Abstract
Purpose
Cystic echinococcosis (CE) is one of the most neglected tropical diseases as per WHO which has an immense public health significance. Diagnosis of CE is difficult as specific clinical signs are manifested only after the hydatid cyst attains a considerable size. Immunodiagnosis is a reliable method of diagnosing CE.
Methods
SDS-PAGE was performed for the hydatid cyst fluid antigens. The antigen purity was tested by Western blotting and four different immunoassays were evaluated using these two antigens in sheep and buffalo in diagnosis of CE.
Results
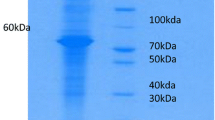
SDS-PAGE revealed four bands of 72, 64, 48 and 24 kDa for crude antigen and a single 72 kDa band for purified antigen. Among sheep sera, ELISA was most sensitive (70%) using crude antigen and also while using the purified antigen (80%). In case of buffaloes, ELISA, DID and CIEP were more sensitive (83.3%) using crude antigen, whereas DID and CIEP were more sensitive (83.3%) using purified antigen.
Conclusion
In sheep, while using the crude antigen ELISA was the most sensitive assay and IHA was the least sensitive assay. While using the purified antigen also, ELISA was the most sensitive and others were absolutely specific except for IHA being less sensitive. In buffaloes, using crude antigen, all the immunoassays CIEP, DID and ELISA were highly sensitive in diagnosing CE infection except IHA, whereas using the purified antigen, both CIEP and DID were more sensitive than ELISA and IHA which were comparatively less sensitive in detecting CE in buffalo sera.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystic echinococcosis is a parasitic zoonosis that has a worldwide distribution across human and livestock populations. CE is an often-neglected zoonotic disease which has an immense public health significance [1]. Larval stage (metacestode) of Echinococcus granulosus, a dog tapeworm is responsible for CE that affects ungulates (Sheep, cattle, buffalo, goat, horse pig) mostly and man is an accidental host for this parasite [2, 3]. E. granulosus is generally an intestine dweller of canids and among the ungulates, sheep is the most affected intermediate host worldwide with a very high incidence rates and prevalence rates. In specific, milk production, fleece quality and fertility are largely affected in sheep. E. granulosus and E. multilocularis are responsible for cystic echinococcosis and alveolar echinococcosis, respectively, which are the most dreadful forms of echinococcosis with regard to medical and public health relevance in humans [4]. WHO declared CE as a worldwide Neglected Tropical Disease (NTD) as this disease is a major problem in both human and livestock across many areas worldwide [5].
E. granulosus being a canid intestine inhabitant, the tapeworm eggs are frequently shed in the canid faeces from the gravid proglottids. On accidental ingestion of these eggs by a suitable host, most probably through contaminated water and food, they hatch in the small intestine releasing an oncosphere. Commonest means of disease transmission are contaminated vegetables, fruits and water that are consumed by human beings [6]. This oncosphere is capable of penetrating the intestinal wall and thus invades other organs through blood circulation with the liver and lung being the organs of predilection [7]. Following the invasion, oncospheres develop into hydatid cysts bearing protoscoleces inside and these cysts increase in size over time responsible for myriad of clinical complications in the host [8].
CE has been recorded in all continents except for Antarctica, with prevalence levels as high as 5–10% in parts of Argentina, Peru, East Africa, Central Asia and China [9]. Nations where animal husbandry practices are predominant have recorded a greater prevalence of CE relatively and people there are at a high risk of contracting CE infection [10]. CE when neglected reflects huge socio-economic losses as both livestock and humans are affected and livestock production losses, like liver condemnation in slaughter houses, carcass weight reduction, decline in milk production and fertility, are often noticed in CE cases [11]. Further, the treatment and management of CE is often expensive and time consuming with a very thin probability of recovery unless proper surgical intervention is made. CE accounts for a huge US$ 193, 529, 740 annual monetary losses to man globally [12].
India is a nation largely dependent on agriculture and animal husbandry, thus harbours a large number of diseases especially in the rural setting. As the sheep–dog lifecycle can easily sustain in Indian conditions with both the dogs and livestock being at close vicinity often contaminating the pastures, CE is a huge concern in India. The conditions in India are perfect for establishment and transmission of hydatidosis in both livestock and humans [13]. States with highest recorded CE prevalence were Saurashtra, Andhra Pradesh and Tamil Nadu especially among the food animals. A prevalence of 4.35% was recorded in Bangalore urban district stray dog population [14]. In South India, 6.5% and 5.8% prevalence were reported in sheep and goat, respectively, at a local slaughter house [15]. Unusually high seroprevalence rate was recorded in dog handlers [16]. In Andhra Pradesh, 118 cases of CE were recorded in hospitals of central and southern epidemic zone during 2009–11 that implied the widespread and emerging nature of the CE infection in AP [17].
As most of the cases of CE are being under reported, WHO placed CE under NTD`s category [18]. Most of the NTD`s can be readily diagnosed by either clinical signs or simple laboratory tests, whereas CE diagnosis is often complicated as no pathognomonic clinical signs are manifested in early stages of the disease which are only evident after years of infection. Initially, CE might be diagnosed by imaging techniques, like X-ray or Ultrasound, which are not feasible under field conditions and in addition are unreliable owing to a large misdiagnosis due to abscess, tumour, calculi and other cysts [12]. For an early and relatively more accurate diagnosis of CE, currently available diagnostic procedures are Indirect fluorescence antibody test (IFAT), Immunoelectrophoresis (IEP), Enzyme Linked Immunosorbent Assay (ELISA), Double Immunodiffusion (DID), Indirect Haemagglutination test (IHA) and Counter immunoelectrophoresis (CIEP) [19]. Cross-reactivity with other parasitic species was a major drawback of these techniques and SDS-PAGE and immunoblotting techniques are regarded as more reliable and recent diagnostic approaches which can negate the earlier mentioned short comings [20].
CE serology works can be traced back to decades and almost every known serological assay has been put to use in the diagnosis of CE. A proper inexpensive serological assay when developed could be employed as a mass screening and surveillance tool in the diagnosis of CE in animals. Serological assays are comparatively more reliable when the right antigen and technique are employed. The efficiency of diagnosing CE infection is largely dependent on the antigen used and the immunological technique employed [21]. The hydatid cyst fluid is a super-rich source of a myriad of antigenic fractions [22,23,24,25,26,27]. Among several reported fractions, antigen 5 (Ag5) and antigen B (AgB) are most efficient purified antigenic fractions with lesser cross-reactivity with other helminths. Even WHO also recommends the use of AgB for serodiagnosis of CE for more accurate and efficient diagnosis [28,29,30].
As per the works done over years, immunoassays, like IHA [31], ELISA [32, 33], Latex Agglutination Test [34] and CIEP [35], are normally employed in diagnosing CE in animals. Hydatid cyst fluid antigens were used in various immunoassays in India with varied rates of sensitivity and specificity. However, reports indicate that the crude hydatid cyst fluid antigen resulted in high cross-reactivity with other parasitic infections, like Coenurus and Cysticercus [36], that led to the quest and development of a purified hydatid cyst fluid antigen. Purified antigen was reportedly more immunogenic compared to the earlier crude antigen that encouraged the application of purified antigen over the crude antigen. Henceforth, the present study was done to evaluate four different immunological techniques (IHA, CIEP, DID and ELISA) using a crude hydatid cyst fluid antigen and a purified hydatid cyst fluid antigen between two species, viz., sheep and buffaloes. The sensitivity and specificity of the earlier mentioned assays were studied, the two different hydatid cyst fluid antigens were compared and their diagnostic efficiency was evaluated in between sheep and buffalo species.
Materials and Methods
Hydatid cyst fluid was collected from hydatid cysts that were collected from the liver and lungs of sheep. Hydatid cysts (N = 80) were collected from the slaughter house from sheep carcasses that were aged between 10 and 14 months. This collected hydatid cyst fluid used in the preparation of two different hydatid cyst fluid antigens. Serum samples for this present study were collected from sheep (n = 150) and buffaloes (n = 50) and they were separated as true positives and negatives based on the presence of hydatid cysts in their liver and lungs observed during post mortem examination.
Microscopic Examination
For the preparation of antigen, only fertile hydatid cysts were used after examination of the cyst fluid for the presence of protoscoleces. Microscopic examination of the cyst fluid was done initially by a wet film examination as per the protocol described by Mohanty [37] followed by Haematoxylin and Eosin staining procedure for a better visualization of the hydatid cyst fluid contents as per the procedure described by Thompson [38]. Another staining procedure, Lugol`s iodine staining was also performed for the cyst fluid as per the method by Patanvadia [39] to verify the use of this staining along with wet film examination, as this technique is rapid and easy comparatively.
Antigen Preparation
Two different antigens were prepared from the sheep hydatid cyst fluid, viz., crude hydatid cyst fluid antigen and a purified hydatid cyst fluid antigen. The crude antigen was prepared as per the method described by Zamani [40] with slight modifications. Briefly, the cyst fluid from all the fertile hydatid cysts was pooled and was centrifuged at 5000 rpm for two minutes. The supernatant was carefully collected and was filtered through a 0.4 µm filter initially followed by a 0.2 µm filter. The filtrate obtained was the crude hydatid cyst fluid antigen and was stored at – 20 ℃ with sodium azide as preservative.
A purified hydatid cyst fluid antigen was also prepared from the hydatid cyst fluid. In this technique of purified antigen preparation, Sephadex size exclusion chromatography was employed based on the procedure of Sbihi [41] with slight modifications. A Sephadex G-50 column was prepared by allowing the powder to swell overnight and stacking it into a column of 10 cm length. The filtered hydatid cyst fluid was passed through the column after equilibrating the column thrice with Tris–EDTA-NaCl buffer. Numerous fractions were collected into separate Eppendorf tubes and were stored at 4 ℃ until further use.
Estimation of Protein
The protein content of the prepared antigens was estimated by two different techniques, viz., Bradford method of protein estimation and Nanodrop method. Bradford method of protein estimation was performed as per the procedure described by Bradford [42] with minor modifications. A Nanodrop spectrophotometer (NanodropLITE) was used for estimation of protein content.
Protein Precipitation
For an enhanced SDS-PAGE results, the protein content of the cyst fluid antigenic fractions was further increased by protein precipitation techniques. The protein precipitation was performed by TCA precipitation method and acetone precipitation method. The TCA precipitation procedure was performed as described by Luis Sanchez [43] and the acetone precipitation method was based on the procedure mentioned by the Thermo Scientific Pierce [44].
Protein Profiling of the Cyst Fluid Antigens
The protein profile of both the prepared hydatid cyst fluid antigens was obtained by subjecting them to SDS-PAGE as per the method of Laemmli [45]. Briefly, a 12.5% SDS-PAGE was performed and was run at 80v for stacking gel and 100v for resolving gel. The gel was stained with Coomassie Brilliant Blue and destained with a destaining solution overnight until proper protein bands were visible.
For a better visualization of the proteins of lower molecular weight, Urea PAGE was also performed for the cyst fluid antigens as per the method described by Rivera [46]. Urea PAGE was performed similar to SDS-PAGE, but the resolving gel was 18% gel with 3gms of Urea which was slightly warmed for the urea to get dissolved.
Raising of Hyperimmune Serum
Hyperimmune sera were raised against both the crude hydatid cyst fluid antigen and the purified hydatid cyst fluid antigens in New Zealand White rabbits as per the method described by Jeyathilakan [2]. Briefly, the blood was collected from the rabbits prior to administration of antigen and was preserved as control. Based on the protein content of the cyst fluid antigens, the antigen was mixed with equal quantity of Freund`s complete adjuvant into a fine emulsion and was injected subcutaneously into the rabbits. After 14 days, a booster dose was given to the rabbits with an emulsion of a cyst fluid antigen and Freund`s incomplete antigen. After 10 days, the rabbits were bled by ear vein puncture and blood was collected and the serum was collected from it and was preserved at – 20 ℃ in 0.1 ml aliquots as hyper immune serum.
Immunochemical Characterization of the Cyst Fluid Antigens
The antibody titre of the raised hyperimmune serum against both the hydatid cyst fluid antigens was estimated by an Indirect ELISA as per the method of Vatankhah [47] with slight modifications. Further, the antigens were evaluated by Double immunodiffusion [48], Counter immunoelectrophoresis [49] and Indirect Haemagglutination test [47].
The purity of the prepared hydatid cyst fluid antigens was tested by Western blotting with the raised hyperimmune sera against them as per the method described by Jeyathilakan [2] with slight modifications. Briefly, the crude and purified antigens were subjected to SDS-PAGE and the obtained protein bands were transferred onto a nitrocellulose membrane [50] using an Electrotransfer Mini system (GeNei™) and the membrane was then washed and followed by blocking for one hour at 37 ℃ with TBS-T, washed thrice with a washing buffer, incubated with hyperimmune serum overnight at 4 ℃ and washed thrice with a washing buffer followed by incubation with Anti-rabbit IgG HRP conjugate for 60 min at 37 ℃ and a final washing with wash buffer. Finally, the membrane was placed in DAB substrate until the appearance of colour reaction.
The serum samples that were collected from sheep and buffaloes were initially segregated as known positives and negatives based on the presence or absence of hydatid cysts in their carcass.
Double Immunodiffusion (DID)
Screening of sheep and buffalo sera was initially done by DID as per the method described by Hudson [48] with a few modifications. Briefly, a 1% molten agarose solution was poured onto the slide that was reconstituted in NS and two pairs of wells were punched after solidification. The bottom of the wells was sealed with molten agarose and antigens were added in the central wells and the sera were added to the peripheral wells, respectively. The slides were then left at refrigerated temperature overnight in a humid chamber and were examined the next day for development of precipitation line that indicates positive reaction.
Counter Immunoelectrophoresis (CIEP)
The sheep and buffalo sera were screened by CIEP as per the method of Jeyathilakan [2]. Briefly, slides were loaded with a 1.5% agarose solution reconstituted in normal saline and two pairs of wells were punctured 0.5 cm apart. Antigen was loaded in the centre wells and the test sera were loaded in one peripheral well and the hyper immune sera in another peripheral well. The slides were placed in an electrophoretic chamber and were run for 45 min at 50 V and were examined for formation of precipitation line for a positive reaction.
Indirect Haemagglutination Test (IHA)
The serum samples were also screened by IHA as per the method described by Golassa [31] with slight modifications. Briefly, tanned RBCs were prepared from fresh sheep blood and were further sensitized with crude and purified cyst fluid antigens separately. These sensitized RBCs were added equally in all the wells of a microtitre plate and dilutions of the test sera were also added to the wells and they were read after 30, 40, 50 and 60 min.
Enzyme Linked Immunosorbent Assay (ELISA)
Serum samples were also screened by ELISA as per the procedure of Vatankhah [47] with minor modifications. Briefly, the wells of a clean ELISA plate were coated with hydatid cyst fluid antigens separately such that the protein concentration is 1 µg/50 ml in Carbonate-Bicarbonate buffer (pH 9.6). The plates were kept at 4 ℃ overnight and were washed three times thoroughly with a wash buffer (PBST). The wells were then blocked for 45 min at 37 ℃ with a block buffer (PBST with 2%BSA) followed by washing. Further, hyper immune serum was made into dilutions and added in all wells as triplicates and was incubated at 37 ℃ for 2 h followed by washing. To those wells, anti-rabbit IgG HRP conjugate was added and incubated at 37 ℃ for 90 min. Finally, the wells were incubated for 15 min by adding TMB substrate and the colour development was stopped by adding stop solution to all the wells. The ELISA plate was then immediately read under an ELISA reader.
IAEC Reference Number
(281/go/ReBi/S/2000/CPCSEA/CVSc/TPTY/16/VPH/2020 dated 30.01.2020).
Results
CE diagnosis cannot be done based on imaging techniques as various false positives occur that can be attributed to other fluid cysts, tumours and abscesses in the body [51]. Hydatid cyst fluid antigens which are crude and purified were prepared from fertile hydatid cysts. The collected hydatid cysts were separated into fertile and sterile based on the protoscoleces by microscopic examination. Out of 125 hydatid cysts that were collected from sheep, 92 cysts were fertile and 33 cysts were sterile without any protoscoleces. Studies on the antigenic potential of hydatid cyst fluid revealed that the fluid from the sterile cysts has very low antigenic activity [52] Thus, only the fertile cysts that had the protoscoleces were utilized as the source for antigen extraction. The protein concentration of the crude antigen was estimated by Nanodrop and Bradford method of protein estimation and the mean protein concentration was estimated to be 2.11 mg/ml. Purified hydatid cyst fluid antigen was extracted from the hydatid cyst fluid by Sephadex size exclusion chromatography and the protein concentration was estimated to be 1.85 mg/ml. Further, the protein profile of the crude and purified antigens was studied by subjecting them to SDS-PAGE. The crude antigen revealed four different proteins of 72, 64, 48 and 24 kDa molecular weight and the purified antigen revealed a single 72 kDa protein on SDS-PAGE (Figs. 1, 2).
Further, Urea PAGE was also employed as it further enhances the visualization of lower molecular weight proteins and similar protein profile was observed with the protein bands being sharper. While using the crude antigen, Urea PAGE also revealed a similar protein profile with 72, 64, 48 and 24 kDa protein bands, whereas using purified antigen, a single 72 kDa band was observed; however, the bands were very clear, sharp and lesser noise than normal PAGE. As urea acts as a further denaturing agent, lower molecular proteins were resolved well and the higher weight proteins showed a slower migration [46].
Hyper immune serum that was raised against both the antigens was examined for the antibody titre by an Indirect ELISA and the antibody titre was estimated to be 1:6000. As sufficient antibody conc. was determined, the hyper immune serum was used as a positive control in the immunoassays.
Among the 150 sheep sera, 20 sera were marked positive and among the 50 buffalo sera, 6 were marked positive based on the hydatid cyst presence. The four mentioned immunoassays were evaluated by using both the crude and purified antigen, positive sheep sera (n = 20), negative sheep sera (n = 130), positive buffalo sera (n = 6) and negative buffalo sera (n = 44).
The sensitivity of the immunoassays was measured based on the PM examination of carcass while slaughter. Sera from animals that had the presence of hydatid cyst during Post-Mortem Examination were considered as gold positive standard and the ones without any cyst were considered as gold negative standard, respectively. Various immunoassays detected the sheep and buffalo sera as positive and negative at different rates as given in Table 1. Based on these results, the sensitivity and diagnostic efficacies of every immunoassay have been calculated.
Among the buffalo sera, the sensitivity values for DID, CIEP, ELISA and IHA by using the crude antigen were 83.33%, 83.33%, 83.33% and 50.00%, respectively, whereas the sensitivity values were 83.33%, 83.33%, 66.66% and 66.66% by using the purified antigen. Among the sheep sera, the sensitivity values for DID, CIEP, ELISA and IHA by using the crude antigen were 60.00%, 60.00%, 70.00% and 55.00%, respectively, whereas the sensitivity values using the purified antigen were 60.00%, 60.00%, 80.00% and 60% for the earlier mentioned immunoassays (Tables 2 and 3) (Figs. 3, 4, 5 and 6).
The diagnostic efficacy values of the immunoassays DID, CIEP, ELISA and IHA while using the crude antigen were estimated to be 92.66%, 92.66%, 94.00% and 90.66% and while using the purified antigen 94.66%, 94.66%, 97.33% and 60.00%, respectively, in sheep sera samples. Regarding the buffalo sera, the diagnostic efficacy values of DID, CIEP, ELISA and IHA while using crude antigen were 96.00%, 96.00%, 98.00% and 88.00% and while using the purified antigen the values were 98.00%, 98.00%, 92.00% and 96.00%, respectively (Tables 2 and 3).
Among the four immunoassays that were evaluated, ELISA showed a higher sensitivity in diagnosing the sheep sera while using both the crude and purified antigen in the assay (Fig. 7). In case of buffalo sera, while crude antigen was used, ELISA was the most sensitive assay along with DID and CIEP; however, while using the purified antigen, DID and CIEP were comparatively more sensitive assays (Fig. 8). Similarly, in case of diagnostic efficacy, in sheep sera, ELISA showed a higher diagnostic efficacy and IHA showed the least diagnostic efficacy (Fig. 9). In case of buffalo sera, ELISA was the assay with highest diagnostic efficacy while the crude antigen was employed; however, using the purified antigen revealed that CIEP and DID were better assays with higher diagnostic efficacy (Fig. 10).
Discussion
CE is one of the most neglected zoonotic diseases across the globe affecting both the humans and animals. Diagnosis of CE at an early stage proved to be crucial in preventing the adversities of this disease. Henceforth, early diagnosis could be done through immunological techniques and in this regard the antigen development proved to be crucial in curbing down the cross-reactivity issues with some antigens [53]. In this study, the cyst fluid was examined microscopically for the presence of protoscoleces to separate the fertile hydatid cysts for antigen extraction. A similar examination was reported prior antigen preparation by several authors [37, 54]. Similar staining procedures were reported to be effective by a few similar studies [55,56,57].
The crude antigen was extracted in this study by a centrifugation followed by double filtration through 0.4 and 0.2 µm filters successfully. Similar method was reported by Itagaki [58] and Kanwar [59]. A lyophilization and reconstitution technique of crude antigen preparation was reported by Nasrieh [60]. Further Rajabiyoun [61] reported an ultrasonic disintegrator-based antigen preparation method which was a different technique to that of this study. Further, a purified hydatid cyst fluid antigen was prepared by Sephadex G-50 size exclusion chromatography in this study and similar chromatographic antigen extraction was reported by several authors [25, 41, 62].
The protein profile of the prepared hydatid cyst fluid antigens was estimated by SDS-PAGE and Urea PAGE. SDS-PAGE of the crude antigen revealed 72, 64, 48 and 24 kDa molecular weight protein bands and similar band profile was obtained even in the Urea PAGE with sharper bands. For the purified antigen a single 72 kDa protein band was observed in both the PAGE techniques. In a similar study by d`Amelio [63] the crude antigen revealed 67, 52, 29 and 13 kDa proteins on SDS-PAGE which were completely different set of bands to that of this present study. Also Kanwar [59] reported the presence of 15 protein bands of 8–116 kDa weight in a similar study on cyst fluid antigens. Similarly, Itagaki [58] reported a slightly similar protein profile with eight protein bands of 96, 90, 66, 38, 27, 14 and 8 kDa protein bands on SDS-PAGE of hydatid cyst fluid antigens. Several authors [21, 27, 61] reported a similar protein profile with similar molecular weight protein bands in SDS-PAGE profiling of hydatid cyst fluid crude antigens. In a similar report by Hassanain [64] different protein bands of 100, 60, 49.5 and 20 kDa molecular weight were observed. Also, Pagnozzi [65] in a study on antigen 5 reported two bands of 57 and 67 kDa molecular weight, whereas 20–24 kDa bands were observed in the protein profile of antigen B.
In a similar study by Jeyathilakan [2] CIEP showed a 94.67% sensitivity and 74.67% specificity in diagnosing CE in sheep using antigen B which indicates that the sensitivity was higher than the results of present study, but the specificity values were higher in this study compared to that of Jeyathilakan [2]. A lower rate of sensitivity and specificity using crude hydatid cyst fluid were reported by Sekar [66] and Raman [35] compared to that of the results obtained in the present study. Further, Ravinder [49] reported that CIEP showed 78.5% sensitivity in diagnosing CE but the assay was quite effective as the specificity recorded was 100% in diagnosing CE in sheep and buffalo sera similar to that of the results in this study.
In a report by Maleki [67] an antigen B and another pure antigen of hydatid cyst fluid origin were evaluated with sensitivity of 86.7% and 83.35% for pure antigen and specificity values of 68.9% and 87.8% for the antigen B which were slightly lower than the results that were obtained in this present study. Between DID and CIEP all the diagnostic parameters were quite similar with the only difference being the time taken for appreciating a clear precipitation line between antigen and antibody wells. CIEP has the edge of developing a line within one hour of electrophoresis, whereas DID takes 12–24 h for a clear cut line formation. ELISA was another assay employed in this present study with appreciable sensitivity and specificity across both the species suing both the crude and purified antigens. As per the reports by Golassa [31] in a similar study, ELISA recorded 96% sensitivity and 83.3% specificity using a crude antigen in diagnosing CE in buffaloes where the reported sensitivity is more than that of the present study; however, the specificity recorded in this study is quite higher than that of Golassa [31]. Similarly, Larrieu [68] reported a higher sensitivity of ELISA (96.00%) than that of this study, but the reported specificity was low (83.33%) when compared to the specificity values obtained in this study. Similar reports were reported by Golassa [31] where IHA sensitivity was 87.20% and specificity was 80.9% in diagnosing CE in cattle which were higher than those of the results recorded in this study. Similarly, low levels of IHA sensitivity and specificity were reported by Ibrahem (69) compared to the results obtained in the present study.
Conclusion
CE is one of the most neglected parasitic zoonoses that impacts the health of both humans and animals across the globe. Early diagnosis is very crucial in determining the final impact of the disease and for an accurate and early diagnosis, immunological techniques are to be employed. HCF is a source for several antigens and in this study a crude antigen and a purified antigen were evaluated between which the purified antigen resulted in better diagnostic efficiency leading to the conclusion that a purified hydatid cyst fluid antigen would curb the cross-reactivity issues of crude antigen. Further, among the four immunoassays that were evaluated, in sheep ELISA was the most sensitive assay and IHA was the least sensitive assay. In case of buffaloes, CIEP and DID were moderately sensitive. In this regard, there is an immense scope to develop a universal immunoassay that would fit all the species that are susceptible and also an antigen with least cross-reactivity has to be developed in order to have an accurate and early diagnosis of cystic echinococcosis. The immunoassays with high specificity could be employed for mass screening of CE, whereas a further confirmation could be done with the more sensitive assays.
Availability of Data and Material
The data and material pertaining to the study would be produced on request.
References
Mandal S, Mandal MD (2012) Human cystic echinococcosis: epidemiologic, zoonotic, clinical, diagnostic and therapeutic aspects. Asian Pac J Trop Med 5(4):253–260. https://doi.org/10.1016/s1995-7645(12)60035-2
Jeyathilakan N, Basith AS, John L, Chandran ND, Dhinakarraj G (2014) Anion exchange chromatography for purification of antigen B of cystic echinococcosis. Int J Chromatogr Sep Tech 5(6):1. https://doi.org/10.4172/2157-7064.1000254
Sangaran A, Lalitha J (2009) Prevalence of hydatidosis in sheep and goats in and around Chennai. Tamilnadu J Vet Anim Sci 5(5):208–210
Eckert J, Deplazes P (2004) Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 17(1):107–135. https://doi.org/10.1128/cmr.17.1.107-135.2004
Getaw A, Beyene D, Ayana D, Megersa B, Abunna F (2010) Hydatidosis: prevalence and its economic importance in ruminants slaughtered at Adama municipal abattoir, Central Oromia. Ethiopia Acta tropica 113(3):221–225. https://doi.org/10.1016/j.actatropica.2009.10.019
Wang Q, Huang Y, Huang L, Yu W, He W, Zhong B, Li W, Zeng X, Vuitton DA, Giraudoux P, Craig PS (2014) Review of risk factors for human echinococcosis prevalence on the Qinghai-Tibet Plateau, China: a prospective for control options. Infect Dis Poverty 3(1):1–8. https://doi.org/10.1186/2F2049-9957-3-3
Regassa F, Molla A, Bekele J (2010) Study on the prevalence of cystic hydatidosis and its economic significance in cattle slaughtered at Hawassa Municipal abattoir. Ethiopia Trop Anim Health Prod 242(5):977–984. https://doi.org/10.1007/s11250-009-9517-2
Abdulhameed MF, Robertson ID, Al-Azizz SA, Habib I (2019) Neglected zoonoses and the missing opportunities for one health education: the case of cystic echinococcosis among surgically operated patients in Basrah. Southern Iraq Diseases 7(1):4. https://doi.org/10.3390/diseases7010004
WHO Fact Sheet, Echinococcosis (2020). https://www.who.int/news-room/fact-sheets/detail/echinococcosis
Shahnazi M, Jafari A, Javadi M, Saraei M (2013) Fertility of hydatid cysts and viability of protoscoleces in slaughtered animals in Qazvin. Iran J Agric Sci 5(1):141. https://doi.org/10.5539/jas.v5n1p141
Assefa H, Mulate B, Nazir S, Alemayehu A (2015) Cystic echinococcosis amongst small ruminants and humans in central Ethiopia. Onderstepoort J Vet Res 282(1):1–7. https://doi.org/10.4102/ojvr.v82i1.949
Budke CM, Deplazes P, Torgerson PR (2006) Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis 12(2):296. https://doi.org/10.3201/eid1202.050499
Singh BB, Singh G, Sharma R, Sharma JK, Aulakh RS, Gill JP (2013) Human hydatidosis: an under discussed occupational zoonosis in India. Helminthologia 50(2):87–90. https://doi.org/10.2478/s11687-013-0113-7
Rema Prathiush P, Eugene DSP, Javare Gowda KA (2008) Diagnosis of Echinococcus granulosus infection in dogs by a coproantigen sandwich ELISA. Veterinarski arhiv. 78(4):297–305
Sangaran A, Arunkumar S, John L (2014) Incidence of hydatidosis in slaughtered sheep and goats. Indian J Vet Anim Sci Res 2:156–158
Nikale NV (2014) Prevalence of echinococcosis in dogs and humans. MAFSU, Nagpur
Md Khader Faheem N, Nusrath N, Syama Sundara Rao B, Raja Ram G, Sushma C, Subramanyam Y, Ramesh K (2013) The scenario of Hydatid cyst disease in epidemic areas of Andhra Pradesh—evaluation and analysis. Int J Res Dev Health. 1(3):120–128
Giri A, Giri M, Giri BK (2012) Hydatidosis/cystic. Emerging and re-emerg. Infect Dis 30:112. https://doi.org/10.4103/2F2229-5070.105174
Constantea N, Ciobanca P (2007) Studiul clinic pentru imunatatirea metodelor de diagnostic de laborator si profilaxia chistului hidatic. Rev Rom Parasitol 17:48–49
Doiz O, Benito R, Gil J, Rojas A, Carmen Rubio M, Osuna A (2002) Pre-and postsurgical detection of IgG, IgM, and IgA specific to hydatidosis by ELISA with purified antigen enriched with the 5/B antigen complex. J Clin Lab Anal 16(6):295–298. https://doi.org/10.1002/jcla.10056
Zhang W, Li J, McManus DP (2003) Concepts in immunology and diagnosis of hydatid disease. Clin Microbiol Rev 16(1):18–36. https://doi.org/10.1128/2FCMR.16.1.18-36.2003
Oriol R, Williams JF, Pérez E, Oriol C (1971) Purification of lipoprotein antigens of Echinococcus granulosus from sheep hydatid fluid. Am J Trop Med Hyg 20(4):569–574. https://doi.org/10.4269/ajtmh.1971.20.569
Pozzuoli R, Musiani P, Arru E, Patrono C, Piantelli M (1974) Echinococcus granulosus: evaluation of purified antigens’ immunoreactivity. Exp Parasitol 35(1):52–60. https://doi.org/10.1016/0014-4894(74)90006-x
Piantelli M, Pozzuoli R, Arru E, Musiani P (1977) Echinococcus granulosus: identification of subunits of the major antigens. The J Immunol Res 119(4):1382–1386
Musiani P, Piantelli M, Lauriola L, Arru E, Pozzuoli R (1978) Echinococcus granulosus: specific quantification of the two most immunoreactive antigens in hydatid fluids. J Clin Path 31(5):475–478. https://doi.org/10.1136/jcp.31.5.475
Kanwar JR, Kaur Kanwar R, Grewal AS, Vinayak VK (1994) Significance of detection of immune-complexed 8 kDa hydatid-specific antigen for immunodiagnosis of hydatidosis. FEMS Immunol Med Microbiol 9(3):231–236. https://doi.org/10.1111/j.1574-695X.1994.tb00498.x
Burgu A, Doğanay A, Gönenç B, Sarimehmetoğlu H, Kalinbacak F (2000) Analysis of fluids of hydatid cysts from sheep by SDS-PAGE, and determination of specific antigens in protein structure by western blotting. Turkish J Vet Anim Sci 5:493–500
Rogan MT, Craig PS, Zeyhle E, Romig T, Lubano GM, Deshan L (1991) Evaluation of a rapid dot-ELISA as a field test for the diagnosis of cystic hydatid disease. Trans R Soc Trop Med Hyg 85(6):773–777. https://doi.org/10.1016/0035-9203(91)90451-4
Sbihi Y, Janssen D, Osuna A (1996) Serologic recognition of hydatid cyst antigens using different purification methods. Diagn Microbiol Infect Dis 24(4):205–211. https://doi.org/10.1016/0732-8893(96)00061-2
Shirazi S, Madani R, Hoghooghi Rad N, Ranjbar Bahadori S (2016) Isolation and purification of Echinococcus granulosus antigen B from hydatid cyst fluid using three different methods. Arch Razi Inst 71(2):103–108. https://doi.org/10.22034/ari.2016.106448
Golassa L, Abebe T, Hailu A (2011) Evaluation of crude hydatid cyst fluid antigens for the serological diagnosis of hydatidosis in cattle. J Helminthol 85(1):100–108. https://doi.org/10.1017/S0022149X10000349
Craig PS, Hocking RE, Mitchell GF, Rickard MD (1981) Murine hybridoma-derived antibodies in the processing of antigens for the immunodiagnosis of hydatid (Echinococcus granulosus) infection in sheep. Parasitology 83(2):303–317. https://doi.org/10.1017/s0031182000085310
Kittelberger R, Reichel MP, Jenner J, Heath DD, Lightowlers MW, Moro P, Ibrahem MM, Craig PS, O’Keefe JS (2002) Evaluation of three enzyme-linked immunosorbent assays (ELISAs) for the detection of serum antibodies in sheep infected with Echinococcus granulosus. Vet Parasitol 110(1–2):57–76. https://doi.org/10.1016/S0304-4017(02)00308-4
Gómez FM, Rodriguez SH, López-Cózar IN, Carretero RC (1980) Serological tests in relation to the viability, fertility and localization of hydatid cysts in cattle, sheep, goats and swine. Vet Parasitol 7(1):33–38. https://doi.org/10.1016/0304-4017(80)90007-2
Raman M, Chellappa DJ (1998) Serodiagnosis of hydatidosis in sheep by counter immunoelectrophoresis in Chennai, India. Indian J Anim Sci 68(11):1169–1170
Shepherd JC, McManus DP (1987) Specific and cross-reactive antigens of Echinococcus granulosus hydatid cyst fluid. Mol Biochem Parasitol 25(2):143–154. https://doi.org/10.1016/0166-6851(87)90003-x
Mohanty S, Behera B, Sasmal PK, Praharaj AK (2016) Staining of hydatid elements: a useful adjunct in the diagnosis of hydatid disease. Trop Doct 46(3):174–176. https://doi.org/10.1177/0049475515613240
Thompson SW, Hunt RD (1966) Selected histochemical and histopathological methods
Patanvadia D, Kruwala Y, Lakhani S, Date V, Lakhani J (2011) Hydatid cyst in the spleen: a rare presentation. Indian J Med Microbiol 29(2):192. https://doi.org/10.4103/0255-0857.81778
Zamani Z, Assmar M, Pyazak N, Wafaie K, Assadian M, Amirkhani A (2001) A novel method of purification of specific hydatid cyst antigen. Med J Islam Repub Iran 15(1):49–53
Sbihi Y, Gil JR, Alvarez PA, Orduna A, Rodríguez-Torres A, Osuna A (2003) Development of a dipstick dye immunoassay for diagnosing hydatidosis. J Clin Lab Anal 17(6):219–222. https://doi.org/10.1002/2Fjcla.10097
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1–2):248–254. https://doi.org/10.1006/abio.1976.9999
Luiz Sanchez (2001). TCA protein precipitation protocol. www.its.caltach.edu. Protocols.
Microsoft word—TR0049.1doc; thermofisher.com
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Rivera CE, Rosales JD, Freites-Perez JC, Rodriguez E (2018) Very low molecular weight proteins electrophoresis protocol. Bio-Protoc 20:e3093. https://doi.org/10.21769/BioProtoc.3093
Vatankhah A, Assmar M, Shokrgozar MA, Hoseini ST, Rastaghi AE (2004) Introduction of an indirect haemagglutination test as a rapid diagnostic method in comparison with Elisa using antigen b for diagnosis of human hydatid disease. Iran J Public Health 33(4):16–25
Hudson L, Hay FC (1989) Practical immunology, 3rd edn. Backwell Scientific, Hoboken
Ravinder PT, Parija SC (1997) Counter current immuno electrophoresis test for detection of hydatid antigen in the fluid from hydatid cysts: a preliminary report. Acta Trop 66(3):169–173. https://doi.org/10.1016/s0001-706x(97)00036-3
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci 76(9):4350–4354. https://doi.org/10.1073/pnas.76.9.4350
Njeruh FM, Okelo GB, Gathuma JM (1989) Usefulness of indirect haemagglutination (IHA) and enzyme-linked immunosorbent assay (ELISA) in the diagnosis of human hydatidosis. East Afr Med J 66(5):310–314
Kagan IG, Agosin M (1968) Echinococcus antigens. Bull World Health Organ 39(1):13
Hira PR, Bahr GM, Shweiki HM, Behbehani K (1990) An enzyme-linked immunosorbent assay using an arc 5 antigen for the diagnosis of cystic hydatid disease. Ann trop med parasitol 84(2):157–162. https://doi.org/10.1080/00034983.1990.11812449
Tabatabai M, Ismaili MH, Sami M, Fardin R, Kadivar R (1975) Effect of ovine hydatid cyst fluid on the cardiovascular and respiratory systems in sheep. Ann Parasitol Hum Comp 50(1):7–15. https://doi.org/10.1051/parasite/1975501007
Clavel A, Varea M, Doiz O, López L, Quílez J, Castillo FJ, Rubio C, Gomez-Lus R (1999) Visualization of hydatid elements: comparison of several techniques. J Clin Microbiol 37(5):1561–1563. https://doi.org/10.1128/2Fjcm.37.5.1561-1563.1999
Abdel-Baki AA, Almalki E, Al-Quarishy S (2018) Prevalence and characterization of hydatidosis in Najdi sheep slaughtered in Riyadh city. Saudi Arabia Saudi J Biol Sci 25(7):1375–1379. https://doi.org/10.1016/j.sjbs.2018.04.011
Panda R, Kommu S, Gaddam R (2017) Hepatic hydatid cysts in sheep and visualization of hydatid elements. J Pharm Innov 6(10):13
Itagaki T, Sakamoto T, Berasain P, Maisonnave J, Yarzabal L (1994) Immunoblot analysis of hydatid cyst fluid antigens using sera of unilocular hydatidosis in cattle and sheep. J. Fac. Agric. Lwate Uni. 22(1):25–30. https://doi.org/10.4103/2F1735-1995.196612
Kanwar JR, Kaushik SP, Sawhney IM, Kamboj MS, Mehta SK, Vinayak VK (1992) Specific antibodies in serum of patients with hydatidosis recognised by immunoblotting. J med Microbiol 36(1):46–51. https://doi.org/10.1099/00222615-36-1-46
Nasrieh MA, Abdel-Hafez SK (2004) Echinococcus granulosus in Jordan: assessment of various antigenic preparations for use in the sero diagnosis of surgically confirmed cases using enzyme immuno assays and the indirect haemagglutination test. Diagn Microbiol Infect Dis 48(2):117–123. https://doi.org/10.1016/j.diagmicrobio.2003.09.018
Rajabiyoun M, Hashemitabar GR, Tavakool Afshari J (2006) Detection of hydatid fluid and protoscolices antigens in sheep with hydatidosis. Iran. J. Vet. Res. 7(2):59–64. https://doi.org/10.22099/ijvr.2006.2664
Fadwa M, Yaman AIA, Knobloch M (1989) Isolation and partial characterization of species specific and cross reactive antigens of Echinococcus granulosus cyst fluid. I Mol Biochem 37:101–108. https://doi.org/10.1016/0166-6851(89)90106-0
d’Amelio R, Pontesilli O, Dayal R, De Rosa F, Barnet M, Teggi A, Brighouse G, Lambert PH (1985) Characterization of parasite antigens from human hydatid cyst fluid by SDS-PAGE and IEF. Med Microbiol Immunol 174(1):43–50. https://doi.org/10.1007/BF02123670
Hassanain MA, Shaapan RM, Khalil FA (2016) Sero-epidemiological value of some hydatid cyst antigen in diagnosis of human cystic echinococcosis. J. Paras. Dis. 40(1):52–56. https://doi.org/10.1007/2Fs12639-014-0443-5
Pagnozzi D, Biosa G, Addis MF, Mastrandrea S, Masala G, Uzzau S (2014) An easy and efficient method for native and immunoreactive Echinococcus granulosus antigen 5 enrichment from hydatid cyst fluid. PLoS ONE 9(8):e104962. https://doi.org/10.1371/2Fjournal.pone.0104962
Sekar M, Appaji Rao VN, Ramadass P, Raghavan N (1989) Comparative study of serological diagnostic test in hydatidosis in sheep. Cheiron 18:10–14
Maleki F, Sarafpoor S (2014) Assessment of pure and B hydatid cyst fluid antigens for the diagnosis of hydatidosis. J Gorgan Univ Med Sci 16(2):74–81
Larrieu E, Costa MT, Cantoni G, Alvarez R, Cavagion L, Labanchi JL, Bigatti R, Araya D, Herrero E, Alvarez E, Mancini S (2001) Ovine Echinococcus granulosus transmission dynamics in the province of Rio Negro, Argentina, 1980–1999. Vet Paras 98(4):263–272. https://doi.org/10.1016/s0304-4017(01)00442-3
Ibrahem MM, Craig PS, McVie A, Ersfeld K, Rogan MT (1996) Echinococcus granulosus antigen B and seroreactivity in natural ovine hydatidosis. Res Vet Sci 61(2):102–106. https://doi.org/10.1016/s0034-5288(96)90082-x
Acknowledgements
The corresponding author would like to thank the Department of Veterinary Public Health and Epidemiology, Department of Veterinary Parasitology, Department of Veterinary Microbiology and State Level Diagnostic Laboratory Facility and College of Veterinary Science, Tirupati for their assistance along the course of this study.
Funding
This research study has been funded by the Department of Veterinary Public Health and Epidemiology, College of Veterinary Science and Sri Venkateswara Veterinary University, Tirupati, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors of this paper declare that there is no conflict of interest.
Ethical Approval
The entire protocol and procedures were presented to the Institutional Animal Ethics Committee (IAEC) for approval. The methods followed in this study were ethically reviewed and approved by the committee.
Consent to Participate
All the participants in the survey have given an oral consent agreeing to take the survey.
Consent for Publication
The authors hereby declare consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siddartha, P.V., Babu, A.J., Rao, T.M. et al. A Comparative Evaluation of Four Different Immunoassays in the Diagnosis of Cystic Echinococcosis Using a Crude and Purified Hydatid Cyst Fluid Antigen. Acta Parasit. 67, 1667–1679 (2022). https://doi.org/10.1007/s11686-022-00614-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-022-00614-5