Abstract
The aim of the present work was to apply and evaluate a dipstick assay for the serodiagnosis of human hydatidosis as well as human and experimental trichinosis using camel hydatid cyst fluid (HCF) and Trichinella spiralis muscle larval (TSML) antigens, respectively, and compare this to enzyme-linked immunoelectrotransfer blot (EITB) and Falcon assay screening test-enzyme-linked immunosorbent assay (FAST-ELISA). Sera samples were collected from patients with confirmed hydatidosis and trichinosis and with other parasitic diseases as well as from normal healthy individuals. Also, sera samples were collected from mice experimentally infected with T. spiralis which were sacrificed at different time points post-infection (PI). HCF and TSML antigens were used in EITB after separation and characterization of their antigenic components using 5–22.5% sodium dodecyl sulphate-polyacrylamide gel electrophoresis under non-reducing condition. For the diagnosis of hydatidosis, the sensitivity, specificity and diagnostic accuracy of the dipstick assay and EITB were 100, 91.4 and 95.1% while those of FAST-ELISA were 96.2, 100 and 98.4%, respectively. For the diagnosis of human trichinosis, the sensitivity, specificity and diagnostic accuracy of the dipstick assay and EITB were 100% while those of FAST-ELISA were 85.7%. FAST-ELISA proved to be more sensitive in the early diagnosis of experimental T. spiralis infection (100% sensitivity from the second week PI) than the dipstick and EITB (100% sensitivity from the third week PI). All tests retained their sensitivity till the 12th week PI. Since the dipstick assay is extremely easy to perform with a visually interpreted result within 15 min, in addition to being both sensitive and specific, the test could be an acceptable alternative for use in clinical laboratories lacking specialized equipment and the technological expertise needed for EITB and FAST-ELISA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most pronounced problems in controlling the morbidity and mortality caused by different parasites is limited access to tools for early, rapid and effective diagnosis in order to provide proper treatment (Tsang and Wilkins 1991). Moreover, one of the main reasons why the control programs have failed is because of a long delay between sample collection, analysis and control implementation (Mamuti et al. 2002). Different serological tests based on antibody detection are widely used but the need for special kits, the technical problems involved in their adequate preparation, reading of results and the time-consuming incubation steps are several of their disadvantages.
The Falcon assay screening test-enzyme-linked immunosorbent assay (FAST-ELISA) is rapid and sensitive and can easily be performed in the place of standard ELISA with the advantages of minimizing reporting time and manpower (Azab et al. 1999). A number of modifications of the ELISA have been described in efforts to produce a rapid more field-applicable assay. The dot-ELISA is a highly versatile solid-phase (nitrocellulose which avidly binds proteins) immunoassay for antigen or antibody detection. Enzyme immunoelectrotransfer blot (EITB) (western immunoblotting) was reported to be the most sensitive serological assay for the confirmation of a diagnosis of hydatidosis (Verastegui et al. 1992) and trichinosis (Robert et al. 1996).
The dipstick assay was first proposed by Pappas (1986) as a potential improvement of dot-ELISA. Nitrocellulose filter paper cut into strips, dotted with antigen or specific antibody and then fixed to flat, pliable plastic strip using water-insoluble glue in order to resist breakage and render it easy to handle, is prepared and incubated with tested samples. It was used by Allan et al. (1993) for the detection of Taenia species’ coproantigens in the stool. Thereafter, Van Etten et al. (1994) had recorded the potential use of this test for the detection of circulating cathodic antigen in urine of schistosomiasis mansoni patients. Then, it was used by Al-Sherbiny (1996) for the detection of antibodies specific to Schistosoma species. In all scenarios, the assay is extremely easy to apply, rapid (15 min), field-portable, inexpensive, reagent-conservative and does not need special laboratory equipment.
Cystic echinococcosis (CE), caused by infection with the larval stage of Echinococcus granulosus, is of public health importance not only in areas of endemicity but also in countries without endemicity due to the migration of infected people and livestock exchange which create new areas of endemicity (Mamuti et al. 2002). Human hydatidosis usually manifests as space-occupying, fluid-filled unilocular cyst(s) commonly located in the liver and/or lung. Diagnosis relies predominantly on the interpretation of results from both radiological imaging techniques and immunodiagnosis (Craig 1997).
Trichinosis remains an important zoonotic food-borne parasitic disease, affecting man and many other mammals, of worldwide distribution (Boulos et al. 2001). The pig is the main source of infection to man. In Egypt, the disease was not given much attention until several cases were reported in different localities (Azab et al. 1999). Trichinosis can be associated with severe neurological, ocular and cardiovascular complications and may end in the death of the patient. It is of such varied symptomatology that it resembles other conditions such as nephritis, typhoid, encephalitis, myositis and tetanus. Diagnosis of Trichinella spiralis infection by trichinoscopy and a digestion method is not sufficiently sensitive and too time-consuming for routine use (Abou-Zakham et al. 1990). As described before, many immunological methods have been used in the last years in its diagnosis and proved to be helpful. The aim of the present work is to apply and evaluate the dipstick assay in the diagnosis of hydatidosis and trichinosis in comparison to EITB and FAST-ELISA.
Materials and methods
Study samples
Clinical study
A clinical study was carried out on a total of 78 patients categorized into five groups as follows:
-
1.
Group I consisted of 26 patients of both sexes, i.e. ten males and 16 females ranging from 5–70 years of age, with surgically confirmed CE in either the liver (14) or lung (5) or mixed-organ infection (7) i.e. liver, lung or spleen with or without the kidneys. The preoperative examination was done clinically, by different imaging techniques (X-ray, ultrasound, computerized axial tomography) and serologically with an indirect haemagglutination test (IHAT) for CE (Fumouze Laboratories Diagnostics, France). The cysts were routinely subjected to post-operative histopathological examination and confirmed as hydatid cysts.
-
2.
Group II consisted of seven male patients infected with trichinosis, aged between 35 and 50 years. They were diagnosed serologically by positive ELISA for trichinosis.
-
3.
Group III consisted of 30 patients with different parasitic diseases (five patients each): hydatidosis, trichinosis, schistosomiasis mansoni, toxoplasmosis, amoebiasis and fascioliasis. This group was further subdivided into two subgroups. Groups IIIa and IIIb consisted of 25 patients with parasitic infections other than hydatidosis and trichinosis.
-
4.
Group IV consisted of 15 patients with different parasitic diseases to be tested with the dipstick assay only (three patients each): ascariasis, alveolar echinococcosis, onchocerciasis, bancroftian filariasis and cysticerosis.
-
5.
Group V consisted of ten apparently healthy, age-matched individuals of both sexes (three males and seven females). Patients examined in this study were selected from those referred to the Diagnostic and Research Unit in the Parasitology Department, Faculty of Medicine, Ain Shams University and from the General Surgery and Tropical Diseases Department, Ain Shams University Hospitals.
The investigations were performed in accordance with the Ministry of Health and Human Services guidelines for Clinical Research and Treatment under a protocol approved by the Egyptian Reference Diagnostic Centre, and the Egyptian Organization for Biological Products and Vaccines.
Sera of group II and IV were provided by Centres for Disease Control, Atlanta, Georgia.
Experimental study
The experimental study was carried out on a total of 40 female Swiss albino mice, 6–8 weeks old, weighing 25–30 g obtained from the Theodore Bilharz Research Institute, Cairo. Mice were experimentally infected with T. spiralis. T. spiralis larvae were prepared by digestion of infected pig’s muscles (obtained from a Cairo abattoir). Infected pork was minced then treated with the digestive fluid (0.05% HCl/0.1% pepsin in distilled water) by stirring at 37°C. The digested product was sieved and the larvae were collected on a 50-μm sieve, washed repeatedly, centrifuged and counted microscopically. Mice were infected per os with 150–200 muscle larvae using a 0.5-ml syringe equipped with a blunt, curved, 18-gauge dosing needle. During the study, 19 mice died and the remaining 21 mice were randomly allocated to seven groups, according to the date of sacrifice and blood samples collection: group 1, 5 days; group 2, 7 days; group 3, 10 days; group 4, 2 weeks; group 5, 3 weeks; group 6, 8 weeks; group 7, 12 weeks post-infection (PI). Infection was confirmed in all groups by muscle biopsy (diaphragm) for larvae.
Sera of all studied groups were separated from blood samples and stored at −70°C until used in all diagnostic assays. Sera samples of groups III and V were also examined by serological examinations to exclude hydatidosis (using IHAT) and trichinosis (using ELISA).
Dipstick assay
Crude camel hydatid cyst fluid (HCF) was prepared according to Rogan et al. (1990) and T. spiralis crude larval muscle (TSML) antigens according to Ruitenberg et al. (1975). The dipstick was prepared according to Al-Sherbiny (1996). Briefly, the antigen [0.1 μg/mm per μl phosphate buffered saline (PBS)] was dotted onto nitrocellulose paper (NC) (Bio-Rad, USA) using Bio-Rad sheet Mini-Protean II multi-screen apparatus. Antigen-free PBS and normal human serum (diluted 1:100) were dotted and used as negative and positive controls, respectively. NC was incubated for 2 h at room temperature, washed, dried, attached to a double-face adhesive tape supported with an inert perspex matrix and cut into 2-mm reagent strips. The sticks were incubated in diluted patients’ sera (1:25) for 7 min, washed 5 times with PBS-Tween 20 and then incubated with peroxidase-conjugated goat antihuman IgG for 7 min. The sticks were then washed and incubated with diaminobenzidine substrate (Sigma) for 2 min. The reactions were stopped by washing with distilled water and left to dry at room temperature. A dark purple band indicates a positive reaction.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis
HCF and TSML antigens were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) under non-reducing condition using gradient gel (5%–22.5%) according to Laemmli (1970). After termination of electrophoresis the separated proteins on the gel were either stained with silver stain to identify their molecular weights or transferred to NC.
Enzyme-linked immunoelectrotransfer blot
EITB was performed according to Wen and Craig (1994). Briefly, separated proteins of HCF and TSML antigens, transferred to NC, were cut into strips and incubated with diluted human sera (1:50) for 1 h. After washing, the strips were incubated with peroxidase conjugated goat antihuman IgG, washed and incubated with diaminobenzidine substrate for 10 min. The reaction was stopped by distilled water.
Falcon assay screening test-enzyme-linked immunosorbent assay
FAST-ELISA was done according to Hancock and Tsang (1986), where samples were considered positive if their optical density was greater than the mean of healthy control samples plus 3 SD.
Statistical analysis
Data were statistically analysed using SPSS version 6 to obtain descriptive (mean and SD) and analytical statistics (sensitivity, specificity, diagnostic accuracy and positive and negative predictive values of each assay). To compare the results of different serological tests, a paired χ2-test (McNemar’s) was used.
Results and discussion
Traditionally, HCF has been used as a source of antigen for the serodiagnosis of hydatidosis. In the present study, crude camel HCF was used because of the less likely cross-reactions of host (camel) antigens with immunoglobulin in human sera as mentioned by El-Zayyat et al. (1999). As shown in Table 1 all hydatidosis patients (group I) were positive according to both dipstick and EITB (on the basis of the presence of one or more bands) (100% sensitivity), while only 25 were positive according to FAST-ELISA (96.2% sensitivity) ( P <0.05) as shown in Table 2. On the other hand, none of the ten healthy controls (group V) were positive according to any of the three tests. When examining sera of patients with parasitic diseases other than hydatidosis, groups IIIa and IV, examined by the dipstick assay, and group IIIa examined only by EITB, revealed false-positive reactions, indicating a specificity of 91.4% for both tests (data not shown). FAST-ELISA showed no false-positive reactions with sera of patients of group IIIa indicating 100% specificity.
As regards the dipstick assay, no similar studies have been done to compare it to other diagnostic assays. Nevertheless, Misterlio et al. (1995) reported a sensitivity of 100% of the dot immunobinding assay in the diagnosis of hydatidosis, and this assay is similar to the dipstick assay except that a coloured conjugate is used in the former instead of the non-coloured conjugate and substrate in the latter. On the other hand, both Romia et al. (1992) and Pappas (1986) reported a lower sensitivity of dot-ELISA in the diagnosis of hydatidosis (88.9 and 96%, respectively); this could be attributed to the different source of antigen used (sheep HCF). There is a substantial body of evidence demonstrating that hepatic cysts are more likely to elicit an immune response than pulmonary cysts and that more than one test is needed to diagnose hydatid pulmonary cysts (Rogan et al. 1990). The results of these previous studies highlight the importance of the dipstick assay, used in our study, for the conclusive diagnosis of hepatic and extra-hepatic cysts, as well as low and high titers of IHAT for hydatidosis and single and multisided cysts (Fig. 1a). The specificity of the dipstick assay (91.4%) nearly agrees with that reported by Rogan et al. (1990) for dot-ELISA (90.3%).
Reactions of sera from a hydatidosis patients (1–26) and normal controls ( C1–10), and b patients with parasitic diseases other than hydatidosis, in the dipstick assay. The dark band shows a positive reaction. F Fascioliasis, Sm schistosomiasis mansoni, Tox toxoplasmosis, Tr trichinosis, Am amoebiasis, W.banch bancroftian filariasis, Onch onchocerciasis, E. multi multilocul alveolar hydatidosis, Cyst. cysticercosis, As. ascariasis
False-positive reactions were observed with cysticercosis, alveolar hydatidosis, trichinosis, schistosomiasis mansoni and fascioliasis sera (Fig. 1b). The false-positive reactions of the dipstick may be due, in part, to the planned inclusion in the study of serum specimens having high titers of antibodies to these related parasitic infections, and to the use of a crude and complex hydatid fluid preparation containing shared helminth antigens (Mamuti et al. 2002). While some authors advocate the use of purified or partially purified HCF in serologic systems (Rogan et al. 1990), others reported that the use of crude HCF antigens is as useful as purified antigens and is more practical for use especially in endemic areas (El-Zayyat et al. 1999). It is very likely that an increase in the sensitivity of a serological test for hydatidosis will be accompanied by a decrease in specificity (Siracusano et al. 1991).
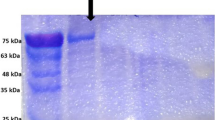
As regards EITB in the current study, the high sensitivity (100%) was similarly recorded by Kanwar et al. (1992) and nearly agrees with the findings of Liance et al. (2000) (97% sensitivity). The high sensitivity of this test was not surprising as its limit of detection was as low as a few picograms of antibodies (Maddison et al. 1989). The specificity of EITB was 91.4% which is lower than the 100% recorded by Varastegui et al. (1992). These workers stated that the high specificity of this test can possibly be due to the high resolution of HCF antigenic components. The SDS-PAGE profile of camel HCF antigen revealed a pattern of bands ranging from 12–200 kDa. Those of diagnostic importance as revealed by EITB were the 200-, 138-, 56-, 45- to 50-, 27-, 23-, 16- and 12-kDa bands (Fig. 2)
Immunoblot of sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) fractionated camel hydatid cyst fluid antigen probed with sera from: hydatidosis patients (1–26), normal control ( C1–5) and patients with parasitic diseases other than hydatidosis; ➙ shows non-specific 83-kDa band. Trich trichinosis; for other abbreviations, see Fig. 1
Previous studies by many authors recognized two HCF antigens; the thermolabile antigen A (arc 5) which was found to be a 60 kDa molecule and the thermostable antigen B which separates into three immunogenic bands of 23, 16 and 12 kDa (Kharebov et al. 1997). Comparing this report with the data presented here, it seems likely that the 56-kDa band is the subunit of antigen A and the 23-, 16- and 12-kDa components are the subunits of antigen B. When each antigenic band was considered separately, the 56-kDa band was the one that showed the highest sensitivity and specificity (100%). This result suggests the potential use of this antigenic fraction in the specific serologic diagnosis of hydatidosis. Babba et al. (1994) indicated the role played by this subunit as a major target for the host immune response.
The 23-, 16- and 12-kDa components of antigen B were also detected by EITB at 46, 46 and 53% sensitivity, respectively, and 100% specificity for all. Reports concerning recognition of antigen B subunits by human hydatidosis sera in immunoblotting have indicated some variability. Siracusano et al. (1991) recorded 46 and 33% sensitivity for 16- and 12-kDa bands, respectively. Moreover, Verastegui et al. (1992) recorded 58, 59 and 65% sensitivity for 23-, 16- and 12-kDa bands, respectively. In the current study, EITB showed a sensitivity of 64.5, 0 and 71% with respect to the 23-, 16- and 12-kDa bands in the diagnosis of hepatic cysts, pulmonary cysts and multiple-organ infection, respectively. It has been reported that hyaline hydatid cysts often found in pulmonary cases do not release 12- and 16-kDa antigens (Shepherd and McManas 1987), which may contribute to the observed low antibody response to them.
As regards FAST-ELISA in the present study, the test was 100% sensitive in diagnosing hepatic and pulmonary cysts and 85.7% sensitive in diagnosing multiple-organs infection. The high specificity of FAST-ELISA (100%) in diagnosing hydatidosis was similarly reported by Kaur et al. (1999) although they reported a lower sensitivity (82.3%). The variation in the sensitivity and specificity of the immunodiagnostic tests for hydatidosis carried out by different authors could be attributed to the source and nature of the antigen used, the stages and localization of hydatid cysts and also perhaps to subspecies and strain differences of E. granulosus in different countries or even maybe within a country (Lucas and Bandera. 1996).
In the current study, the dipstick assay and EITB consistently detected seven out of seven trichinosis patients (group II) (100% sensitivity), whereas FAST-ELISA failed to detect one infection (85.7% sensitivity) ( P >0.05). None of the ten healthy controls (group V) was positive according to any of the three tests. When sera of patients with parasitic diseases other than trichinosis were examined, i.e. group IIIb and IV by dipstick and group IIIb only by EITB, no false-positive reactions were revealed (100% specificity), while sera of five patients of group IIIb were positive according to FAST-ELISA (85.7% specificity) ( P >0.05) (Tables 2, 3; Figs. 3, 4).
Reactions of sera from trichinosis patients (1–7), normal controls (1–10) and patients with parasitic diseases other than trichinosis in the dipstick assay. Trich Trichinosis, Hydatid hydatidosis, Fasciola Fascioliasis, S.m. schistosomiasis mansoni, Toxo. toxoplasmosis, Amoeb. amoebiasis, W. Bancrofti bancroftian filariasis, Onchocerca onchocerciasis, E. multilo c alveolar hydatidosis, Ascaris ascariasis
The high sensitivity of the dipstick as well as EITB agrees with the report of Su and Prestwood (1991) that both dot-ELISA and EITB confirmed an experimental T. spiralis infection in swine at 100% sensitivity, even in those found to be negative by a digestion procedure. In a study comparing dot- and conventional ELISA, Chan and Ko (1988) highlighted the value of dot-ELISA in reaching a specific diagnosis of trichinosis (100% sensitivity and specificity).
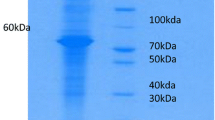
In the present study, EITB was found to be more sensitive than FAST-ELISA in the diagnosis of trichinosis. This result confirms the recent findings of Gomez-Priego et al. (2000) that ELISA could detect only 26 (76%) out of 34 trichinosis patients that were diagnosed serologically by EITB. It is becoming increasingly apparent that the EITB is more precise than ELISA for the detection of infectious diseases because positive reactions are determined by colour reaction and also by the antigen profile, not by the measurement of optical density (Katti 2001). The SDS-PAGE profile of TSML antigen revealed a pattern of bands ranging in molecular weight from 14–120 kDa. Bands of diagnostic importance for trichinosis as revealed by EITB were the 87-, 52-, 45- and 40-kDa ones (Fig. 4). These proteins were found to have an important role in eliciting a strong IgG humoral immune response in trichinosis patients as they represent major targets for the host immune response (Gold et al. 1990). Using a monoclonal antibody produced against T. spiralis, Rodriguez-Perez et al. (1989) identified a 45-kDa antigenic band, in TSML antigen, as the most strongly recognized band. Moreover, Robert et al. (1996) found that the human IgG response to TSML antigen recognized 47-, 55- and 90-kDa bands at a sensitivity of 100%. On the other hand, although Robert et al. (1996) was able to differentiate T. spiralis infection from other parasitic infections (100% specificity) by EITB, bands which had the same molecular weights as T. spiralis bands were observed using sera from patients with autoimmune diseases that can mimic the clinical symptoms and biological signs of trichinosis. They suggested that these antigens could represent shared epitopes between the host and parasites such as heat shock proteins. On the other hand, the sensitivity of FAST-ELISA in the present study (85.7%) almost agrees with the recorded sensitivity of 88.2% found by Morakote et al. (1992). Also, it supports the opinion of Costantino et al. (2001) that the use of ELISA as a sole immunoserological technique can lead to a misdiagnosis of this parasitic infection. False-positive reactions of FAST-ELISA were observed with hydatidosis, toxoplasmosis and fascioliasis sera by El-Temsahi et al. (1992).
In the present study, the sensitivity of the dipstick assay in detecting T. spiralis antibodies was investigated in mice experimentally infected with T. spiralis and sacrificed at different time points PI. With FAST-ELISA, antibodies were first detected in all mice (100% sensitivity) 14 days PI (group 4) while antibodies were detected by dipstick and EITB in all mice 21 days PI (group 5) (Table 4, Fig. 5a, b). All tests retained their sensitivity, thereafter, till the 12th week PI. So, FAST-ELISA detected T. spiralis infection somewhat earlier than the other two tests which apparently reflects the point at which the maximum invasion of muscles by larvae occurs (Beaver et al. 1984). Dipstick and EITB detected T. spiralis infection 3 weeks PI which could be explained by the notion that antibodies against T. spiralis reach their peak by the fourth week PI (Beaver et al. 1984). Meanwhile, this result contradicts that of Su and Prestwood (1991) who reported that dot-ELISA detected a low experimental T. spiralis infection in swine with 100% sensitivity by the fifth to sixth week of infection. This discrepancy could be due to the difference in the host used or the difference in the infection intensity. Serrano et al. (2001) reported that the IgG response against T. spiralis showed a significant relation with the infecting dose and intensity of infection.
Sera of mice experimentally infected with T. spiralis (1–21), at different time points post infection, reactions in immunoblot of fractionated TSML antigens ( a) and in the dipstick assay ( b). Dipsticks were dotted with: human immunoglobulin (positive reference)( a), antigen-free phosphate buffered saline (negative reference) ( b), and corresponding antigen ( c)
One of the advantages associated with the dipstick as well as EITB is the use of a nitrocellulose membrane as a solid phase for antigen binding rather than polystyrene beads as used in FAST-ELISA, and this may explain why they showed a higher sensitivity (100%) in the diagnosis of hydatidosis and trichinosis. Nitrocellulose firmly binds proteins and has a large surface area due to its porosity (Boctor et al. 1987), reducing the possibility of proteins leaching during the washing steps. In spite of the equally high sensitivity, specificity and diagnostic accuracy of the dipstick assay and EITB, the latter needs a well-equipped laboratory, sophisticated equipment and highly trained personnel (Versatagui et al. 1992). It should be noted, however, that FAST-ELISA detected T. spiralis infection somewhat earlier than the dipstick and EITB.
An ideal diagnostic test should be simple and easy to perform and to interpret, in addition to being both sensitive and specific. The dipstick is a simple antigen- and serum-conservative test that requires only micrograms of parasite antigen and 10 μl of patient’s sera. The assay can be completed in 15 min with results obtained visually, and hundreds of samples can be tested at a time to suit a variety of diagnostic needs, such as epidemiological surveys. This test, which can be carried out while the patients waits, would be more practical than current diagnostic techniques, especially in rural areas and district hospitals. Additionally, patients can see the results themselves, which would increase treatment-compliance rates. From an epidemiological point of view, a dipstick assay allows control measures to be implemented in situ. The test has its merit in being both a qualitative (screening large numbers of blood samples) and quantitative assay (determining the end-point titration of an individual’s sera) (Zimmerman et al. 1985). Antigens kept frozen on nitrocellulose strips at −20°C retain antigenicity for a long time (Pappas et al. 1986), hence an added advantage for the rapid routine use of this test in the laboratory. Genetically engineered antigens undoubtedly will greatly enhance the overall utility of dipsticks, providing parasitologists with a commercially important stock for the detection of human diseases endemic in different areas.
The results of the present study point to a clear conclusion that this dipstick assay is a valuable immunodiagnostic test for the diagnosis of hydatidosis and trichinosis, with a high sensitivity (100%) and fairly high specificity (91.4 and 100%, respectively). Further studies should concentrate on maximizing the specificity of this test through the use of highly purified antigens.
References
Abou-Zakham AA, Romia SA, EI-Naggar HAL, ElKhouly EI (1990) Evaluation of immunodiagnostic tests in detection of trichinosis in experimentally infected rats. J Egypt Soc Parasitol 20(2):5 73–8
Al-Sherbiny M (1996) Field applicable method for detection of antibodies to Schistosoma species and genus specific antigens using dipsticks. J Egypt Ger Soc Zool 20(A):81–97
Allan JC, Mencos F, Garcia-Novel J, Sarti E, Flisser A, Wang Y, Liu D, Craig PS (1993) Dipstick dot ELISA for the detection of Taenia coproantigens in humans. Parasitology 107:79–85
Azab ME, Fekry AA, Abbas MS, Khalifa KE, Tawfik RA (1999) Evaluation of FAST-ELISA for the diagnosis of experimental trichinosis. J Egypt Soc Parasitol 29(1):247–259
Babba H, Messedi A, Masmoudi S, Zribi M, Grillot R, Ambriose-Thomes P, Beyrouti L, Sohnoun Y (1994) Diagnosis of human hydatidosis. Comparison between imagery and six serologic techniques. Am J Trop Med Hyg 50:64–68
Beaver PC, Jung RC, Cupp EW (1984) Amphistomate and distomate flukes. In: Clinical parasitology, 9th edn. Lea and Febrig, Pa., pp 451–538
Boctor FN, Stek MJ Jr, Peter JB, Kamal R (1987) Simplification and standardization of dot-ELISA for human schistosomiasis mansoni. J Parasitol 73(3):589–92
Boulos LM, Ibrahim IR, Negm AY, Aly SM (2001) Detection of coproantigen in early trichinellosis. Parasite 8(2):136–139
Chan SW, Ko RC (1988) Comparison between standard ELISA and dot-ELISA for serodiagnosis of human trichinosis. Trans R Soc Trop Med Hyg 82(6):892–894
Costantino SN, Malmassari SL, Dalla Fontana ML, Diamante MA, Venturiello SM (2001) Diagnosis of human trichinellosis: pitfalls in the use of a unique immunoserological technique. Parasite 8(2):144–146
Craig PS (1997) Immunodiagnosis of Echinococcus granulosus and a comparison of techniques for diagnosis of canine echinococcosis. In: Anderson FL, Onhelli H, Kachain M (eds) Compendium on cystic echinococcosis. Brigham Young University, Provo, Utah
El-Temsahi MM, Abu-Samra LM, EI-Mansoury ST, Barakat RM, Awadalla HN (1992) Evaluation of enzyme-linked immunosorbent assay and counter-current immunoelectrophoresis in diagnosis of experimental trichinosis. J Egypt Soc Parasitol 22(1):9–15
El-Zayyat EA, Ramzy, RM, Abdel-Baki MH, Rifaat MM, Helmy H, Abdel Hamid DM (1999) Human cystic echinoccosis: diagnostic value of different antigenic fractions of hydatid cyst fluid with different specific immunoglogulin G subclasses by enzyme-linked immunoelectrotransfer blot. J Egypt Soc Parasitol 29(3):817–830
Gold AM, Despommier DD, Buck SW (1990) Partial characterization of two antigens secreted by L1 larvae of Trichinella spiralis. Mol Biochem Parasitol 41:187–195
Gomez-Priego A, Crecencio-Rosales L, de-La-Rosa JL (2000) Serological evaluation of thin-layer immunoassay-enzyme-linked immunosorbent assay for antibody detection in human trichinellosis. Clin Diagn Lab Immunol 7(5):810–812
Hancock K, Tsang VCW (1986) Development and optimization of the FAST-ELISA for detecting antibodies to Schistosoma mansoni. J Immunol Methods 92:167–176
Kanwar JR, Kaushik SP, Sawhney LMS, Kamboj MS, Mehtab SK, Vinayak EK (1992) Specific antibodies in serum of patients with hydatidosis recognized by immunoblotting. J Med Microbiol 36:46–51
Katti MK (2001) Are enzyme-linked immunosorbent assay and immunoblot assay independent in immunodiagnosis of infectious disease? Clin Infect Dis 32(1):1114
Kaur M, Mahajan RC, Malla N (1999) Diagnostic accuracy of rapid enzyme-linked immunosorbent assay for the diagnosis of human hydatidosis. Indian J Med Res 3(110):18–21
Kharebov A, Nahmias J, El-On, J (1997) Cellular and humoral immune responses of hydatidosis patients to Echinococcus granulosus purified antigens. Am J Trop Med Hyg 57(5):619–625
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Liance M, Janin V, Bresson-Hadni S, Vuitton DA, Houin R, Piarroux R (2000) Immunodiagnosis of Echinococcus infections: confirmatory testing and species differentiation by a new commercial Western Blot. J Clin Microbiol 38(10):3718–21
Lucas MS, Bandera CC (1996) Echinococcus granulosus in Spain: strain differentian by SDS-PAGE of somatic and axcretory/secretory proteins. J Helminthol 70:253–261
Maddison SE, Slemenda SD, Shantz PM, Fried JA, Wilson NI, Tsang, VCW (1989) A specific diagnostic antigen of Echinococcus granulosus with an apparant molecular weight of 8KDa. Am J Trop Med Hyg 40(4):337–383
Mamuti W, Yamasaki H, Saka Y, Nakao M, Lightowlers MW, Ito A (2002) Usefulness of hydatid cyts fluid of Echinococcus granulosus developed in mice with secondary infection for serodiagnosis of cystic echinococcosis in human. Clin Diagn Lab Immunol 9(3):573–576
Misterlio G, Gentili M, Falaglani P, Roncarolo D, Riva G, Tinelli M (1995) Dot immunobinding assay as a new diagnostic test for human hydatid disease. Immunol Lett 47(1/2):79–85
Morakote N, Sukhavat K, Khamboonruang C, Siriprasert V, Suphawiteyanukul S, Thamasonthi W (1992) Persistence of IgG, IgM, and IgE antibodies in human trichinosis. Trop Med Parasitol 43:167–9
Pappas MG (1986) Rapid serodiagnosis of parasitic infections by Dot-EL1SA using dipsticks. Trans R Soc Trop Med Hyg 80:1006
Pappas MG, Schantz PM, Cannon LT, Wahlquist SP (1986) Dot-ELISA for the rapid serodiagnosis of human hydatid disease. Diagn Immunol 4(6):271–276
Robert F, Weil B, Kassis N, Dupouy-Camet, J (1996) Investigation of immunofluorescence cross-reactions against Trichinella spiralis by western blot analysis. Clin Diagn Lab Immunol 3(5):575–577
Rodrigues-Perez J, Gomez-Garcia V, Rodriguez-Osorio M, Rojas-Gonzalez J, Gomez-Morales, MA (1989) Differentation between Trichinella spiralis and T.pseudospiralis infection larvae by a monoclonal antibody. J Helminthol 63(4):275–279
Rogan MT, Morris DL, Pritchard DL, Perkins AC (1990) Echinococcus granulosus: The potential use of specific radiolabelled antibodies in diagnosis by immunoscintigraphy. Clin Exp Immunol 80:225–231
Romia SA, Youssef ME, Handoussa AE, Rizk HM, Sallam SM (1992) Dot-ELISA as a diagnostic test in hydatid disease. J Egypt Soc Parasitol 22(3):603–610
Ruitenberg EJ, Steerenberg PA, Brosi BJM (1975) Microsystem for the application of ELISA in the serodiagnosis of Trichinella spiralis infection. Med Ned 4:30–3
Serrano FJ, Perez-Martin JE, Carron A, Navarrete I (2001) Comparison of IgG, IgG1 and IgG2 responses to Trichinella spiralis Trichinella britovi in swine. Parasite 8(2):133–135
Shepherd JC, McManas DP (1987) Specific and crossreactive antigens of Echinococcus grqnulosus hydatid cyst fluid. Mol Biochem Parasitol 25:143–154
Siracusano A, Ioppolo S, Notargiacomo S (1991) Detection of antibodies against Echinococcus granulosus major antigens and their subunits by immunoblotting. Trans R Soc Trop Med Hyg 85:239–243
Su XZ, Prestwood A K (1991) A dot-ELISA mimicry western blot test for the detection of swine trichinellosis. J Parasitol 77:76–82
Tsang, VCW, Wilkins PP (1991) Immunodiagnosis of schistosomiasis screen with FAST-ELISA and confirm with inununoblot. Clin Lab Med 11:1029–1039
Van Etten L, Folman CC, Eggelte TA, Kremsner PG, Deelder AM (1994) Rapid diagnosis of schistosomiasis by antigen detection in urine with a reagent strip. J Clin Microbiol 32:2404–2406
Verastegui M, Moro P, Guevara A, Rodriguez T, Mirand E, Gilman, RH (1992) Enzyme-linked immunoelectrotransfer blot test for diagnosis of human hydatid disease. J Med Microbiol 67:129–143
Wen H, Craig PS (1994) Immunogolbulin G subclasses in human cystic and alveolar echinococcosis. Am J Trop Med Hyg 51(6):741–748
Zimmerman GL, Nelson MJ, Clark CRB (1985) Diagnosis of bovine fascioliasjs by a dot enzyme-linked immunosorbent assay: a rapid microdiagnostic technique. Am J Vet Res 46:1513–1515
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Sherbiny, M.M., Farrag, AA.M.M.K., Fayad, M.H. et al. Application and assessment of a dipstick assay in the diagnosis of hydatidosis and trichinosis. Parasitol Res 93, 87–95 (2004). https://doi.org/10.1007/s00436-004-1076-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-004-1076-x