Abstract
The dog tapeworm Echinococcus granulosus is the causative agent of cystic hydatid disease in domestic/wild herbivores animals and man. Accurate immunodiagnosis of the infection requires highly specific and sensitive antigens. The aim of this study was to develop and evaluate immunoassays with principles of precipitation, agglutination for the identification of buffaloes infected with hydatid cyst which would allow the monitoring of animals from endemic areas and identifying infected animals prior to slaughter. The immunoassays were developed and validated using hydatid specific, non-cross reactive low molecular weight 8 kDa hydatid cyst fluid protein. Sera used for the assay validations were obtained from 200 buffaloes infected naturally with hydatid cyst and 200 non-infected buffaloes. The diagnostic sensitivity with latex agglutination test was 98.67 %. It should be useful for the conformation of hydatid cyst infected individual sheep.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystic echinococcosis (CE) is a zoonotic parasitic infection of many mammalian species caused by the larvae of Echinococcus granulosus which is a small tapeworm. The definitive hosts which include dogs, hyenas, jackals and other canids carry the adult tapeworms sub clinically. Dogs are particularly important in zoonotic transmission due to their close association with human beings. Intermediate hosts affected with the larval stage of the tapeworm, hydatid cysts are initially asymptomatic; however, the growth of the larvae, which form cysts in vital organs such as the liver and lungs, can lead to illness, decreased production and possibly death (Moro and Schantz 2009). It causes severe economic loss and public health problem to both human beings and livestock in many temperate and tropical areas of the world including India. The global annual monetary loss due to CE in man has accounted for US$ 193, 529, 740 (Budke 2006). The cases of CE in humans and domesticated animals such as sheep, cattle, buffaloes, pigs and wild animals are being increasingly reported from different parts of India including Tamil Nadu, India (Parija and Sheela Devi 1999; Raman and John 2003). In livestock, infection with hydatid cyst is asymptomatic and diagnosis is made usually at necropsy. Lahmar et al. (2007) reported ultra-sonography in animals, but a precise diagnosis of CE was not possible. CE serology has a very long history and almost all serological assays have been used in the diagnosis of disease.
Serology is the method generally used for prevalence surveys in developing countries like India. Development of an inexpensive accurate serological assay could be of importance as a surveillance tool for diagnosis and seroepidemiology of hydatidosis in animals. In addition, such an assay could serve as a screening instrument for live animals prior to export and in the identification and elimination of isolated focal reservoirs of infection during the consolidation phase of control programme (Dueger et al. 2003). Antibody detection remains the method of choice for diagnosis of hydatid cyst in buffaloes. Since the detection of circulating Echinococcus granulosus antigens in sera of animals is less sensitive and more time consuming than anti-body detection (Parija 1998).Indirect haemagglutination test (Golassa et al. 2011), counter immunoelectrophoresis (Raman and Chellappa 1998), ELISA (Craig and Rickard 1981; Kittelberger et al. 2002; Gatti et al. 2007), latex agglutination test (Gomez et al. 1980) and EITB (Dalmasso et al. 2012) are the immuno diagnostic methods used in animals. Various immunodiagnostic tests for hydatidosis in man and animals have been attempted in India, including Tamil Nadu, India (Dhar et al. 1996; Parija 1998; Raman and Chellappa 1998; Jeyathilakan 2007) using hydatid cyst fluid antigens with varied sensitivity and specificity.
However these assays using crude hydatid antigens have been non-specific due to cross reaction with Cysticercus, Coenurus and other helminthic infections (Shepherd and McManus 1987; Siracusano and Bruschi 2006). In order to overcome these difficulties various novel tests using purified antigens are essential for confirmative diagnosis of hydatidosis in man and animals. Currently, the antigen B, hydatid cyst fluid and these properties have encouraged the preferential use of this antigen over other hydatid antigens, in the sero diagnosis of CE (Maddison et al. 1989; Fernandez et al. 1996; Mamuti et al. 2006; Jiang et al. 2012). Native antigens and their purified subunit fractions are more reliable for serodiagnostic purposes than recombinant proteins and synthetic peptides (Zhang et al. 2012). Hence the present study was envisaged to evaluate latex agglutination test (LAT) using native hydatid specific non cross reactive 8 kDa cyst fluid antigen for diagnosis of cystic echinococcosis in Buffaloes.
Materials and methods
Antigen preparation
Hydatid cysts for this study were collected from Buffaloes slaughtered at Corporation Slaughter House in Perambur, Chennai, India. From the collected hydatid cysts fluid was aspirated fluid was pooled together and kept in a glass beaker for settling of brood capsules, protoscolices and dead tissues. The supernatant was collected by centrifugation at 10,000 rpm for 30 min poured into a 1000 Da cut off membrane (Sigma, USA) and dialysed against three changes of distilled water at 4 °C. concentrated using polyethylene glycol-6000 (SRL, India). The immunodominant 8 kDa antigen was prepared from hydatid cyst fluid by anion exchange chromatography using DEAE-Sepharose fast flow as per the method described by Gonzalez et al. (1996) with minor modifications. The hydatid cysts were collected and processed as mentioned above.
The conductivity of hydatid fluid was adjusted with conductivity buffer followed by antigen fractions were eluted with elution buffer (Jeyathilakan 2007). The fractions were extensively dialysed against phosphate buffered saline (pH 7.2) and concentrated with polyethylene glycol-6000. The concentrated protein was antigen B. The protein content of concentrated antigen B was estimated as per Smith et al. (1985).
The antigen B was resolved in 12.5 % SDS-PAGE (Laemmli 1970) to identify the 8 kD a protein band. The 8 kDa protein band strips were excised from gels. They were immersed in 2 % glutaraldehyde for 60 min. The strips were destained completely at 4 °C and pulverized with PBS (pH 7.2). The material was centrifuged at 15 000 rpm at 4 °C for 30 min. The supernatant was collected. The procedure was repeated many times to collect 8 kDa antigen (Jeyathilakan 2007). The pools of supernatant were concentrated by polyethylene glycol with dialysis tubing (cut of 1000 Da, Sigma, USA). The 8 kDa protein content was estimated as per Smith et al. (1985).
Hyperimmune sera were raised against hydatid cyst fluid antigen, 8 kDa antigen in adult New Zealand white rabbits (3–4 kg). Eight rabbits were divided into four groups and each antigen was given to two rabbits. The antigens were prepared by emulsifying 1 ml of purified protein (1 mg/ml) with 1 ml of Freund’s complete adjuvant (Sigma, USA). The rabbits were given 0.5 ml of this emulsion in all the four limbs intramuscularly. After 4 weeks, the rabbits were boosted with 0.5 ml emulsion of antigen and Freund’s incomplete adjuvant mix in all the limbs intramuscularly. The rabbits were bled by ear vein puncture 10 days after the last injection. About 5 ml of blood was collected from each rabbit and sera separated at 4 °C. The hyper immune sera collected were tested for antibody titer and stored in 100 µl aliquots with Merthiolate as preservative at −20 °C. Before inoculation of antigen, the rabbits were bled by ear vein puncture to obtain known negative control sera and stored at −20 °C with preservative.
Serum samples
The serum samples for the evaluation of immunological assays were collected from Buffaloes slaughtered at Perambur Slaughter House, Chennai, India. A total of 200 known positive serum samples were collected from sheep showing the presence of hydatid cysts in visceral organs and 200 known negative serum samples collected from hydatid cyst free sheep and kept as gold standards. The hyper immune serum raised in rabbit against 8 kDa antigen and normal rabbit serum was kept as positive control and negative control respectively.
Western blot analysis
The purity of the 8 kDa protein was tested by Western blot with hyperimmune sera raised against hydatid cyst fluid antigen. SDS-PAGE (12.5 %) of 8 kDa protein from hydatid cyst fluid was carried out on a mini protein-3 electrophoresis apparatus (Biorad, USA) using 1 mm thickness gel using a discontinuous system as described by Laemmli (1970). The 8 kDa protein bands were then transferred to PVDF membrane as described by Towbin et al. (1979) using Mini Trans-Blot Electrophoretic Transfer Cell (Biorad, USA). PVDF membranes with resolved 8 kDa protein were incubated with hyperimmune sera raised against hydatid cyst fluid antigen. The PVDF membranes were probed with 1:1000 anti rabbit IgG HRP conjugate (Sigma, USA) for 1 h at 37 °C. The membranes were again washed three times with washing buffer for 5 min each and treated with substrate diaminobenzidine solution till the appearance of reaction.
Latex agglutination test (LAT)
Latex agglutination test was carried out as per the method described by Dey et al. (2007) with some modifications.
Ten per cent suspension of dyed latex particle coated with Hydatid cyst fluid antigen (25 mg/ml) using 0.06 m carbonate–bicarbonate buffer, pH 9.6 kept at 37 ° C for 6 h with constant shaking. Sensitized bead centrifuged at 6800×g for 3 min and pellet resuspended as 1 % suspension in phosphate buffer saline containing 5 mg/ml of bovine serum albumin. Latex beads were left at 37 °C overnight with constant shaking. Latex beads centrifuged as before and pellet resuspended in PBS contain 0.5 mg/ml of BSA and 0.1 % sodium azide as 0.25 % suspension. Latex agglutination test was carried out by taking 20 μl of serum and 20 μl of latex sensitized beads coated antigen were placed in a slide and they were mixed with toothpick, slide was rotated for 5 min and observed for the reaction. Agglutination of latex particles within 2–3 min of the test was considered as positive and when latex particle remains as a homogenous suspension, the samples were considered negative.
Result and discussion
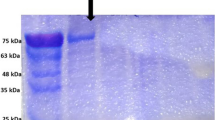
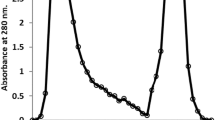
On characterisation Hydatid cyst fluid antigen three different bands were obtained (Fig. 1). The protein content was 0.987 mg/ml. Purification of hydatid cyst fluid was carried out isolated single 8 kDa (Fig. 2).Western blot analysis was carried (Fig. 3). These tests were evaluated with 8 kDa antigen, positive control, negative control, 200 hydatid positive buffalo sera and 200 hydatid negative buffalo sera using Latex Agglutination Test. Positive of LAT indicated by agglutination (Fig. 4). The sensitivity, specificity, positive predictive value, negative predictive value and efficiency of the tests were assessed. LAT showed 98.67, 88.00, 89.16, 98.51 and 93.33 % sensitivity, specificity, positive predictive value, negative predictive value and efficiency respectively (Table 1).
Hydatid cyst fluid (HCF) is a complex mixture of glycol lipoproteins, carbohydrates and salts. Crude HCF has a high sensitivity, ranging typically from 75 to 95 % (Zhang et al. 2012). However, its specificity is often unsatisfactory and cross-reactivity with sera from patients infected with other cestode (89 %), nematode (39 %) and trematode (30 %) species is commonly observed (Eckert and Deplazes 2004). Hence, the crude HCF is specifically recommended for mass serological screening and it has now become more frequent to purify components such as the lipoproteins antigen B and antigen 5, the most relevant components of HCF for diagnostic purposes. The 8 kDa antigen has been shown to be hydatid specific. Antigen B which comprises 8 and 24 kDa may have the opportunity to accumulate in the cyst fluid after being secreted by the parasite in such a way that the protein has the chance to aggregate into a form that is more immunogenic before the antigen gains contact with the host immune system (Mamuti et al. 2006).
Various authors have used different protocols to isolate 8 kDa antigen from hydatid cyst fluid (Kanwar and Kanwar 1994; Ioppolo et al. 1996; Ibrahem et al. 1996; Ito et al. 1999; Kittelberger et al. 2002), but the quantity of antigen available from the above methods was scanty. Therefore the method described by Gonzalez et al. (1996) using DEAE-Sepharose fast flow was followed and it resulted in production of a large quantity of antigen. The hydatid cyst fluid antigen was purified by anion exchange chromatography using DEAE-Sepharose fast flow in the present study.
The results of the present study were higher than those of Dueger et al. (2003) who observed 91.4 % sensitivity. In another study Moro et al. (1997) found 73 % sensitivity and 89 % specificity and Simsek and Koroglu (2004) reported 88 % sensitivity and 84 % specificity. The variation of sensitivity and specificity in diagnosing CE in sheep could be due to strain variation, nature of antigen, level of antibody in the serum etc. (Dueger et al. 2003).The false positive diagnosis was due to unspecific granulomas, pseudotuberculosis, emphysema and fatty degeneration and false negative diagnoses were due to small intra parenchymal cysts (Larrieu et al. 2001). The selective expression of EgAgB 8 kDa monomers in different hosts and/or different level of host immune response.
It is possible that the differential expression of AgB 8 kDa monomers in different developmental stages of the parasite might be relevant to the different biological functions of each individual monomer in the host parasite interactions (Mamuti et al. 2006). Further, the antigen B, 8 kDa is a highly immunogenic major component of hydatid cyst fluid and these properties have encouraged the preferential use of this antigen over other hydatid antigens in the serodiagnosis of CE (Fernandez et al. 1996; Mohammadzadeh et al. 2012). LAT assay can be applied to practical use for screening studies and for the detection of the infected buffalo for surveillance and evaluating control programmes.
References
Budke CM (2006) Global socio economic impact of cystic echinococcosis. Emerg Infect Dis 12:296–303
Craig PS, Rickard MD (1981) Studies on the specific immunodiagnosis of larval cestodes infections of cattle and sheep using antigens purified by affinity chromatography in an enzyme linked immunosorbent assay (ELISA). Int J Parasitol 11:441–449
Dalmasso RL, Molinar Min AR, Gennero S, Stella MC, Ram-bozzi L (2012) Seroprevalence of cystic echinococcosis in small small ruminants from hypoendemic Northern Italy. Small Ruminant Res 106:S18–S20
Dey S et al (2007) Recombinant Ag based latex agglutination test for rapid sero diagnosis of leptospirosis. Vet Res Commun 31:9–15
Dhar S, Singh BP, Raina OK (1996) Hybridoma derived antibodies for the diagnosis of Echinococcus granulosus infection. J Vet Parasitol 10:153–157
Dueger EL, Verastgui M, Gilman RH (2003) Evaluation of enzyme linked immuno electro transfer blot for ovine hydatidosis relative to age and cyst characteristic in naturally infected sheep. Vet Parasitol 114:284–293
Eckert J, Deplazes P (2004) Biological, epidemiological and clinical aspects of echinococcosis, a zoonoses of increasing concern. Clin Microbiol Rev 17:107–135
Fernandez V, Ferreira HB, Fernandez C, Zaha A, Nieto A (1996) Molecular characterisation of a novel 8-kDa subunit of Echinococcus granulosus antigen B. Mol Biochem Parasitol 77:247–250
Gatti A, Alvarez AR, Araya R, Mancini S, Herrero E, Santillan G, Lar-rieu E (2007) Ovine echinococcosis I. Immunological diagnosis by enzyme immunoassay. Vet Parasitol 143:112–121
Golassa L, Abebe T, Hailu A (2011) Evaluation of crude hydatid cyst fluid antigens for the serological diagnosis of hydatidosis in cattle. J Helminthol 85:100–108
Gomez FM, Rodriguez SH, Lopez-Cozar IN, Carretero RC (1980) Sero-logical tests in relation to the viability, fertility and localization of hydatid cysts in cattle, sheep, goats and swine. Vet Parasitol 7:33–38
Gonzalez G, Nieto A, Fernandez C, Orn A, Wernstedet C, Hellman U (1996) Two different 8 kDa monomers are involved in the oligomeric organization of the native Echinococcus granulosus antigen B. Parasite Immunol 18:587–596
Ibrahem MM, Craig PS, McVIE A, Ersfeld K, Rogan MT (1996) Echinococcus granulosus antigen B and seroreactivity in natural ovine hydatidosis. Res Vet Sci 61:102–106
Ioppolo S, Notargiacomo S, Profumo E, Franchi C, Ortona E, Rig-ano R, Siracusano A (1996) Immunological responses to antigen B from Echinococcus granulosus cyst fluid in hydatid patients. Parasite Immunol 18:571–578
Ito A, Liang MA, Schantz PM, Gottstein B, Liu YH, Chai JJ, Sami K, Nazmiye AB, Joshi DD, Lightowlers MW, Pawlowski ZS (1999) Differential serodiagnosis for cystic and alveolar echinococcosis using fractions of Echinococcus granulosus cyst fluid (antigen B) and E. mul-tiiocularis protoscolex (EM18). Am J Trop Med Hyg 60:188–192
Jeyathilakan N (2007) Antigenic profile and immunodiagnosis of cystic echinococcosis. Ph.D. Thesis submitted to Tamil Nadu Veterinary and Animal Sciences University, Chennai
Jiang L, Zhang YG, Liu MX, Feng Z (2012) Analysis on the reactivity of five subunits of antigen B family in serodiagnosis of echinococcosis. Exp Parasitol 131:85–91
Kanwar JR, Kanwar R (1994) Purification and partial immunochemical characterization of a low molecular mass, diagnostic Echinococcus granulosus immunogen for sheep hydatidosis. FEMS Immunol Med Microbiol 9:101–108
Kittelberger R, Reichel MP, Jenner J, Heath DD, Lightowlers MW, Moro P, Ibrahem MM, Craig PS, O’Keefe JS (2002) Evaluation of three enzyme linked immunosorbent assay (ELISAs) for the detection of serum antibodies in sheep infected with Echinococcus granulosus. Vet Parasitol 110:57–76
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of Bacteriophage T4. Nature 227:680–685
Lahmar S, Chehida FB, Petavy AF, Hammou H, Lahmar J, Ghanny A, Gharbi H, Sarciron AME (2007) Ultra sonographic screening for cystic echinococcosis in sheep in Tunisia. Vet Parasitol 143:42–49
Larrieu E, Costa M, Cantoni G, Alvarez R, Cavagion L, Labanchi J, Bigatti R, Raya D, Herrero E, Mancini S, Cabrera A (2001) Ovine Echinococcus granulosus transmission dynamics in the province of RioNegro, Argentina. Vet Parasitol 98:263–272
Maddison SE, Slemenda SB, Schantz PM, Fried JA, Wilson M, Tsang VCW (1989) A specific diagnostic antigen of Echinococcus granulosus with an apparent molecular weight of 8 kDa. Am J Trop Med Hyg 40:377–383
Mamuti W, Sako Y, Nakao M, Xiao N, Nakaya K, Ishikawa Y, Yamasaki H, Lightowlers MW, Ito A (2006) Recent advances in characterisation of Echinococcus antigen B. Parasitol Int 55:S57–S62
Mohammadzadeh T, Sakob Y, Sadjjadia SM, Sarkaric B, Ito A (2012) Comparison of the usefulness of hydatid cyst fluid, native antigenB and recombinant antigen B8/1 for serological diagnosis of cystic echinococcosis. Trans R Soc Trop Med Hyg 106:371–375
Moro P, Schantz PM (2009) Echinococcosis: a review. Int J Infect Dis 13:125–133
Moro P, Verastegui M, Gilman RH, Fallon N, Bernal T, Gavidia C, Gorzale ZH, Malgni V, Morris HR, Duegar E (1997) Enzyme linkedimmunoelectro transfer blot assay for diagnosis of hydatidosis in sheep. Vet Rec 140:605–606
Parija SC (1998) A review of some simple immuno assays in the serodiagnosis of cystic hydatid disease. Acta Trop 70:17–24
Parija SC, Sheela Devi C (1999) Current concepts in the diagnosis of cystic echinococcosis in humans and livestock and intestinal echinococcosis in canine hosts. J Vet Parasitol 13:93–102
Raman M, Chellappa DJ (1998) Sero diagnosis of hydatidosis in sheep by counter immuno electrophoresis in Chennai, India. Ind J Anim Sci 68:1169–1170
Raman M, John L (2003) Prevalence of hydatidosis in sheep and goats in Chennai, India. Ind J Anim Res 37:57–58
Shepherd JC, McManus DP (1987) Specific and cross-reactive antigens of Echinococcus granulosus hydatid cyst fluid. Mol Biochem Parasitol 25:143–154
Simsek S, Koroglu E (2004) Evaluation of enzyme linked immunosorbent assay (ELISA) and enzyme linked immunoelectrotransfer blot (EITB) for immunodiagnosis of hydatid diseases in sheep. Acta Trop 92:17–24
Siracusano A, Bruschi F (2006) Cystic echinococcosis: progress and limits in epidemiology and immunodiagnosis. Parassitologia 48(65L):6
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Proven-zano MD, Fuji Moto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Towbin H, Staehelin T, Gordon J (1979) Electrophorectic transfer of proteins from polyacrylanide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Zhang W, Wen H, Li J, Lin R, McManus DP (2012) Immunology and immunodiagnosis of cystic echinococcosis: an update. Clin Develop Immuno. doi:10.1155/2012/101895
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sheeba, A., Sangaran, A. & Latha, B.R. Diagnosis of cystic echinococcosis in buffaloes by native 8 kDa antigen using latex agglutination test (LAT). J Parasit Dis 40, 1401–1405 (2016). https://doi.org/10.1007/s12639-015-0700-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12639-015-0700-2