digested with the same restriction enzyme. The recombinant plasmid was confirmed by DNA sequencing and transformed into Escherichia coli BL21. The expression of CsTP31.8-glutathione-S-transferase (GST) fusion protein was induced by isopropyl-β-d-thiogalactopyranoside (IPTG). After induction, the bacteria cells were collected and sonicated. The fusion protein was purified by using affinity column of GSTrapFF (Amersham Biosciences, USA) according to the user manual. The GST tag was cleaved with thrombin (Pharmacia). The efficiencies of CsTP31.8 expression and purification were identified by 15% sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and visualized by Coomassie brilliant blue G-250. The final concentration of purified CsTP31.8 was determined by the method of Bradford on nucleic acid/protein analysator (Beckman, USA).
Acquirement of rat anti-recombinant CsTP31.8 immune sera and Western blotting analysis
Approximately 100 μg of recombinant CsTP31.8 was mixed with equal volume of complete Freund’s adjuvant. Sprague–Dawley rats were immunized with the mixture. After 2 weeks, the rats were immunized again with 100 μg of CsTP31.8 mixed with incomplete Freund’s adjuvant. After one more pulse with CsTP31.8 alone at fifth week, the immune sera were collected at sixth week from rats. The titer of the antisera to CsTP31.8 was 1:12,800 determined by enzyme-linked immunosorbent assay (ELISA).
The recombinant CsTP31.8 protein were subjected to 15% SDS-PAGE and then electrotransferred to a polyvinylidene difluoride membrane at 100 V for 1 h in a Trans-Blot transfer cell (BioRad, CA). The membrane was incubated in the immune sera (1:100 dilution) and subsequently in horseradish peroxidase-conjugated goat anti-rat IgG (1:5,000 dilution; Bethyl, USA). At last, the membrane was developed color with diaminobenzidine substrate solution.
Immunolocalization of CsTP31.8 at adult worm of C. sinensis
Sectioned worms in paraffin wax were deparaffinized and incubated in the rat anti-CsTP31.8 sera (1:100 dilution). Preimmune rat sera were employed to make a negative control. The sections were subsequently incubated in fluorenscein isothiscyanate (FITC)-conjugated anti-rat IgG (1:50; Boster). The sections were observed under fluorescence microscope.
Results
Sequence analysis of CsTP31.8
The cDNA sequence of CsTP31.8 was identified by BLASTx. The full cDNA sequence (GenBank accession number, ABK60086) contained a complete ORF of 828 base pairs (bp) which encoded a putative protein of 275 amino acids.
The MW of CsTP31.8 was predicted to be 31.8 kDa. Its theoretical isoelectric point (pI) was 4.33. The estimated half-life of CsTP31.8 was more than 10 h in E. coli. The instability index of CsTP31.8 was computed to be 35.57. This classified the protein as stable.
BLASTx showed that CsTP31.8 had more than 40% identity to tegumental proteins from other platyhelminth parasites. It was predicted that there were conserved domains of calcium-binding EF hand and dynein light chain type 1 in CsTP31.8 by Interpro Scan program. The alignments were 96.8 and 89.9%, respectively.
Transcript analysis of CsTP31.8 at life-stage of C. sinensis
CsTP 31.8 transcripts were detected in cDNA library of adult worm or metacercariae of C. sinensis but not in that of egg (Fig. 1).
Transcript analysis of CsTP31.8 at life-stage of C. sinensis by using PCR with specific primers of the complete encoding sequence of CsTP31.8. Lane 1 DNA marker (2,000, 1,000, 750, 500, 250, and 100 bp); lane 2 positive control; lane 3 negative control; lane 4 adult worm cDNA library; lane 5 metacercariae cDNA library; lane 6 egg cDNA library
Expression and purification of CsTP31.8
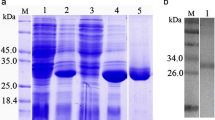
The recombinant CsTP31.8 was expressed as a fusion protein with a GST tag in E. coli BL21 after induction by IPTG. The fusion protein of GST-CsTP31.8 was about 58 kDa on polyacrylamide gel stained by 0.1% Coomassie brilliant blue R250. The MW was consistent with the predicted MW of 57.8 kDa (MW of GST is 26 kDa). After purification, other proteins of E. coli BL21 were removed. GST was completely removed (Fig. 2). The concentration of purified CsTP31.8 was 1.8 mg/ml.
Expression and purification of recombinant CsTP31.8 in Escherichia coli BL21 identified by 15% SDS-PAGE staining by 0.1% Coomassie brilliant blue R250. Lane 1 Protein molecular weight markers; lane 2 lysate of the bacteria with pGEX-4T-1 vector induced with IPTG; lane 3 lysate of the bacteria with recombinant pGEX-4T-1-CsTP31.8 induced with IPTG; lane 4 GST-CsTP31.8 fusion protein purified with affinity column of GSTrapFF; lane 5 recombinant CsTP31.8 without GST tag
Western blotting analysis of recombinant CsTP31.8
The purified CsTP31.8 could be probed by rat anti-CsTP31.8 sera in the Western blot analysis (Fig. 3).
Western blotting analysis of recombinant CsTP31.8 by using rat preimmune sera or anti-CsTP31.8 sera as primary antibody. Lane 1 Protein molecular weight markers; lane 2 purified recombinant CsTP31.8 reacted with rat preimmune sera; lane 3 purified recombinant CsTP31.8 reacted with rat anti-CsTP31.8 sera
Immunolocalization of CsTP31.8 at adult worm of C. sinensis
The analysis of immunohistochemical localization by using rat anti-CsTP31.8 sera showed that CsTP31.8 was located at the tegument of adult C. sinensis. CsTP31.8 wasn’t observed in the internal tissues of the C. sinensis. CsTP31.8 could not be detected in sections incubated with preimmune rat sera (Fig. 4).
Immunohistochemical localization of CsTP31.8 at adult C. sinensis by using rat anti-CsTP31.8 sera as primary antibody and FITC-conjugated anti-rat IgG as secondary antibody. The left images showed the tegument and underlying tissues of adult worm under fluorescence microscope. The right images showed the same part under optical microscope. Sera from nonimmunized rats were used to make negative control. The localization of CsTP31.8 is shown in a and the negative control is shown in b
Discussion
In this work, we studied a novel tegumental protein CsTP31.8 from C. sinensis. We identified its complete encoding sequence from cDNA library. It contains 828 bp which encoded a putative protein of 275 amino acids. Its physico-chemical properties were analyzed by bioinformatics. It showed around 40% identity to tegumental proteins of other species. There might be conserved domains of calcium-binding EF hand and dynein light chain type 1 in it. The alignments were 96.8 and 89.9%. The transcripts of CsTP31.8 could be detected in cDNA libraries of adult worm and metacercariae. CsTP31.8 was immunolocalized at the tegument of adult C. sinensis as predicted.
The identity between CsTP31.8 and membrane-associated antigens or tegumental antigens of other species was about 40%. There probably were calcium-binding EF-hand and dynein light chain type 1 domains in CsTP31.8. Such domains were usually involved in the structure of membrane-associated or tegumental antigens from Schistosoma japonicum, Schistosoma mansoni, and Fasciola gigantica (Jeffs et al. 1991; Ruiz de Equino et al. 1999; Fitzsimmons et al. 2004; Hoffmann and Strand 1997). The result of immunolocalization showed that CsTP31.8 was located at the tegument of adult C. sinensis. The results of both bioinformatics analysis and actual immunolocalization of CsTP31.8 indicated that the novel protein was one of the tegumental proteins from C. sinensis.
The tegument is a dynamic host-interactive layer involved in nutrition, immune evasion and modulation, excretion, and signal transduction. The tegument constitutes the parasite–host interface (Jones et al. 2004; Van Hellemond et al. 2006). Many proteins identified in the tegument of S. japonicum, S. mansoni, and F. gigantica were potentially molecules for vaccine development and for immunological diagnosis (Jeffs et al. 1991; Waine et al. 1994; Xavier et al. 1998). It was documented that the tegument showed the strongest staining in the immunohistochemical staining of C. sinensis with C. sinensis-infected animal sera (Cho et al. 1986; Choi et al. 1981; Hong et al. 2001). The transcripts of CsTP31.8 were detected in the stage of metacercariae and adult worm, so that if CsTP31.8 might be defined as an important antigen of C. sinensis, it could be developed as a vaccine not only for intermediate host but also for definitive host.
In a previous study, another tegumental protein from C. sinensis was identified, and its value as a molecule for serologic diagnosis was evaluated in our lab (Zhou et al. 2007). The identification of this novel tegumental protein CsTP31.8 would enhance our understanding of host–parasite interaction as well as provide another potential antigen for diagnosis and vaccine development.
In summary, we identified a novel tegumental protein CsTP31.8 of C. sinensis. Basic information of CsTP31.8 was obtained. Further studies will be focused on its ultrastructure location and value as a molecule for diagnosis and vaccine development of clonorchiasis.
References
Cho BS, Eom KS, Rim HJ (1986) Comparative studies on the antibody levels in clonorchiasis using IFAT with sera and blood collected on filter paper. Korea Univ Med J 23(3):23–32
Choi WY, Jin YK, Lee OR, Kim WG (1981) Analysis of protein components at various stages of Clonorchis sinensis. Korean J Parasitol 19(1):8–17
Fang YY, Pan B, Shi XC, Chen ZZ, Lin RX, Huang SY, Zhang XC, Deng ZH, Zhang QM, Liu YY, He Q (2000) Comparative analysis of two surveys of distribution of human parasites in Guangdong province. Strait J Prevent Med 6(2):32–33 (in Chinese)
Fitzsimmons CM, Stewart TJ, Hoffmann KF, Grogan JL, Yazdanbakhsh M, Dunne DW (2004) Human IgE response to the Schistosoma haematobium 22.6 kDa antigen. Parasite Immunol 26(8–9):371–376
Hoffmann KF, Strand M (1997) Molecular characterization of a 20.8 kDa Schistosoma mansoni antigen. J Biol Chem 272:14509–14515
Hong SJ, Kim TY, Song KY, Sohn WM, Kang SY (2001) Antigenic profile and localization of Clonorchis sinensis proteins in the course of infection. Korea J Parasitol 39(4):307–312
Jeffs SA, Hagan P, Allen R, Correa-Oliveira R, Smithers SR, Simpson AJ (1991) Molecular cloning and characterisation of the 22-kilodalton adult Schistosoma mansoni antigen recognized by antibodies from mice protectively vaccinated with isolated tegumental surface membranes. Mol Biochem Parasitol 46(1):159–167
Jones MK, Gobert GN, Zhang L, Sunderland P, McManus DP (2004) The cytoskeleton and motor proteins of human schistosomes and their roles in surface maintenance and host-parasite interactions. Bioessays 26:752–765
Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, Fang YY (2005) Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis 5:31–41
Pan B, Fang YY, Yang WS (2000) Current situation and control strategy of parasitic diseases in Guangdong province. Ann Bull Soc Parasitol Guangdong 22:85–89 (in Chinese)
Ruiz de Equino AD, Machin A, Casais R, Castro AM, Boqa JA, Martin-Alonso JM, Parra F (1999) Cloning and expression in Escherichia coli of a Fasciola hepatica gene encoding a calcium-binding protein. Mol Biochem Parasitol 101:13–21
Song LX, Chen SY, Yu XB, Wu ZD, Xu J, Yang G, Zheng NC, Hu XC, Guo LC, Dai JF, Xu J, Ji CN, Gu SH, Ying K (2004) Molecular cloning and characterization of cDNA encoding a ubiquitin-conjugating enzyme from Clonorchis sinensis. Parasitol Res 94:227–232
Van Hellemond JJ, Retra K, Brouwers JFHM, Balkom BWM, Yazdanbakhsh M, Shoemaker CB, Tielens AGM (2006) Functions of the tegument of schistosomes: clues from the proteome and lipidome. Int J Parasitol 36:691–699
Waine GJ, Becker MM, Scott JC, Kalinna BH, Yang W, McManus DP (1994) Purification of a recombinant Schistosoma japonicum antigen homologous to the 22-kDa membrane-associated antigen of S. mansoni, a putative vaccine candidate against schistosomiasis. Gene 142(2):259–263
Xavier EM, Lucena-Silva N, Werkhauser RP, Franco GR, Santos RA, Simpson AJ, Abath FG (1998) The tegument of Schistosoma mansoni: genes, antigens and the host–parasite relationship. Mem Inst Oswaldo Cruz 93(Suppl 1):85–86
Yang G, Yu XB, Wu ZD, Xu J, Song LX, Zhang HM, Hu XC, Zheng NC, Guo LC, Xu J, Dai JF, Ji CN, Gu SH, Ying K (2005) Molecular cloning and characterization of a novel adenylate kinase 3 gene from Clonorchis sinensis. Parasitol Res 95:406–412
Zheng NC, Xu J, Wu ZD, Chen JZ, Hu XC, Song LX, Yang G, Ji CN, Chen SY, Gu SH, Ying K, Yu XB (2005) Clonorchis sinensis: molecular cloning and functional expression of novel cytosolic malate dehydrogenase. Exp Parasitol 109:220–227
Zhou ZW, Hu XC, Huang Y, Hu HX, Ma CL, Chen XX, Hu FY, Xu J, Lu FL, Wu ZD, Yu XB (2007) Molecular cloning and identification of a novel Clonorchis sinensis gene encoding a tegumental protein. Parasitol Res 101:737–742
Acknowledgments
This study was supported by a grant from the key science and technique program of Guangdong province (number 2004A30801004) and the national high technology research and development program of China (863 Program; number 2006AA02Z422) to X. Yu.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, Y., Zhou, Z., Hu, X. et al. A novel tegumental protein 31.8 kDa of Clonorchis sinensis: sequence analysis, expression, and immunolocalization. Parasitol Res 102, 77–81 (2007). https://doi.org/10.1007/s00436-007-0728-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-007-0728-z