Abstract
Ingestion of the larval nematode Angiostrongylus cantonensis can cause the human eosinophilic meningitis known as angiostrongyliasis. Analysis of the extracts and excretory-secretory (ES) products of A. cantonensis larvae and adult stages on gelatin substrate zymography demonstrated the presence of distinct gelatinolytic enzymes. In worm extracts, inhibitor studies showed that the metalloproteinases revealed in L1 (23 kDa), L3 (66, 42 and 30 kDa), young adult worm (72 and 94 kDa) and adult worm (72 and 94 kDa). In ES products, the L1 revealed one low (42 kDa) and two high (105 and 94 kDa) molecular weight proteolytic bands that degraded gelatin in substrate gels. The L3 revealed three low (66, 50, and 30 kDa) and one high (105 kDa) molecular weight proteolytic bands. Inhibitor studies confirmed that the 105 and 94 proteolytic bands of the L1, and the 50 and 30 kDa proteolytic bands of the L3 classification were metalloproteinases. These metalloproteinases secreted in the infective larvae may be associated with the parasite dissemination or pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The parasitic nematode Angiostrongylus cantonensis, which dwells in the rat pulmonary artery, is the most common infectious cause of eosinophilic meningitis worldwide (Kliks and Palumbo 1992). The adult worm of males typically measure 14–15 mm in length, and has a tail with copulatory bursa and long spicules. Females typically measure 24–26 mm in length, and present a characteristic barber-pole appearance (Lindo et al. 2002). The nematode has a complex life cycle (Alicata 1965). Eggs hatch in the lungs, and first-stage larvae (L1) pass out in the faeces, to subsequently enter a molluscan intermediate host where they moult twice. The third stage larvae (L3) of A. cantonensis typically measure 425–524 μm in length and 23–34 μm in width. The posterior end of the tail always terminates in a fine point (Ash 1970). The infective L3 orally infect the final host and are carried in the blood to the central nervous system, where they moult twice to become immature adults. Once reaching the branches of the pulmonary artery, they grow rapidly, attain sexual maturity, and release eggs (Alicata 1965).
The production of proteolytic enzymes and their release as excretory-secretory (ES) products have been reported for various helminthes (Matthews 1982; McKerrow and Doenhoff 1988; McKerrow et al. 1990). Cysteine proteases have been found in Ancylostoma caninum (Harrop et al. 1995) and Toxocara canis (Loukas et al. 1998). Serine proteinases have been identified in Anisakis simplex (Morris and Sakanari 1994). Metalloproteinases have also been identified in a variety of helminthes including Brugia malayi (Petralanda et al. 1986), T. canis (Robertson et al. 1989), Strongyloides stercoralis (McKerrow et al. 1990), Nippostrongylus brasiliensis (Healer et al. 1991), Dirofilaria immitis (Richer et al. 1992), Trichuris suis (Hill et al. 1993), A. caninum (Hawdon et al. 1995), Caenorhabditis elegans (Wada et al. 1998) and Gnathostoma spinigerum (Uparanukraw et al. 2001).
Matrix metalloproteinases (MMPs) are a family of at least 20 zinc metallo-endopeptidases in vertebrates that regulate cell-matrix composition. They have been divided into subgroups according to their structure and function (Matrisian 1992). These enzymes are thought to participate in extracellular matrix (ECM) remodeling and degradation, and have been implicated in important roles during organ morphogenesis, embryonic development and pathological processes (Stetler-Stevenson et al. 1993; Sato and Seiki 1996).
We have previously shown that the A. cantonensis could induce host MMP-9 production in the brain (Lai et al. 2004; Lee et al. 2004). However, knowledge about the MMPs in nematodes is limited and whether the proteinases can produce from A. cantonensis remains unclear. The present study sought to investigate the activity of metalloproteinases in the extracts and ES products of A. cantonensis.
Materials and methods
Parasite preparation
The L3 (infective larvae for mammalian host) of A. cantonensis originally obtained from the field mollusk host that were propagated for several months in our laboratory by cycling through rats and snails (Biomphalaria glabrata). The larvae within tissues were recovered using a modification of the method of Parsons and Grieve (1990). Briefly, the shells were crushed, the tissues were homogenized, and digested in a pepsin-HCl solution (pH 1–2,500 I.U. pepsin/g tissue), and incubated with agitation in a 37°C water bath for 2 h. Host cellular debris was removed from the digest by centrifugation at 1,400g for 10 min. The larvae in the sediment were collected by serial washing in double-distilled water and counted under the microscope. Immature adult worms were dissected from rat brains on day 25 post-inoculation (PI). Each brain was torn into small pieces and homogenized separately in 15 ml of 0.25% sodium citrate in phosphate-buffered saline (PBS) followed by centrifugation. Adult worms were obtained as described previously (Joshua et al. 1995). L1 (infective larvae for mollusk host) were obtained by pepsin:HCl digestion of lungs obtained at 40 days PI, followed by purification from lung tissue by pelleting through 100% isopaque (Pharmacia, New York, NY) (Bessarab and Joshua 1997).
Parasite extracts
Worms were thoroughly washed in PBS to free them of enzyme, ground in liquid nitrogen, homogenized in PBS in an ice bath and subjected to repeated brief sonications. The success of this procedure in completely breaking worms apart was confirmed microscopically. The extracts were centrifuged at 12,000g for 10 min.
In vitro cultivation of A. cantonensis
For the experiments, five groups were used. All experiments utilized RPMI-1640 medium (Sigma, USA). The groups were: control (no worm); L1, 2000 L1 cultured in medium; L3, 2000 L3 cultured in medium; young adult, 60 young adult worms cultured in medium; adult, 60 adult worms (30 males and 30 females) cultured in medium. The control medium or worm-containing media were incubated at 37°C for 72 h in 200 μl of sterile medium, pH 7.2, containing 100 IU/ml penicillin and 100 μg/ml streptomycin. The motility of the worms was checked microscopically. The medium was changed every 24 h by pelleting the worms at 150g for 5 min and resuspending them in fresh complete medium. The culture medium was checked for bacterial contamination during each experiment. Bacterial contamination was prevented prior to the experiments by passing the medium through a 0.2 μm filter (Millipore, Eschborn, Germany) and storing the filtrate at −70°C before use.
Experimental animals and infection
Five-week-old male rodents, BALB/c mice and Sprague-Dawley rats, were purchased from the National Laboratory Animal Center, Taipei, Taiwan. They were maintained at 12 h light/dark cycle photoperiods, provided with Purina Laboratory Chow and water ad libitum, and kept in our laboratory for more than one week before the experimental infection. The rodents were prohibited food and water for 12 h before infection. They were infected with 60 A. cantonensis L3 by oral inoculation and sacrificed on day 25 post-infection (PI). Control rodents received only water and were also sacrificed on day 25 PI.
Gelatin substrate zymography
The A. acntonensis samples (extracts and cultured fluids, 20 μg protein) were diluted 1:1 in sample buffer (1% SDS, 2% glycerol, 10% bromophenol blue and 0.5 M Tris–HCl, pH 6.8). Samples were loaded on 7.5% (mass/volume) SDS-polyacrylamide gels that had been copolymerized with 0.1% gelatin (Sigma, USA). Stacking gels were 4% (mass/volume) polyacrylamide and did not contain gelatin substrate. Electrophoresis was performed in running buffer (25 mM Tris, 250 mM glycine, 1% SDS) at room temperature at 120 V for 1 h. The gel was washed two times at room temperature for 30 min each in 2.5% Triton X-100, and then washed two times with double-distilled water for 10 min each. The gel was incubated in reaction buffer (50 mM Tris–HCl, pH 7.5, containing 200 mM NaCl, 10 mM CaCl2, 0.02% Brij-35, 0.01% NaN3) at 37°C for 18 h. The gel was stained with 0.25% Coomassie Brilliant Blue R-250 (Sigma, USA) for 1 h and destained in 15% methanol/7.5% acetic acid. Gelatinase activity was detected as unstained bands on a blue background.
Inhibition of gelatinase on gelatin zymography
Samples were resolved by SDS/PAGE on 7.5% polyacrylamide gels impregnated with 0.1% gelatin (Sigma, USA). Following electrophoresis, gels were soaked in 2.5% Triton-X-100 to replace SDS, washed twice with water, then incubated at 37°C for 18 h in MMP activation buffer (50 mM Tris, pH 8.0, 5 mM CaCl2). In control experiments, calcium was replaced with 10 mM ethylenediamine tetraacetic acid (EDTA, Sigma, USA) in the activation buffer. For inhibitor studies, either 20 μM leupeptin (Sigma, USA), 2 mM phenylmethanesulphonyl fluoride (PMSF, Sigma, USA), 5 mM 1,10-phenanthroline (Sigma, USA), solvent was added to the Triton and activation buffers. Zymography gels were stained with Coomassie Brilliant Blue and destained in 15% methanol/7.5% acetic acid. Proteins with gelatinolytic activity were revealed as clear bands on a blue background.
Results
Gelatinase activity from worm extracts
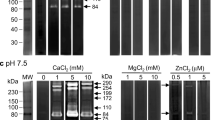
Angiostrongylus cantonensis L1 extracts had a restricted profile when examined by gelatin substrate SDS-PAGE. Three dominant bands at 94, 42 and 23 kDa. In L3 extracts, five gelatinase bands were evident at 94, 86, 66, 42 and 30 kDa. In extracts from both young and adult worms two gelatinase bands were observed at 94 and 72 kDa (Fig. 1a). Inhibitor studies showed the 23 kDa L1 gelatinase band share characteristics of metalloproteinase/cysteine-proteinase/serine-proteinase, and the 42 kDa band was serine-proteinase. In L3 gelatinase bands, the 66, 42 and 30 kDa bands showed share characteristics of metalloproteinase/cysteine-proteinase. The 72 and 94 kDa gelatinase bands in the young adult worm and adult worm showed share characteristics of metalloproteinase/cysteine-proteinase (Fig. 1b–e).
Gelatinase activity from worm extracts. a L1 of Angiostrongylus cantonensis extracts have a restricted profile when examined by gelatin substrate SDS-PAGE, with three dominant bands at 94, 42 and 23 kDa; five gelatinase bands at 94, 86, 66, 42 and 30 kDa of the L3; two gelatinase bands at 94 and 72 kDa in young adult and adult worms. b–e In L1 gelatinase bands, the 23 kDa band was significantly inhibited by 1,10-phenanthroline, leupeptin or PMSF; the 42 kDa band was significantly inhibited by PMSF. In L3 gelatinase bands, the 66, 42 and 30 kDa bands were significantly inhibited by 1,10-phenanthroline or leupeptin. The 72 kDa gelatinase bands in the young adult worm and adult worm were significantly inhibited by EDTA and 1,10-phenanthroline, partially inhibited by leupeptin. The 94 kDa gelatinase bands in the young adult worm and adult worm were significantly inhibited by leupeptin, partially inhibited by EDTA or 1,10-phenanthroline. Other gelatinase bands from worm extracts could not be inhibited by EDTA, 1,10-phenanthroline, leupeptin or PMSF
Gelatinase activity from worm ES products
Analysis of L1 ES products revealed one low (42 kDa) and two high (105 and 94 kDa) molecular weight proteolytic bands that degraded gelatin in substrate gels. Similar analysis of L3 products revealed three low (66, 50, and 30 kDa) and one high (105 kDa) molecular weight proteolytic bands. In contrast, gelatinase activities were not detected in the ES obtained from young adult and adult worms (Fig. 2a). Inhibitor studies showed the 105 and 94 kDa L1 gelatinase bands, and 50 and 30 kDa L3 gelatinase bands were metalloproteinases (Fig. 2b–e).
Gelatinase activity from worm secretory products. a The analysis of the excretory-secretory products of the L1 revealed one low (42 kDa) and two high (105 and 94 kDa) molecular weight proteolytic bands that degraded gelatin in substrate gels. In addition, the L3 revealed three low (66, 50, and 30 kDa) and one high (105 kDa) molecular weight proteolytic bands. The 105 and 94 kDa gelatinase bands of the L1, and the 50 and 30 kDa gelatinase bands of the L3 were inhibited by EDTA (b), 1,10-phenanthroline (c), and GM6001 (f), but not affected by leupeptin (d) or PMSF (e)
Differentiation the gelatinases from worms and hosts
The gelatinase bands of 94 (MMP-9) and 72 (MMP-2) kDa were detected in 60 A. cantonensis-infected mice but not in rats or following culturing of 60 worms. Cerebrospinal fluid from mice infected with A. cantonensis contains metalloproteinase. However, MMPs were not detected in experiments utilizing 60 larvae, 60 young adult worms, or 60 adult worms (Fig. 3).
Discussion
Proteinases serve a variety of functions in parasite development including the facilitation of host tissue invasion, digestion of host proteins, inhibition of blood clotting, molting and evasion of the host immune response (McKerrow 1989). In the present study, we observed metalloproteinases secretion in the L3 (50 and 30 kDa). These molecular weights and responses to the inhibitory agents are consistent with the classification of these species as metalloproteinases. These bands were observed in gelatin zymography after proteolysis by ES products from A. cantonensis. This means that metalloproteinases secreted by A. cantonensis L3 exhibit diversity based on the differential migration in polyacrylamide gels. These enzymes may play a role in the parasite penetration of the stomach or intestinal wall of the host, although more directed investigations will be needed to confirm these roles.
Cerebrospinal fluid from mice infected with A. cantonensis also contains metalloproteinase. Earlier studies in our laboratory demonstrated that, following infection with 60 A. cantonensis, MMP-9 can be induced in ICR mice (Lai et al. 2004) and BALB/c mice (Lee et al. 2004). Presently, at least 2000 larvae (L1 or L3) were required for the detection of the enzyme activity. In contrast, MMPs were not detected in 60 A. cantonensis of L1, L3, young adult worms or adult worms, respectively. These data can differentiate the metalloproteinases in this study secreted by the L3, young adult or adult worms, and previous studies (Lai et al. 2004; Lee et al. 2004) secreted by the mice. Moreover, our findings strongly support the suggestions that the production of metalloproteinases is by the host rather than the parasite (Hotez et al. 1985; Petralanda et al. 1986; Knox and Kennedy 1988; Lackey et al. 1989).
Proteinase secreted by the infective larvae facilitates penetration of skin or intestinal walls of humans (Tort et al. 1999). Metalloproteinase-mediated degradation of ECM components is also a feature of some helminthes (Petralanda et al. 1986). Ingestion of larval nematode A. cantonensis can cause the human disease known as angiostrongyliasis (Alicata 1965). After ingestion, A. cantonensis larvae can be invasive, penetrating host stomach or intestinal wall (Wang et al. 1991). Thus, we suggest that metalloproteinases secreted in the infective larvae may be associated with the parasite dissemination or pathogenesis.
References
Alicata JE (1965) Biology and distribution of the rat lungworm, Angiostrongylus cantonensis, and its relationship to eosinophilic meningoencephalitis and other neurological disorders of man and animals. Adv Parasitol 3:223–248
Ash LR (1970) Diagnostic morphology of the third-stage larvae of Angiostrongylus cantonensis, Angiostrongylus vasorum, Aelurostrongylus abstrusus, and Anafilaroides rostratus (Nematoda: Metastrongyloidea). J Parasitol 56:249–253
Bessarab IN, Joshua GW (1997) Stage-specific gene expression in Angiostrongylus cantonensis: characterisation and expression of an adult-specific gene. Mol Biochem Parasitol 88:73–84
Harrop SA, Sawangjaroen N, Prociv P, Brindley PJ (1995) Characterization and localization of cathepsin B proteinases expressed by adult Ancylostoma caninum hookworms. Mol Biochem Parasitol 71:163–171
Hawdon JM, Jones BF, Perregaux MA, Hotez PJ (1995) Ancylostoma caninum: metalloprotease release coincides with activation of infective larvae in vitro. Exp Parasitol 80:205–211
Healer J, Ashall F, Maizels RM (1991) Characterization of proteolytic enzymes from larval and adult Nippostrongylus brasiliensis. Parasitology 103:305–314
Hill DE, Gamble HR, Rhoads ML, Fetterer RH, Urban JF Jr (1993) Trichuris suis: a zinc metalloprotease from culture fluids of adult parasites. Exp Parasitol 77:170–178
Hotez PJ, Trang NL, McKerrow JH, Cerami A (1985) Isolation and characterization of a proteolytic enzyme from the adult hookworm Ancylostoma caninum. J Biol Chem 260:7343–7348
Joshua GW, Perler FB, Wang CC (1995) Orphon spliced-leader sequences form part of a repetitive element in Angiostrongylus cantonensis. Nucl Acids Res 23:1030–1035
Kliks MM, Palumbo NE (1992) Eosinophilic meningitis beyond the Pacific Basin: the global dispersal of a peridomestic zoonosis caused by Angiostrongylus cantonensis, the nematode lungworm of rats. Soc Sci Med 34:199–212
Knox DP, Kennedy MW (1988) Proteinases released by the parasitic larval stages of Ascaris suum, and their inhibiton by antibody. Mol Biochem Parasitol 28:207–216
Lackey A, James ER, Sakanari JA, Resnick SD, Brown M, Bianco AE, McKerrow JH (1989) Extracellular proteases of Onchocerca. Exp Parasitol 68:176–185
Lai SC, Twu JJ, Jiang ST, Hsu JD, Chen KM, Chiaing HC, Wang CJ, Tseng CK, Shyu LY, Lee HH (2004) Induction of matrix metalloproteinase-9 in murine eosinophilic meningitis caused by Angiostrongylus cantonensis. Ann Trop Med Parasitol 98:715–724
Lee HH, Chou HL, Chen KM, Lai SC (2004) Association of matrix-metalloproteinase-9 in eosinophilic meningitis of BALB/c mice caused by Angiostrongylus cantonensis. Parasitol Res 94:321–328
Lindo JF, Waugh C, Hall J, Cunningham-Myrie C, Ashley D, Eberhard ML, Sullivan JJ, Bishop HS, Robinson DG, Holtz T, Robinson RD (2002) Enzootic Angiostrongylus cantonensis in rats and snails after an outbreak of human eosinophilic meningitis, Jamaica. Emerg Infect Dis 8:324–326
Loukas A, Selzer PM, Maizels RM (1998) Characterisation of Tc-cp1–1, a cathepsin L-like cysteine protease from Toxocara canis infective larvae. Mol Biochem Parasitol 92:275–289
Matrisian LM (1992) The matrix-degrading metalloproteinases. Bioessays 14:455–463
Matthews BE (1982) Behaviour and enzyme release by Anisakis sp larvae (Nematoda: Ascaridida). J Helminthol 56:177–183
McKerrow JH (1989) Parasite proteases. Exp Parasitol 68:111–115
McKerrow JH, Doenhoff MJ (1988) Schistosoma proteases. Parasitol Today 12:334–339
McKerrow JH, Brindley P, Brown M, Gam AA, Staunton C, Neva FA (1990) Strongyloides stercoralis: identification of a protease that facilitates penetration of skin by the infective larvae. Exp Parasitol 70:134–143
Morris SR, Sakanari JA (1994) Characterization of the serine protease and serine protease inhibitor from the tissue-penetrating nematode Anisakis simplex. J Biol Chem 269:27650–27656
Parsons JC, Grieve RB (1990) Effect of egg dosage and host genotype on liver trapping in murine larval toxocariasis. J Parasitol 76:53–58
Petralanda I, Yarzabal L, Piessens WF (1986) Studies on a filarial antigen with collagenase activity. Mol Biochem Parasitol 19:51–59
Richer JK, Sakanari JA, Frank GR, Grieve RB (1992) Dirofilaria immitis: proteases produced by third- and fourth-stage larvae. Exp Parasitol 75:213–222
Robertson BD, Bianco AT, McKerrow JH, Maizels RM (1989) Toxocara canis: proteolytic enzymes secreted by the infective larvae in vitro. Exp Parasitol 69:30–36
Sato H, Seiki M (1996) Membrane-type matrix metalloproteinases (MT-MMPs) in tumor metastasis. J Biochem 119:209–215
Stetler-Stevenson WG, Aznavoorian S, Liotta LA (1993) Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol 9:541–573
Tort J, Brindley PJ, Knox D, Wolfe KH, Dalton JP (1999) Proteinases and associated genes of parasitic helminths. Adv Parasitol 43:161–266
Uparanukraw P, Morakote N, Harnnoi T, Dantrakool A (2001) Molecular cloning of a gene encoding matrix metalloproteinase-like protein from Gnathostoma spinigerum. Parasitol Res 87:751–757
Wada K, Sato H, Kinoh H, Kajita M, Yamamoto H, Seiki M (1998) Cloning of three Caenorhabditis elegans genes potentially encoding novel matrix metalloproteinases. Gene 211:57–62
Wang LC, Chao D, Chen ER (1991) Experimental infection routes of Angiostrongylus cantonensis in mice. J Helminthol 65:296–300
Acknowledgement
This study was supported by a grant (NSC-90–2320–B040–021) from the National Science Council, Republic of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, S.C., Jiang, S.T., Chen, K.M. et al. Matrix metalloproteinases activity demonstrated in the infective stage of the nematodes, Angiostrongylus cantonensis. Parasitol Res 97, 466–471 (2005). https://doi.org/10.1007/s00436-005-1484-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-005-1484-6