Abstract
Induction of gelatinase in eosinophilic meningitis of BALB/c-strain mice was caused by Angiostrongylus cantonensis. Time-course studies showed that the molecular weight of 94-kDa gelatinase was detected at day 10 post-inoculation (PI), and reached a high intensity from days 15 to 25 PI. The 94-kDa gelatinase activity was clearly inhibited by EDTA and 1,10-phenanthroline, but not by leupeptin and phenylmethanesulphonyl fluoride. When immunoblots were performed using specific antiserums against the 94-kDa gelatinase B (matrix metalloproteinase-9; MMP-9) with cerebrospinal fluid (CSF), the 94-kDa immunopositive band was MMP-9. Immunohistochemistry studies demonstrated MMP-9 localisation within eosinophils and macrophages. The increased MMP-9 activity was closely associated with the rapid rise of CSF eosinophils, and the inflammatory reaction of the subarachnoid space. In contrast to changes in MMP-9, MMP-2 activity was constitutive and unaffected in this parasitic meningitis. These results show that MMP-9 was associated with eosinophilic meningitis, and that the enzyme may be a useful marker for angiostrongyliasis meningitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Angiostrongylus cantonensis is a parasitic nematode and a zoonotic parasite, whose mature adults reside in the pulmonary arteries of rats. Eggs hatch in the lungs, and first-stage larvae pass out in the faeces, then enter a molluscan intermediate host where they moult twice. The infective third-stage larvae orally infect the final host, and are carried in the blood to the central nervous system (CNS), where they moult twice to become immature adults and enter the subarachnoid space. In a permissive host (rodents), immature adults migrate from the brain to the lungs (Alicata 1965; Muller 1975). However, in non-permissive hosts (humans and mice), the immature adults remain in the CNS of the hosts, and this infection is the main cause of eosinophilic meningitis and eosinophilic meningoencephalitis (Hsu et al. 1990; Ismail and Arsura 1993). Mice infected with A. cantonensis-caused pathogenesis of eosinophilic meningitis reach a peak at around 3 weeks and, in parallel with this pathogenesis, infected mice showed a gradual increase in cerebrospinal (CSF) eosinophilia, reaching a peak at the same time (Sugaya and Yoshimura 1988; Sasaki et al. 1993).
Matrix metalloproteinases (MMPs) are a family of zinc metallo-endopeptidases that regulate cell-matrix composition (Matrisian 1992). The MMPs are produced as zymogens, with a signal sequence and propeptide segment that must be removed during activation. The propeptide domain contains a conserved cysteine, which chelates the zinc in the active site (Birkedal-Hansen et al. 1993). These proteinases play an essential role in the remodelling of connective tissue under physiological and pathological conditions, and as modulators of inflammation. An emerging body of evidence points to MMPs as important factors in the pathogenesis of meningitis. Increased levels of MMP-9 were detectable in CSF samples from patients suffering from viral (Kolb et al. 1998), bacterial (Gijbels et al. 1992; Kieseier et al. 1999) or fungal (Matsuura et al. 2000) meningitis. Infiltration of leukocytes into the subarachnoid space was associated with breakdown of the blood–brain barrier (BBB; Quagliarello et al. 1986; Quagliarello and Scheld 1992). MMP-9 is important for leukocyte migration, and there exists an inflammatory reaction due to its ability to degrade basement membranes and components of the extracellular matrix (ECM) such as collagens, elastin and aggrecan (Dubois et al. 1999).
Knowledge about the roles of MMPs in parasitic meningitis is limited. Thus, the aim of the present study was to use a mouse model of parasitic meningitis caused by A. cantonensis to investigate the activity of specific MMPs in relation to eosinophilic meningitis.
Materials and methods
Experimental animals
Five-week-old male mice, BALB/c strain, were purchased from the National Laboratory Animal Center, Taipei, Taiwan. They were maintained at 12-h light/dark cycle photoperiod, provided with Purina Laboratory Chow and water ad libitum, and kept in our laboratory for more than 1 week before the experimental infection.
Larval preparation
Third-stage (infective) larvae of A. cantonensis were obtained from naturally infected giant African snails, Achatina fulica, collected from fields in Taichung, central Taiwan. The larvae within tissues were recovered using the method of Parsons and Grieve (1990), with slight modifications. Briefly, the shells were crushed, the tissues were homogenised and digested in a pepsin-HCl solution (pH 1–2, 500 IU pepsin/g tissue), and incubated with agitation at 37°C in a water bath for 2 h. Host cellular debris was removed from the digest by centrifugation at 1,400 g for 10 min. The larvae in the sedimented material were observed under the microscope. The morphological criteria for identification of the third-stage larvae of A. cantonensis are 425 to 524 μm in length, and 23 to 34 μm in width. The posterior end of the tail always terminates as a fine point, as has been reported by Ash (1970). To determine if the larvae found were A. cantonensis, we fed larvae to rats and then examined their brains 2–3 weeks later for evidence of infection.
Animal infection
A total of 90 mice were randomly allocated to six groups: D0, D5, D10, D15, D20, and D25. Mice were not given food or water for 12 h before infection. The mice of the experimental groups (D5, D10, D15, D20, and D25) were infected with 60 A. cantonensis larvae by oral inoculation on day 0, and sacrificed on days 5, 10, 15, 20 and 25 post-inoculation (PI) respectively. The control mice (D0) received only water, and they were sacrificed on day 25 PI.
In vitro cultivation of young adult worms
Young adult worms were recovered from the brain on day 20 PI. Each brain was cut into pieces, and homogenised separately in 15 ml of 0.25% sodium citrate in phosphate-buffered saline (PBS), followed by centrifugation. A total of 60 young adult worms were incubated in 3 ml of sterile RPMI-1640 medium (Sigma, USA) containing antibiotics (100 IU/ml penicillin, 250 μg/ml streptomycin, and 25 μg/ml nystain) for 5 days. The young adult worm-cultured RPMI-1640 medium was analysed by gelatin zymography.
Eosinophil counts in the CSF
Five mice from each of the D0, D5, D10, D15, D20 and D25 groups were sacrificed and their brains removed into a 35-mm dish. The cranial cavity and cerebral ventricles (lateral, third and fourth ventricles) were rinsed with 1 ml PBS. The washing solution was collected into a centrifuge to spin at 400 g for 10 min. The resultant sedimented material was then gently mixed with 100 μl Unopette (Becton Dickinson vacutainer system, USA) and 2 μl acetic acid to count eosinophils with a hemacytometer (Marienfeld, Germany). Mean values of total eosinophil numbers were expressed as mean±SD, and were considered significant at P<0.05.
Worm recovery
Experimental and control mice were killed by cervical dislocation. Each brain was cut into pieces and homogenised separately in 15 ml of 0.25% sodium citrate in PBS, followed by centrifugation. Larval counts were measured under 25× magnification of a dissecting microscope. Mean values were expressed as mean±SD, and were considered significant at P<0.05.
Histology
The mouse brains were fixed separately in 10% neutral buffered formalin for 24 h. The fixed specimens were dehydrated in a graded ethanol series (50, 75, and 100%) and xylene, then embedded in paraffin at 55°C for 24 h. Several serial sections were cut at 5-μm thickness for each organ from each mouse. Paraffin was removed by heating the sections for 5 min at 65°C. These sections were dewaxed by washing three times for 5 min each in xylene, then rehydrated through 100, 95 and 75% ethanol for 5 min each, and finally rinsed with distilled water. After staining with haematoxylin (Muto, Japan) and eosin (Muto, Japan), pathological changes were examined under a light microscope.
Gelatin zymography
The CSF and young adult worm-cultured RPMI-1640 medium were centrifuged at 12,000 g for 10 min to remove debris. Samples were loaded on 7.5% (mass/volume) SDS-polyacrylamide gels that had been co-polymerised with 0.1% gelatin (Sigma, USA). Stacking gels were 4% (mass/volume) polyacrylamide, and did not contain gelatin substrate. Electrophoresis was performed in running buffer (25 mM Tris, 250 mM glycine, 1% SDS) at room temperature at 120 V for 1 h. The gel was washed two times at room temperature for 30 min each in 2.5% Triton X-100, and then washed two times with double-distilled H2O for 10 min each. The gel was incubated in reaction buffer (50 mM Tris-HCl, pH 7.5, containing 200 mM NaCl, 10 mM CaCl2, 0.02% Brij-35, 0.01% NaN3) at 37°C for 18 h. The gel was stained with 0.25% Coomassie brilliant blue R-250 (Sigma, USA) for 1 h, and destained in 15% methanol/7.5% acetic acid. Gelatinase activity was detected as unstained bands on a blue background. Quantitative analysis of the gelatinolytic enzyme was performed with a computer-assisted imaging densitometer system, UN-SCAN-IT gel version 5.1 (Silk Scientific, USA).
Inhibition of gelatinase on gelatin zymography
The CSF was centrifuged at 12,000 g at 4°C for 10 min, and the protein contents of the supernatants were resolved by non-reducing SDS/PAGE on 7.5% polyacrylamide gels impregnated with 0.1% gelatin (Sigma). Following electrophoresis, gels were soaked in 2.5% Triton-X-100 to replace SDS, washed twice with water, then incubated at 37°C for 18 h in MMP activation buffer (50 mM Tris, pH 8.0, 5 mM CaCl2). In control experiments, calcium was replaced with 10 mM ethylenediamine tetraacetic acid (EDTA, Sigma) in the activation buffer. For inhibitor studies, 20 μM leupeptin (Sigma), or 2 mM phenylmethanesulphonyl fluoride (PMSF, Sigma), or 5 mM 1,10-phenanthroline (Sigma), or solvent only were added to the Triton and activation buffers. Zymography gels were stained with Coomassie brilliant blue, and destained in 15% methanol/7.5% acetic acid. Proteins with gelatinolytic activity were revealed as clear bands on a blue background.
Western blot analysis
The CSF was centrifuged at 12,000 g at 4°C for 10 min, and the protein contents of the supernatants were determined with protein assay kits (Bio-Rad, USA), using bovine serum albumin as the standard. An equal volume of loading buffer (62.5 mM Tris-HCl, pH 6.8, 10% glycerol, 2% SDS, 5% 2-mercaptoethanol, 0.05% bromophenol blue) was added to the samples, which contained 30 μg of brain tissue protein. The mixture was boiled for 5 min prior to electrophoresis on SDS-polyacrylamide gel under non-reducing conditions, and electrotransfer to nitrocellulose membranes at a constant current of 190 mA for 90 min. Afterwards, the membrane was saturated with PBS containing 0.1% Tween 20 for 30 min at room temperature. The membrane was allowed to react with goat anti-mouse MMP-9 polyclonal antibody (R&D Systems, USA) diluted 1:100, at 37°C for 1 h. Then, the membrane was washed three times with PBS containing 0.1% Tween 20 (PBS-T), followed by incubation with horseradish peroxidase (HRP)-conjugated rabbit anti-goat IgG (Jackson ImmunoResearch Laboratories, USA) diluted 1:5,000, at 37°C for 1 h to detect the bound primary antibody. The reactive protein was detected by enhanced chemiluminescence (Amersham, UK). To confirm equivalent protein loading, membranes were stripped by incubation in 62.5 mM of Tris-HCl (pH 6.8), 2% SDS, and 100 mM 2-mercaptoethanol at 55°C, subsequently washed with PBS-T, and reprobed with anti-β-actin antibody (dilution 1:500).
Immunohistochemistry
Ten-micrometer, paraffin-embedded sections of brain were prepared and mounted on glass slides. Serial sections were deparaffinised with xylene and a graded series of ethanol. Sections were treated with 3% H2O2 in methanol for 10 min to inactivate endogenous peroxidase, and washed three times with PBS, pH 7.4 for 5 min. Sections were blocked with 3% BSA (bovine serum albumin) at room temperature for 1 h, incubated with goat anti-mouse MMP-9 polyclonal antibody (R&D Systems, USA) diluted 1:50 in 1% BSA, at 37°C for 1 h, and washed three times in PBS for 5 min each. Sections were then incubated with HRP-conjugated rabbit anti-goat IgG (Jackson ImmunoResearch Laboratories, USA) diluted 1:100 in 1% BSA, at 37°C for 1 h, and washed three times in PBS for 5 min each. Subsequently, sections were incubated in DAB (3, 3′-diaminobenzidine; 0.3 mg/ml in 100 mM Tris, pH 7.5, containing 0.3 μl H2O2/ml) at room temperature for 3 min, and washed three times in PBS for 5 min each. Mounted slides (with 50% glycerol in PBS) were examined under a light microscope.
Statistical analysis
Results in the different groups of mice were compared using the nonparametric Kruskal-Wallis test, followed by post-testing using Dunn’s multiple comparison of means. All results were presented as mean±standard deviation (SD); P values<0.05 were considered statistically significant.
Results
Eosinophil changes and young adult worm recovery
CSF eosinophilia for BALB/c-strain mice were found only in infected mice, and not in the uninfected control. The time-course studies showed a mild eosinophilia at day 10 PI, showing a plateau response from days 15 to 25 PI. The eosinophil numbers in the CSF were significantly increased (P<0.05) from days 15 to 25 PI (Fig. 1a). Young adult worms were not found in the uninfected control, or at days 5 and 10 PI. Subsequently, there were 26±3.4 recovered worms on day 15 PI, 36±2.9 recovered worms on day 20 PI, and 32±5.4 recovered worms on day 25 PI. The worm numbers in the brain were significantly higher (P<0.05) from days 15 to 25 PI (Fig. 1b).
Eosinophil changes and young adult worm recovery. a The time-course studies revealed that CSF eosinophils had increased slightly on day 10 PI, and showed a plateau response (*P<0.05) from days 15 to 25 PI. The range bars indicate the standard error of the mean. b Worms were not found in the uninfected control, and on days 5 and 10 PI. The number of worms in the brain significantly increased (*P<0.05) from days 15 to 25 PI
Histological observations
In the brain section with haematoxylin and eosin stain, the uninfected control had no inflammatory cells in the meninges, and the meninges were normal (Fig. 2a, b). In contrast, mice infected with A. cantonensis had mild inflammation in the subarachnoid space on day 10 PI (Fig. 2c). Severe inflammation was observed from days 15 to 25 PI; in particular, eosinophilic and neutrophilic leukocytes infiltrated the edema meninges (Fig. 2d–f).
Histological observations on H&E staining. a, b The uninfected control had no inflammatory cells in the meninges, and the meninges were normal. c Mice infected with A. cantonensis had mild inflammation in the subarachnoid space on day 10 PI. d–f Severe inflammation in the subarachnoid space was observed on days 15, 20, and 25 PI; in particular, eosinophilic and neutrophilic leukocytes infiltrated the edema meninges. P Brain parenchyma, arrowheads leukocytes
Time-course studies of gelatinase activity
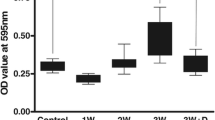
Gelatin zymography showed two gelatinase bands, with a lower molecular weight of about 72 kDa and a higher molecular weight of 94 kDa. The 72-kDa gelatinase was present in all samples. In addition, the 94-kDa gelatinase was detected on day 10 PI, and reached a high intensity from days 15 to 25 PI. In contrast, the young adult worm-cultured RPMI-1640 medium was undetectable (Fig. 3).
Time-course studies for gelatinase activity. a The 94-kDa molecular weight gelatinase bands were detected on day 10 PI, and reached a high intensity from days 15 to 25 PI. The 72-kDa gelatinase was present in all samples, and remained unchanged. In contrast, the young adult worm-cultured RPMI-1640 medium (YA) was undetectable. M Marker. b Quantitative analysis of the gelatinolytic enzyme was performed with a computer-assisted, imaging densitometer system. The relative intensity of the gelatinolytic bands in infected groups showed significant increases (*P<0.05) compared with the uninfected control
Identification of the gelatinases
Gelatin zymography showed that the 94-kDa gelatinase was present in A. cantonensis-infected mice (on day 20 PI), and it was undetectable in the uninfected mice. Discrimination of the 94-kDa gelatinase band was obtained from leupeptin (a cysteine proteinase inhibitor), PMSF (a serine proteinase inhibitor), EDTA (a catalytic-site MMP inhibitor), and 1,10-phenanthroline (an inhibitor of zinc-containing neutral metallo-enzymes). The results of the inhibition studies show that the enzyme was obviously inhibited by EDTA and 1,10-phenanthroline but not by leupeptin or PMSF (Fig. 4a). By Western blot analysis, the molecular weight 94-kDa immunopositive band was detected by anti-MMP-9 antibody in the CSF of the infected mice, whereas no positive signal was observed in uninfected mice (Fig. 4b).
Identification of the gelatinases. a Gelatin zymography showed that the 94-kDa gelatinase was present in A. cantonensis-infected mice (on day 20 PI), and in the uninfected control it was undetectable. The gelatinase was inhibited by EDTA and phenanthroline, but not by leupeptin and PMSF. b Western blot analysis from the control and A. cantonensis-infected mice. The MMP-9 positive band was detected in the CSF of the infected mice. β-actin was used as a loading control
Localisation of the MMP-9 protein
The localisation of MMP-9 in the subarachnoid space of A. cantonensis-infected mice was on day 20 PI; positive signals for MMP-9 could be localised in infiltrating polymorphonuclear and mononuclear cells, including eosinophils (Fig. 5b) and macrophages (Fig. 5d). No positive signal could be detected when normal serum was used instead of the anti-MMP-9 antibody, either in eosinophils (Fig. 5a) or macrophages (Fig. 5c).
Localisation of MMP-9 in the subarachnoid space of mice. No MMP-9 positive signal can be detected with normal serum in the leukocytes, including a eosinophils (arrowheads), and c macrophage (arrowhead). Detection with a polyclonal antiserum against MMP-9 showed MMP-9 localised in polymorphonuclear and mononuclear cells, including b eosinophils (arrowheads), and d macrophages (arrowheads)
Discussion
In human angiostrongyliasis cantonensis, CSF pleocytosis and eosinophilia have been shown to occur several days after infection, peaking at 2–3 weeks PI (Yii 1976). For eosinophilia of the CSF in ICR-strain mice infected with A. cantonensis, the infection provoked a marked CSF eosinophilia starting at around day 12, reaching a peak level on day 20 (Sugaya and Yoshimura 1988). Similarly, the present study shows that eosinphils were significantly increased from days 15 to 25 PI. A 94-kDa molecular-weight gelatinase was detected on day 10 PI, and reached a high intensity from days 15 to 25 PI. The 94-kDa gelatinase belonged to the MMPs family based on its molecular weight, and the inhibition by EDTA and 1,10-phenanthroline but not by leupeptin or PMSF. By Western blot analysis, the 94-kDa immunopositive band was MMP-9. The 72-kDa gelatinase (MMP-2) was present in all samples, and remained unchanged. In contrast to changes in MMP-9, MMP-2 secretion was constitutive and unaffected by A. cantonensis infection. This is in agreement with previous findings in experimental models, and patients with bacterial, viral or fungal meningitis, where MMP-2 was unaffected by the disease while MMP-9 was increased (Paul et al. 1998; Beuche et al. 2000; Leib et al. 2000; Leppert et al. 2000; Matsuura et al. 2000). These studies showed that MMP-9 associated with various types of meningitis, including parasitic meningitis.
The kinetics of the CSF MMP-9 activity correlated with the CSF eosinophilia and young adult worm recovery. The young adult worms cultured in RPMI-1640 medium were analysed by gelatin zymography, without detecting any gelatinase in these samples. These data show that the MMP-9 was from the mouse as induced by A. cantonensis infection, and not from enzymes secreted by the worms.
Various pathogens including bacteria, virus, fungi, rickettsia and parasites may cause meningitis. Viral meningitis evokes a lymphocytic reaction, whereas bacterial meningitis is marked by exudates of polymorphonuclear leukocytes (Damjanov et al. 1996). MMP-9 has been identified as a mediator of brain injury in HIV-associated neurological disease (Liuzzi et al. 2000). Matsuura et al. (2000) also suggested that MMP-9 in the CSF may be a useful marker of encephalitogeneity during the course of subacute meningitis. Angiostrongyliasis of the meninges was chronic meningitis characterised by the aggregation of eosinophils in the subarachnoid space. In our study, the increased activity of MMP-9 was closely associated with a rapid rise of CSF eosinophils. In addition, there is a relation between elevated MMP-9 levels in the CSF and the number of white blood cells in various neurological disorders (Gijbels et al. 1992). For example, MMP-9 in the CSF of HIV-infected patients is associated with cell counts (Sporer et al. 1998). Therefore, it is reasonable to assume that MMP-9 was associated with the inflammatory reaction of angiostrongyliasis.
MMP-9 is produced by the CNS, and inflammatory cells in an inflammatory condition in both animals and humans (Williams et al. 2002). Cells which are capable of producing MMP-9 include monocytes (Welgus et al. 1990), T cells (Leppert et al. 1995), neutrophils (Masure et al. 1991), astrocytes (Wells et al. 1996), microglia (Gottschall and Yu 1995), eosinophils (Okada et al. 1997), macrophages (Nielsen et al. 1996), and endothelial cells (Herron et al. 1986). Okada et al. (1997) showed that MMP-9 was released from eosinophils, which were transmigrating basement membranes, and participating in the pathophysiology of inflammatory diseases. In the present study on immunohistochemistry, MMP-9 was found to be localised in the infiltrated polymorphonuclear and mononuclear cells, including eosinophils and macrophages. These data suggest that infiltrating leukocytes are important sources of MMP-9 in parasitic meningitis.
The present study shows that MMP-9 was induced in eosinophilic meningitis of BALB/c-strain mice caused by A. cantonensis, and the increased MMP-9 activity coincided with the degree of inflammation. This study shows that MMP-9 was associated with eosinophilic meningitis, and the enzyme may be a useful marker in angiostrongyliasis meningitis.
References
Alicata JE (1965) Biology and distribution of the rat lungworm, Angiostrongylus cantonensis, and its relationship to eosinophilic meningoencephalitis and other neurological disorders of man and animals. In: Dawes B (ed) Advances in parasitology. Academic Press, London, pp 223–248
Ash LR (1970) Diagnostic morphology of the third-stage larvae of Angiostrongylus cantonensis, Angiostrongylus vasorum, Aelurostrongylus abstrusus, and Anafilaroides rostratus (Nematoda: Metastrongyloidea). J Parasitol 56:249–253
Beuche W, Yushchenko M, Mäder M, Maliszewska M, Felgenhauer K, Weber F (2000) Matrix metalloproteinase-9 is elevated in serum of patients with amyotrophic lateral sclerosis. Neuroreport 11:3419–3422
Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA (1993) Matrix metalloproteinases: a review. Crit Rev Oral Biol Med 4:197–250
Damjanov I, McCue PA, Chansky M (1996) Nervous system. In: Velker J (ed) Histopathology—a color atlas and textbook. Williams & Wilkins, New York, pp 459–489
Dubois B, Masure S, Hurtenbach U, Paemen L, Heremans H, van den Oord J, Sciot R, Meinhardt T, Hammerling G, Opdenakker G, Arnold B (1999) Resistance of young gelatinase B-deficient mice to experimental autoimmune encephalomyelitis and necrotizing tail lesions. J Clin Invest 104:1507–1515
Gijbels K, Masure S, Carton H, Opdenakker G (1992) Gelatinase in the cerebrospinal fluid of patients with multiple sclerosis and other inflammatory neurological disorders. J Neuroimmunol 41:29–34
Gottschall PE, Yu X (1995) Cytokines regulate gelatinase A and B (matrix metalloproteinase 2 and 9) activity in cultured rat astrocytes. J Neurochem 64:1513–1520
Herron GS, Werb Z, Dwyer K, Banda MJ (1986) Secretion of metalloproteinases by stimulated capillary endothelial cells. I. Production of procollagenase and prostromelysin exceeds expression of proteolytic activity. J Biol Chem 261:2810–2813
Hsu WY, Chen JY, Chien CT, Chi CS, Han NT (1990) Eosinophilic meningitis caused by Angiostrongylus cantonensis. Pediatr Infect Dis J 9:443–445
Ismail Y, Arsura EL (1993) Eosinophilic meningitis. Western J Med 159:623
Kieseier BC, Paul R, Koedel U, Seifert T, Clements JM, Gearing AJH, Pfister HW, Hartung HP (1999) Differential expression of matrix metalloproteinases in bacterial meningitis. Brain 122:1579–1587
Kolb SA, Lahrtz F, Paul R, Leppert D, Nadal D, Pfister HW, Fontana A (1998) Matrix metalloproteinases and tissue inhibitors of metalloproteinases in viral meningitis: upregulation of MMP-9 and TIMP-1 in cerebrospinal fluid. J Neuroimmunol 84:143–150
Leib SJ, Leppert D, Clements J, Täuber MG (2000) Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect Immunol 68:615–620
Leppert D, Waubant E, Galardy R, Bunnett NW, Hauser SL (1995) T cell gelatinases mediate basement membrane transmigration in vitro. J Immunol 154:4379–4389
Leppert D, Leib SL, Grygar C, Miller KM, Schaad UB, Holländer GA (2000) Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood-brain barrier damage and neurological sequelae. Clin Infect Dis 31:80–84
Liuzzi GM, Mastroianni CM, Santacroce MP, Fanelli M, D’Agostino C, Vullo V, Riccio P (2000) Increased activity of matrix metalloproteinases in the cerebrospinal fluid of patients with HIV-associated neurological disease. J Neurovirol 6:156–163
Masure S, Proost P, van Damme J, Opdenakker G (1991) Purification and identification of 91-kDa neutrophil gelatinase: release by the activating peptide interleukin-8. Eur J Biochem 198:391–398
Matrisian LM (1992) The matrix-degrading metalloproteinases. Bioessays 14:455–463
Matsuura E, Umehara F, Hashiguchi T, Fujimoto N, Okada Y, Osame M (2000) Marked increase of matrix metalloproteinase 9 in cerebrospinal fluid of patients with fungal or tuberculous meningoencephalitis. J Neurol Sci 173:45–52
Muller R (1975) Worms and disease. William Heinemann, London
Nielsen BS, Timshel S, Kjeldsen L, Sehested M, Pyke C, Borregaard N, Dano K (1996) 92 kDa type IV collagenase (MMP-9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. Int J Cancer 65:57–62
Okada S, Kita H, George TJ, Gleich GJ, Leiferman KM (1997) Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. Am J Respir Cell Mol Biol 17:519–528
Parsons JC, Grieve RB (1990) Effect of egg dosage and host genotype on liver trapping in murine larval toxocariasis. J Parasitol 76:53–58
Paul R, Lorenzl S, Koedel U, Sporer B, Vogel U, Frosch M, Pfister HW (1998) Matrix metalloproteinases contribute to the blood-brain barrier disruption during bacterial meningitis. Ann Neurol 44: 592–600
Quagliarello VJ, Scheld WM (1992) Bacterial meningitis: pathogenesis, pathophysiology, and progress. N Engl J Med 327:864–872
Quagliarello VJ, Long WJ, Scheld WM (1986) Morphologic alterations of the blood-brain barrier with experimental meningitis in the rat: temporal sequence and role of encapsulation. J Clin Invest 77:1084–1095
Sasaki O, Sugaya H, Ishida K, Yoshimura K (1993) Ablation of eosinophils with anti-IL-5 antibody enhances the survival of intracranial worms of Angiostrongylus cantonensis in the mouse. Parasite Immunol 15:349–354
Sporer B, Paul R, Koedel U, Grimm R, Wick M, Goebel FD, Pfister HW (1998) Presence of matrix metalloproteinase-9 activity in the cerebrospinal fluid of human immunodeficiency virus-infected patients. J Infect Dis 178:854–857
Sugaya H, Yoshimura K (1988) T-cell-dependent eosinophilia in the cerebrospinal fluid of the mouse infected with Angiostrongylus cantonensis. Parasite Immunol 10:127–138
Welgus HG, Campbell EJ, Cury JD, Eisen AZ, Senior RM, Wilhelm SM, Goldberg GI (1990) Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest 86:1496–1502
Wells GM, Catlin G, Cossins JA, Mangan M, Ward GA, Miller KM, Clements JM (1996) Quantitation of matrix metalloproteinases in cultured rat astrocytes using the polymerase chain reaction with a multi-competitor cDNA standard. Glia 18:332–340
Williams PL, Leib SL, Kamberi P, Leppert D, Sobel RA, Bifrare YD, Clemons KV, Stevens DA (2002) Levels of matrix metalloproteinase-9 within cerebrospinal fluid in a rabbit model of coccidioidal meningitis and vasculitis. J Infect Dis 186:1692–1695
Yii CY (1976) Clinical observations on eosinophilic meningitis and meningoencephalitis caused by Angiostrongylus cantonensis on Taiwan. Am J Trop Med Hyg 25:233-249
Acknowledgement
This study was supported in part by a grant (NSC-90–2320-B040–015) from the National Science Council, Republic of China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, H.H., Chou, H.L., Chen, K.M. et al. Association of matrix metalloproteinase-9 in eosinophilic meningitis of BALB/c mice caused by Angiostrongylus cantonensis. Parasitol Res 94, 321–328 (2004). https://doi.org/10.1007/s00436-004-1196-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-004-1196-3