Abstract
Background
Cancer-associated fibroblasts (CAFs) consist of heterogeneous cell population in terms of their differentiation potential. The functional differences in tumor progression between CAFs with mesenchymal stem/progenitor cells (MSCs/MPCs) characteristics and CAFs without MSCs/MPCs characteristics are not clarified.
Methods
CAFs and vascular adventitial fibroblasts (VAFs, which contain MSCs/MPCs) were isolated from nine primary lung cancers and were cultured in osteogenic or adipogenic medium to assess their multi-lineage differentiation. Next, we established nine single-cell-derived clones from the primary culture of CAFs and examined their differentiation potential. The effects of each single-cell-derived clone on the proliferation and migration of lung adenocarcinoma cell line, A549, were analyzed.
Results
The nine samples of VAFs and CAFs showed various degrees of osteogenic differentiation. Although the VAFs displayed the ability to undergo adipogenic differentiation, all cases of the CAFs did not. CAFs clones presented varying degrees of osteogenic differentiation. Four clones displayed comparable levels of osteogenic potential with that of the VAFs, and two clones were completely negative. As compared to the CAFs clones that possessed lower osteogenic potential, CAFs clones with higher osteogenic potential did not confer proliferative activity in A549 cells. On the contrary, these clones significantly promoted the migration of A549 cells as compared to the clones with lower osteogenic potential.
Conclusion
Our studies clearly indicate that CAFs derived from lung cancer are heterogeneous population that consists of cells with varying osteogenic potentials and that CAFs with higher osteogenic potential have a greater tumor-promoting function through the enhancement of cancer cell migration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibroblasts are stromal cells, which constitute organ structures together with epithelial cells. The functions and gene expressions of fibroblasts are heterogeneous, but not uniform while their morphologies are same (Koumas et al. 2001). It has been reported that various kinds of fibroblasts with diverse differentiation potentials intermix even within the same organ (Nombela-Arrieta et al. 2011). Mesenchymal stem cells (MSCs) are the most undifferentiated, multipotent cells that can differentiate into several cell types, including osteoblasts, adipocytes, and chondrocytes. However, mesenchymal progenitor cells (MPCs) are more restricted in their differentiation and proceed to form more particular cell types as compared to MSCs. Furthermore, fibroblasts that have no differentiation potentials also exist.
MSCs/MPCs are known to exist in various tissues (Bieback et al. 2004; Campagnoli et al. 2001; Hoshino et al. 2008; Toma et al. 2001). Therefore, MSCs are expected to be a more convenient biomaterial for regenerative therapy. The frequency of MSCs/MPCs within fibroblast population is presumed to differ depending on the kind of tissues (Riekstina et al. 2009). However, specific surface markers to distinguish between MSCs, MPCs, and fibroblasts without their differentiation potentials are not yet determined (Lv et al. 2014).
An issue worth mentioning is that mixed cell populations were used in some previous studies, which made it difficult to confirm whether there were specific MSCs/MPCs within the population (Pevsner-Fischer et al. 2011). Thus, in order to reveal the frequency and existence of MSCs/MPCs in a cell population, analysis using single-cell-derived clones should be necessary (Muraglia et al. 2000; Okamoto et al. 2002).
Within cancer microenvironments, fibroblasts form the main compartment of host stromal cells, and are called cancer-associated fibroblasts (CAFs). CAFs directly communicate with the cancer cells and acquire a specific biological phenotype that plays an important role in tumorigenesis and tumor progression (Cirri and Chiarugi 2012; Ishii et al. 2015; Xing et al. 2010). CAFs consist of fibroblasts from different origins and represent heterogeneous tumor-related functions (Bauer et al. 2010; Sugimoto et al. 2006). MSCs are known to be recruited into cancer tissues and can be one component of the CAFs. MSCs have been reported to have several functions in tumor progression, including proliferation (Scherzad et al. 2015), migration (Karnoub et al. 2007; Martin et al. 2010), oxidant stress resistance (Fiaschi and Chiarugi 2012), stemness (Kabashima-Niibe et al. 2013; Kuhn and Tuan 2010; Pauwels and Rabe 2004), and peripheral cells interaction (Huang et al. 2013; Liu et al. 2011; Ono et al. 2015; Uchibori et al. 2013). Previous reports have confirmed that MSCs/MPCs can be isolated from several human tumor tissues (Ding et al. 2012; Gottschling et al. 2013; Hossain et al. 2015; Hu et al. 2013; Lin et al. 2013; Liotta et al. 2015; McLean et al. 2011; Xu et al. 2011; Yan et al. 2012). However, most of these reports used mixed cell populations to argue about the characteristics of MSCs/MPCs as compared to non-cancerous tissue-derived MSC/MPCs. To identify the actual biological characteristics of MSCs/MPCs residing in the cancer microenvironment, comparison between the populations of CAFs might be necessary.
As for cancer cells, most immature stem cell-like cells, cancer stem cells, are at the apex of a malignant differentiation hierarchy (Magee et al. 2012; Plaks et al. 2015). Past studies suggest that CAFs hierarchy based on the differentiation potential also present, as such hierarchy exists in cancer cells. In this study, we attempted to identify the specific functions of lung cancer tissue-derived CAFs with MSCs/MPCs characteristics by comparison with CAFs without MSCs/MPCs characteristics, using single-cell-derived CAFs clones.
Materials and methods
Cell culture
Human pulmonary vascular adventitia fibroblasts (hVAFs) and cancer-associated fibroblasts (CAFs) were obtained from the surgically resected human lung tissues of lung cancer patients and cultured in mesenchymal stem cell medium (MF) (TOYOBO, Japan) as previously reported (Hoshino et al. 2008). Human lung adenocarcinoma cell line A549 (RIKEN BioResource Center, Japan) were cultured in DMEM F12 HAM (Sigma–Aldrich, St. Louis, MO) supplemented with 10 % fetal bovine serum (FBS) (Life Technologies, Grand Island, NY) in a 5 % CO2 incubator. All specimens were collected after the subjects gave their written informed consent, approved by the Institutional Review Board of the National Cancer Center.
Collection of conditioned medium
A549 cells were cultured for 2 days and replaced by MF medium. After 24 h, the supernatants were harvested and filtered using a 0.45 µm Millex-HV Syringe Filter Unit (Merck Millipore, Darmstadt, Germany). VAFs were treated to this conditioned medium for 3 days followed by osteogenic or adipogenic induction. As for the collection of conditioned medium for each CAFs clone, the semi-confluent CAFs were cultured in serum-free DMEM F12 HAM for 24 h and conditioned medium was obtained.
Osteogenic induction and alkaline phosphatase (ALP)/von Kossa staining
When the hVAFs and CAFs were plated, the medium was replaced with fresh osteogenic induction medium (LONZA, Walkersville, MD). After complete stimulation, alkaline phosphatase was detected by an alkaline phosphatase staining kit (Muto Chemical, Japan), according to the manufacturer’s instructions. Calcium deposition was detected by von Kossa’s method. Cells were fixed with paraformaldehyde buffer, and then stained with silver sulfate under ultraviolet rays. After washing with sodium thiosulfate and water, cells were counterstained with nuclear fast red solution (Sigma–Aldrich). Control cells were subjected to the same assay with MF medium instead of the osteogenic induction medium.
Adipogenic induction and Oil red O staining
The hVAFs and CAFs were plated and cultured until confluence. The cells were then alternately cultured in adipogenic induction medium (LONZA) and adipogenic maintenance mediums (LONZA). After three complete cycles of induction/maintenance medium stimulation, the cells were fixed with paraformaldehyde buffer and incubated with isopropanol. The cells were then stained with oil red O (Sigma–Aldrich) solution and counterstained with Meyer’s hematoxylin.
Measurement of staining-positive area
The slides were photographed by the plate scanner, Nano Zoomer (Hamamatsu Photonics, Japan). More than 3 spots in an area of 0.64 cm2 were selected for analysis from the staining images of each group. Staining-positive areas were characterized by colors, which were blue in case of ALP staining, black in case of von Kossa, or red in case of oil red O, respectively, and measured by Win ROOF (Mitani-corp., Japan) image analysis software. Quantitative values were calculated as rates of staining-positive areas against whole cell areas.
Lifetime extension by lentivirus-mediated gene transfer
For lifetime extension, the lentiviruses were produced by the 293T cells transfected with CSII-CMV-RfA-IRES2-Venus-hTERT plasmid (Neri et al. 2015), pCMV-VSV-G-RSV-Rev (RIKEN BioResource Center), and pCMV-HIV (RIKEN BioResource Center), using Lipofectamine 2000 transfection reagent (Invitrogen, Carlsbad, CA). Vector-containing medium was filtered through a 0.45-µm filter (Merck Millipore), and 8 µg/mL of polybrene (Santa Cruz Biotechnology, Dallas, TX) was added for target cell transduction. The fluorescent of Venus proteins were observed by fluorescence microscope.
Quantitative Real-Time Polymerase Chain Reaction
Cells were suspended in 1 mL of TRIzol (Life Technologies) and total RNA was extracted. cDNA was synthesized using the PrimeScript RT reagent Kit (Takara Bio, Japan), according to the manufacturer’s instructions. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) was performed in a Smart Cycler System (Takara Bio) with SYBR Premix Ex Taq II (Takara Bio). The primers used are shown in supplemental Table 2.
Generation of single-cell derived clones
The hTERT-transfected CAFs were sowed in 96-well Prime Surface plates (Sumitomo Bakelite Corp., Japan) at a concentration of 1.0 × 10 cells/mL, one by one. By visually confirming whether it was a single cell using the light microscope, the fibroblasts were replated on 384 well culture plates (BD Biosciences, Franklin Lakes, NJ). Then, these were repeatedly passaged onto a larger plate until confluency was achieved on the 10-cm dish.
WST-8 cell proliferation assay
A549 cells were seeded in 96-well plates (Corning, Corning, NY). The next day, the medium were replaced with serum-free DMEM F12 HAM or conditioned medium from each CAF-derived clone. Two days later, a cell counting kit-8 (DOJINDO, Japan) was used in accordance with the manufacturer’s instructions. Then, the absorbance was measured at 450 nm wavelength by a micro plate reader, Spectra Max 190 (Molecular Devices Corp., Japan).
Cell scratch assay
A549 cells were seeded in 96-well image lock plates (Essen BioScience, Ann Arbor, MI). The cell single layer was scratched by wound maker (Essen BioScience). After washing with PBS, the medium was exchanged with the conditioned medium for each CAF-derived clone. The photographs of the scratched cells were taken by a microscope with an imaging system, Incucyte (Essen BioScience), at intervals of 6 h until 18 h had passed.
Statistical analysis
Statistical analysis was performed using Microsoft Excel (Microsoft, Redmond, WA). Statistical significance was assessed by student t test. Error bars represent the SEM. P values of <0.05 were considered statistically significant.
Result
Osteogenic and adipogenic differentiation potential of CAFs from lung cancer patients
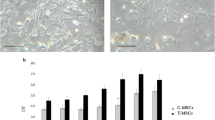
Patient’s characteristics from which CAFs were isolated were summarized in Supplementary Table 1. The cells grown were spindle-shaped and similar to those of the non-cancerous tissue-derived fibroblasts (Supplementary Fig. 1). We used hVAFs as the positive control for lung MSC/MPCs (Hoshino et al. 2008). For visualization of osteogenic differentiation, we performed ALP staining and von Kossa staining (Fig. 1a). The area of ALP-positive CAFs and hVAFs was 13.8 ± 1.0 and 21.2 ± 1.7 %, respectively. CAFs and hVAFs showed equivalent positive areas with von Kossa staining (5.7 ± 0.7 vs. 5.3 ± 0.3 %) (Fig. 1b and Supplementary Fig. 2a, b). After adipogenic induction, hVAFs formed large lipid drops in the cytoplasm, which is a typical feature of adipocytes. In contrast, oil red O-positive cells were not found in all of the CAFs cases (Fig. 1c, d and Supplementary Fig. 2c).
Differentiation potential of CAFs and hVAFs. a The staining images of osteogenic differentiation using ALP and von Kossa methods. b Quantitative analysis of ALP and von Kossa stainings. c The staining images of adipogenic differentiation by oil red O. d Quantitative analysis of oil red O staining. Each result was calculated from the averages of nine specimens. Bar = 100 μm. Values are the mean ± SEM. *p < 0.05; **p < 0.01 between control versus induction
Correlations between osteogenic differentiation of CAFs and clinicopathological factors
We examined the correlation between osteogenic differentiation of 9 CAFs and their clinicopathological factors (Fig. 2). CAFs from patients with a positive smoking history showed significantly higher rate of positive areas with von Kossa staining (p = 0.04) (Fig. 2b). On the other hand, there were no significant correlations between the von Kossa-positive areas and other clinicopathological factors (Fig. 2a, c, d).
Effect of lung adenocarcinoma supernatant on differentiation potential of hVAFs
To examine whether humoral factors secreted from the carcinoma cells inhibit the adipogenic potential of MSCs/MPCs (Fig. 3a), we treated hVAFs with the conditioned medium from A549 cells (A549-CM). The ALP-positive areas were found to be 32.4 ± 4.1 %, which is similar to the result of the control group. In von Kossa staining, the positive area of hVAFs treated with A549-CM was 7.0 ± 1.6 %, and that of the control was 8.7 ± 1.7 % (Fig. 3b, c). There was no significant difference between the two groups.
Changes in the differentiation potential of hVAFs treated by A549 supernatants. a Scheme of experimental protocol. hVAFs were cultured in A549-derived supernatants for 3 days, and then cultured in osteogenic or adipogenic medium. b The staining images of osteogenic differentiation by ALP and von Kossa stainings. c Quantitative analysis of ALP and von Kossa stainings. d The staining images of adipogenic differentiation by oil red O. e Quantitative analysis of oil red O staining. Each result was calculated from three examinations. Bar = 100 μm. Values are the mean ± SEM. *p < 0.05; **p < 0.01 between control versus induction
As for the adipogenic induction, positive area of A549-CM treated hVAFs was significantly lower than the control hVAFs (17.7 ± 3.3 vs. 31.9 ± 5.3 %, p < 0.01) (Fig. 3 d, e).
Generation of single-cell-derived clones from CAFs
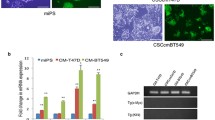
We transduced hTERT gene into specimen number 2 using lentivirus vectors. The increase in the hTERT mRNA expression was confirmed by RT-PCR (Fig. 4a). hTERT-transfected CAFs did not change the cell shape (Fig. 4b). hTERT-transfected CAFs showed higher ALP-positive area (9.3 ± 0. 9 vs. 1.7 ± 0.2 %) and same degree of von Kossa-positive area as compared to control (3.0 ± 0. 4 vs. 2.4 ± 0.2 %) (Fig. 4c, d). We confirmed that CAFs transfected with hTERT gene retained osteogenic potential.
Generation of single-cell-derived CAFs clones. a Quantitative RT-PCR for hTERT mRNA expression of primary CAFs and hTERT- transfected CAFs. b Morphological appearance of CAFs transfected with control vector and hTERT vector. c The staining images of osteogenic differentiation by ALP and von Kossa methods. d Quantitative analysis of ALP and von Kossa stainings. e The method of generation of single-cell-derived CAFs clones by limiting dilution. The single cells were sowed into 96-well plates and confirmed using light microscope (asterisk). Later, the cells were seeded 384-well plates and continued culturing. Bar = 100 μm. Values are the mean ± SEM. *p < 0.05; **p < 0.01 between control versus induction
Next, we performed single cell cloning of hTERT-transfected CAFs by limiting dilution and established 9 CAFs clones (Fig. 4e). Each clone obtained displayed the spindle-shape form; however, the morphology of each clone was slightly different (Supplementary Fig. 4).
Osteogenic differentiation potential of CAFs clones
We performed osteogenic induction of nine CAF clones. Four clones (clone 1, 3, 5, 9) had over 5 % of von Kossa-positive area, which was as much as that of the hVAFs. On the other hand, 2 clones (7, 8) did not show any positive areas (Fig. 5a, b). The values of the other clones were lower than that of the hVAFs. We also calculated ALP-positive areas; however, this result did not correlate with von Kossa-positive areas (Supplementary Fig. 4). The von Kossa staining reflects a more mature and specific osteogenesis than the ALP staining. We collected the supernatants of clones 1, 3, and 9 having a higher osteogenic potential and clones 2, 7, and 8 that had a lower osteogenic potential, for further investigations.
Effect of CAFs clones with higher osteogenic potential on A549 cells proliferation
Proliferation of A549 cells treated with the conditioned medium from each CAFs clone was measured (Fig. 6a). Both higher and lower osteogenic potential clones showed similar effect on the proliferation activity of A549 cells, and no statistical significance was observed (Fig. 6b).
Effect of soluble factors derived from CAFs clones on A549 cells proliferation and migration. a The proliferation of each clones assessed by WST-8 assay. b Average of proliferation of higher and lower osteogenic differentiation clones. c The images of scratched wound area observed at start-up time (left) and 18 h after (right). d Quantitative analysis of wound area rate of each clone. e Averages of wound area rate of clones with higher and lower osteogenic differentiation potentials. Values are the mean ± SEM (n = 6)
Effects of CAFs clones with higher osteogenic potential on A549 cell migration
Next, we performed scratch assay to examine whether A549 cell migration was affected by the CAFs clones-derived condition medium (Fig. 6c). After 18 h, the wound area of the serum-free culture medium (control) was 98.3 ± 0.8 %. The wound area treated with the condition medium from CAF clones 1, 3, and 9 was 89.0 ± 0.4, 87.7 ± 0.2 and, 89.2 ± 0.3 %, respectively. On the other hand, the wound area treated with the condition medium from clone 2, 7, and 8 was 91.8 ± 0.2, 92.8 ± 0.2, and 91.5 ± 0.2 %, respectively (Fig. 6d). The average wound area treated with clones having a higher osteogenic potential was significantly lower than that treated with clones having a lower osteogenic potential (88.7 ± 0.3 vs. 92.0 ± 0.2 %, p < 0.01) (Fig. 6e).
Discussion
In the current study, we found that cultured bulk CAFs derived from lung cancer tissue showed osteogenic but not adipogenic potential. Thus, lung cancer-derived CAFs were considered not to contain MSCs and adipogenic MPCs. Using single-cell-derived clones, we confirmed the presence of osteogenic MPCs and found that there were clones with both higher as well as lower osteogenic differentiation potential among the CAFs.
To explore heterogeneous characters of fibroblasts within mixed population, analysis using clones is an effective method (Hiraoka et al. 2016; Neri et al. 2015). This is the first study to reveal that lung CAFs are configured from cells with varied osteogenic potentials by using CAFs clones. In the current study, we found that clones with a higher osteogenic differentiation potential had a stronger effect on cancer cell migration than those with a lower differentiation potential.
In the current study, we found that clones with a higher osteogenic differentiation potential had a stronger effect on cancer cell migration than those with a lower differentiation potential. This phenomenon may be supported by the results obtained in Fig. 2. The von Kossa-positive area was significantly higher in the CAFs from patients with a smoking history (Fig. 2b). Benzo[a]pyrene, a carcinogen found in cigarette smoke, was reported to inhibit adipogenesis of MSCs (Podechard et al. 2009). Tumor necrosis factor (TNF-α) which is a mediator of cigarette smoke-induced disease (Churg et al. 2002) reportedly promoted osteogenesis of MSCs (Ding et al. 2009). Therefore, smoking might decrease adipogenic potential and enhance osteogenic potential of lung CAFs. Maeda et al., investigated the association between cigarette smoking and the pathological features seen in clinical stage IA lung adenocarcinoma (Maeda et al. 2012). They found that a history of heavy smoking was a statistically significant predictor of histologic vascular invasion. The association between smoking history and the frequency of osteogenic MPCs may explain the underlying mechanism why patients with a smoking history exhibited frequent vascular invasion. Indeed, although not significantly, CAFs from vascular invasion-positive patients displayed a higher von Kossa area (6.7 ± 0.5 vs. 4.3 ± 0.2 %, p = 0.12) (Fig. 2d). On the contrary, no correlations were observed between rates of von Kossa-positive areas and pathological stage of the cancer, indicating that osteogenic MPCs could be recruited into the cancer microenvironment even in the early stages of tumor formation.
The current study revealed that MSCs and adipogenic MPCs are an extremely rare population in our collected CAFs. There are two possibilities for the lack of adipogenic differentiation potential in lung cancer-derived CAFs. One possibility was that primary lung tissue does not have MSCs. Actually, fibroblasts from the non-cancerous tissue did not contain oil red O-positive cells in our study (Supplementary Fig. 3). Another possibility was the recruitment of MSCs/MPCs from other organs or peripheral blood into tumor microenvironments through blood vessels (Kidd et al. 2009). Peripheral-blood-derived MSCs/MPCs were obtained by culture of mononuclear cells in lung cancer patients’ pulmonary arterial blood (Chiba et al. 2008). Effects of cancer-derived humoral factors against differentiation potential of MSCs/MPCs were also reported (Fritz et al. 2011; Tu et al. 2014). Considering these possibilities, we treated the hVAFs to the supernatant from A549 cells and observed decreased adipogenic differentiation levels, although osteogenic differentiation remained. On the contrary, another lung adenocarcinoma cell line, PC9, had no effect on both types of differentiation (data not shown). The regulation of MSCs differentiation is governed by master regulators, such as runt-related transcription factor 2 (RUNX2) for osteogenesis (Komori 2010) and peroxisome proliferator-activated receptor γ (PPARγ) for adipogenesis (Lefterova et al. 2014), respectively. Activation of extracellular signal-regulated kinase (ERK) is known to promote osteogenesis and suppress adipogenesis (Ge et al. 2016). It is possible to think that A549-derived humoral factors which activate ERK signaling could affect to the differentiation potential of hVAFs.
Kuribayashi et al. reported that lung cancer with heterotopic ossification expressed bone morphogenetic protein 2 (BMP2) at high frequency (Kuribayashi et al. 2009). BMP2 is a bone-growth regulatory factor and could be produced by cancer cells. BMP and transforming growth factor-β (TGF-β) pathways reportedly promote cancer cell migration (Hsu et al. 2011; Kang et al. 2010) and expand cancer stem cell (CSCs) population (Choi et al. 2015). Therefore, BMP2-rich tumor microenvironment can differentiate osteogenic potential-positive CAFs into bone (heterotopic ossification) and also might be effective for promoting tumor progression.
ALP and mineralization of calcium, which can be detected by von Kossa staining, are both known as osteogenesis markers (Bonewald 2011). In fact, primary bulk CAFs showed both ALP- and von Kossa-positive areas. However, ALP-positive rates of single-cell-derived CAFs clones were not correlated with von Kossa-positive rates. A similar result was also found in the analysis of single-cell-derived hVAFs clones (Supplementary Fig. 7). During osteogenesis, ALP is upregulated at an early stage and is then downregulated with progressing of bone maturation, which is a mineralization stage (Komori 2010). The discordance between ALP- and von Kossa-positive rates suggested that a hierarchy on differentiation potentials may be present in osteogenic MPCs.
In this study, we proved the presence of fibroblasts with varying osteogenic potentials in CAFs derived from lung cancer tissues through the analysis of single-cell-derived clones. Moreover, osteogenic MPCs with higher differentiation potential promoted the migration of A549 cells to a great extent compared to CAFs with lower differentiation potential. In the tumor microenvironment, heterogeneous CAFs populations that have various degrees of differentiation potential are intermingled, and more tumor-promoting niches could be formed inside the area that osteogenic MPCs were present. Our current study highlights the importance of the classification of CAFs according to the presence of MPCs and non-MPCs phenotypes. Moreover, it provides new insight into the understanding of CAFs hierarchy based on the differentiation potential, as such hierarchy exists in cancer cells. A subject for future studies may be in comprehending the molecular mechanisms how the osteogenic MPCs promote cancer cell migration. The single-cell-derived clonal method used in this study would be a very effective tool for further analyses in this regard.
References
Bauer M, Su G, Casper C, He R, Rehrauer W, Friedl A (2010) Heterogeneity of gene expression in stromal fibroblasts of human breast carcinomas and normal breast. Oncogene 29:1732–1740. doi:10.1038/onc.2009.463
Bieback K, Kern S, Kluter H, Eichler H (2004) Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells 22:625–634. doi:10.1634/stemcells.22-4-625
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26:229–238. doi:10.1002/jbmr.320
Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM (2001) Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98:2396–2402
Chiba H, Ishii G, Ito TK, Aoyagi K, Sasaki H, Nagai K, Ochiai A (2008) CD105-positive cells in pulmonary arterial blood of adult human lung cancer patients include mesenchymal progenitors. Stem Cells 26:2523–2530. doi:10.1634/stemcells.2008-0037
Choi YJ et al (2015) Identifying an ovarian cancer cell hierarchy regulated by bone morphogenetic protein 2. Proc Natl Acad Sci USA 112:E6882–E6888. doi:10.1073/pnas.1507899112
Churg A, Dai J, Tai H, Xie CS, Wright JL (2002) Tumor necrosis factor-alpha is central to acute cigarette smoke-induced inflammation and connective tissue breakdown. Am J Respir Crit Care 166:849–854. doi:10.1164/rccm.200202-097OC
Cirri P, Chiarugi P (2012) Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev 31:195–208. doi:10.1007/s10555-011-9340-x
Ding J et al (2009) TNF-alpha and IL-1beta inhibit RUNX2 and collagen expression but increase alkaline phosphatase activity and mineralization in human mesenchymal stem cells. Life Sci 84:499–504. doi:10.1016/j.lfs.2009.01.013
Ding GX, Shao JL, Ding Q, Fang ZJ, Wu Z, Xu JF, Gao P (2012) Comparison of the characteristics of mesenchymal stem cells obtained from prostate tumors and from bone marrow cultured in conditioned medium. Exp Ther Med 4:711–715. doi:10.3892/etm.2012.642
Fiaschi T, Chiarugi P (2012) Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol 2012:762825. doi:10.1155/2012/762825
Fritz V et al (2011) Bone-metastatic prostate carcinoma favors mesenchymal stem cell differentiation toward osteoblasts and reduces their osteoclastogenic potential. J Cell Biochem 112:3234–3245. doi:10.1002/jcb.23258
Ge C, Cawthorn WP, Li Y, Zhao G, Macdougald OA, Franceschi RT (2016) Reciprocal control of osteogenic and adipogenic differentiation by ERK/MAP kinase phosphorylation of Runx2 and PPARgamma transcription factors. J Cell Physiol 231:587–596. doi:10.1002/jcp.25102
Gottschling S et al (2013) Mesenchymal stem cells in non-small cell lung cancer–different from others? Insights from comparative molecular and functional analyses. Lung Cancer 80:19–29. doi:10.1016/j.lungcan.2012.12.015
Hiraoka C et al (2016) Two clonal types of human skin fibroblasts with different potentials for proliferation and tissue remodeling ability. J Dermatol Sci. doi:10.1016/j.jdermsci.2016.01.009
Hoshino A, Chiba H, Nagai K, Ishii G, Ochiai A (2008) Human vascular adventitial fibroblasts contain mesenchymal stem/progenitor cells. Biochem Biophys Res Commun 368:305–310. doi:10.1016/j.bbrc.2008.01.090
Hossain A et al (2015) Mesenchymal stem cells isolated from human gliomas increase proliferation and maintain stemness of glioma stem cells through the IL-6/gp130/STAT3 pathway. Stem Cells 33:2400–2415. doi:10.1002/stem.2053
Hsu YL, Huang MS, Yang CJ, Hung JY, Wu LY, Kuo PL (2011) Lung tumor-associated osteoblast-derived bone morphogenetic protein-2 increased epithelial-to-mesenchymal transition of cancer by Runx2/Snail signaling pathway. J Biol Chem 286:37335–37346. doi:10.1074/jbc.M111.256156
Hu J et al (2013) Mesenchymal stem-like cells isolated from human esophageal carcinoma and adjacent non-cancerous tissues. Oncol Lett 5:179–184. doi:10.3892/ol.2012.1003
Huang WH, Chang MC, Tsai KS, Hung MC, Chen HL, Hung SC (2013) Mesenchymal stem cells promote growth and angiogenesis of tumors in mice. Oncogene 32:4343–4354. doi:10.1038/onc.2012.458
Ishii G, Ochiai A, Neri S (2015) Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. doi:10.1016/j.addr.2015.07.007
Kabashima-Niibe A et al (2013) Mesenchymal stem cells regulate epithelial-mesenchymal transition and tumor progression of pancreatic cancer cells. Cancer Sci 104:157–164. doi:10.1111/cas.12059
Kang MH, Kim JS, Seo JE, Oh SC, Yoo YA (2010) BMP2 accelerates the motility and invasiveness of gastric cancer cells via activation of the phosphatidylinositol 3-kinase (PI3 K)/Akt pathway. Exp Cell Res 316:24–37. doi:10.1016/j.yexcr.2009.10.010
Karnoub AE et al (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449:U557–U563. doi:10.1038/nature06188
Kidd S et al (2009) Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 27:2614–2623. doi:10.1002/stem.187
Komori T (2010) Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res 339:189–195. doi:10.1007/s00441-009-0832-8
Koumas L, King AE, Critchley HO, Kelly RW, Phipps RP (2001) Fibroblast heterogeneity: existence of functionally distinct Thy 1(+) and Thy 1(−) human female reproductive tract fibroblasts. Am J Pathol 159:925–935. doi:10.1016/S0002-9440(10)61768-3
Kuhn NZ, Tuan RS (2010) Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol 222:268–277. doi:10.1002/jcp.21940
Kuribayashi H et al (2009) Clinicopathological analysis of primary lung carcinoma with heterotopic ossification. Lung Cancer 64:160–165. doi:10.1016/j.lungcan.2008.08.007
Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S (2014) PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol Metab 25:293–302. doi:10.1016/j.tem.2014.04.001
Lin JT, Wang JY, Chen MK, Chen HC, Chang TH, Su BW, Chang PJ (2013) Colon cancer mesenchymal stem cells modulate the tumorigenicity of colon cancer through interleukin 6. Exp Cell Res 319:2216–2229. doi:10.1016/j.yexcr.2013.06.003
Liotta F et al (2015) Mesenchymal stem cells are enriched in head neck squamous cell carcinoma, correlates with tumour size and inhibit T-cell proliferation. Br J Cancer 112:745–754. doi:10.1038/bjc.2015.15
Liu Y et al (2011) Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J Biol Chem 286:25007–25015. doi:10.1074/jbc.M110.213108
Lv FJ, Tuan RS, Cheung KM, Leung VY (2014) Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 32:1408–1419. doi:10.1002/stem.1681
Maeda R, Yoshida J, Ishii G, Hishida T, Nishimura M, Nagai K (2012) Influence of cigarette smoking on survival and tumor invasiveness in clinical stage IA lung adenocarcinoma. Ann Thorac Surg 93:1626–1632. doi:10.1016/j.athoracsur.2012.01.005
Magee JA, Piskounova E, Morrison SJ (2012) Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell 21:283–296. doi:10.1016/j.ccr.2012.03.003
Martin FT et al (2010) Potential role of mesenchymal stem cells (MSCs) in the breast tumour microenvironment: stimulation of epithelial to mesenchymal transition (EMT). Breast Cancer Res Treat 124:317–326. doi:10.1007/s10549-010-0734-1
McLean K et al (2011) Human ovarian carcinoma-associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest 121:3206–3219. doi:10.1172/JCI45273
Muraglia A, Cancedda R, Quarto R (2000) Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci 113(Pt 7):1161–1166
Neri S et al (2015) Cancer cell invasion driven by extracellular matrix remodeling is dependent on the properties of cancer-associated fibroblasts. J Cancer Res Clin Oncol. doi:10.1007/s00432-015-2046-7
Nombela-Arrieta C, Ritz J, Silberstein LE (2011) The elusive nature and function of mesenchymal stem cells. Nat Rev Mol Cell Biol 12:126–131. doi:10.1038/Nrm3049
Okamoto T et al (2002) Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem Biophys Res Commun 295:354–361
Ono M et al (2015) Mesenchymal stem cells correct inappropriate epithelial-mesenchyme relation in pulmonary fibrosis using Stanniocalcin-1. Mol Ther 23:549–560. doi:10.1038/mt.2014.217
Pauwels RA, Rabe KF (2004) Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 364:613–620
Pevsner-Fischer M, Levin S, Zipori D (2011) The origins of mesenchymal stromal cell heterogeneity. Stem Cell Rev Rep 7:560–568. doi:10.1007/s12015-011-9229-7
Plaks V, Kong NW, Werb Z (2015) The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 16:225–238. doi:10.1016/j.stem.2015.02.015
Podechard N, Fardel O, Corolleur M, Bernard M, Lecureur V (2009) Inhibition of human mesenchymal stem cell-derived adipogenesis by the environmental contaminant benzo(a)pyrene. Toxicol In Vitro 23:1139–1144. doi:10.1016/j.tiv.2009.05.011
Riekstina U et al (2009) Embryonic stem cell marker expression pattern in human mesenchymal stem cells derived from bone marrow, adipose tissue, heart and dermis. Stem Cell Rev 5:378–386. doi:10.1007/s12015-009-9094-9
Scherzad A et al (2015) Human mesenchymal stem cells enhance cancer cell proliferation via IL-6 secretion and activation of ERK1/2. Int J Oncol 47:391–397. doi:10.3892/ijo.2015.3009
Sugimoto H, Mundel TM, Kieran MW, Kalluri R (2006) Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther 5:1640–1646
Toma JG, Akhavan M, Fernandes KJ, Barnabe-Heider F, Sadikot A, Kaplan DR, Miller FD (2001) Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol 3:778–784. doi:10.1038/ncb0901-778
Tu B, Peng ZX, Fan QM, Du L, Yan W, Tang TT (2014) Osteosarcoma cells promote the production of pro-tumor cytokines in mesenchymal stem cells by inhibiting their osteogenic differentiation through the TGF-beta/Smad2/3 pathway. Exp Cell Res 320:164–173. doi:10.1016/j.yexcr.2013.10.013
Uchibori R et al (2013) NF-kappa B activity regulates mesenchymal stem cell accumulation at tumor sites. Cancer Res 73:364–372. doi:10.1158/0008-5472.CAN-12-0088
Xing F, Saidou J, Watabe K (2010) Cancer associated fibroblasts (CAFs) in tumor microenvironment. Front Biosci-Landmark 15:166–179. doi:10.2741/3613
Xu X et al (2011) Isolation and comparison of mesenchymal stem-like cells from human gastric cancer and adjacent non-cancerous tissues. J Cancer Res Clin Oncol 137:495–504. doi:10.1007/s00432-010-0908-6
Yan XL et al (2012) Mesenchymal stem cells from primary breast cancer tissue promote cancer proliferation and enhance mammosphere formation partially via EGF/EGFR/Akt pathway. Breast Cancer Res Treat 132:153–164. doi:10.1007/s10549-011-1577-0
Acknowledgments
This work was supported by National Cancer Center Research and Development Fund (23-A-12 and 26-A-16), the Foundation for the Promotion of Cancer Research, 3rd-Term Comprehensive 10-Year Strategy for Cancer Control, Program for the Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation, and JSPS KAKENHI (24659185).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Morphological appearance of non-cancer-associated fibroblasts (NCAFs), CAFs and hVAFs. Microscopic images of NCAFs (left), CAFs (center) and hVAFs (right) (TIFF 13041 kb)
Supplementary Fig. 2
Scatter plots of each specimen’s quantitative differentiation potential. a ALP and b von Kossa for osteogenic differentiation. c Oil red O for adipogenic differentiation of each specimens (TIFF 2476 kb)
Supplementary Fig. 3
Morphology of single-cell derived CAFs clones. Microscopic images of 9 single-cell derived CAFs clones (TIFF 50781 kb)
Supplementary Fig. 4
Quantitative analysis for osteogenic differentiation potential of each CAFs clones by ALP staining. Quantitative analysis of ALP-positive area of each CAFs clones. Values are the mean ± SEM (TIFF 4262 kb)
Supplementary Fig. 5
Differentiation potential of NCAFs. a The staining images of osteogenic differentiation by ALP and von Kossa methods. b Quantitative analysis of ALP and von Kossa. c The staining images of adipogenic differentiation by oil red O. d Quantitative analysis of oil red O staining. Each result was calculated from the averages of 9 specimens. Bar = 100 μm. Values are the mean ± SEM. **P < 0.01 between control vs induction (TIFF 22857 kb)
Supplementary Fig. 6
Scheme summaries. a Brief summary of WST-8 assay. The cells were treated by supernatants of CAFs clones for 2 days. After WST-8 reaction occurred for an hour, absorbance was measured at 450 nm. b Brief scheme of scratch wound healing assay. The monolayer cells were scratched and cultured in CAFs clones. Then, wound healing was observed every 6 h until 18 h (TIFF 6470 kb)
Supplementary Fig. 7
Correlation between ALP-positive area and von Kossa-positive area in single-cell-derived hVAFs clones. Clones of hVAFs were generated by single cell cloning using hTERT gene transfection. The 50 clones that induced osteogenesis were stained by ALP and von Kossa methods. This figure shows a scatter plot of quantitative analysis by ALP and von Kossa for each clone (TIFF 2009 kb)
Rights and permissions
About this article
Cite this article
Suda, Y., Neri, S., Hashimoto, H. et al. Clonal heterogeneity in osteogenic potential of lung cancer-associated fibroblasts: promotional effect of osteogenic progenitor cells on cancer cell migration. J Cancer Res Clin Oncol 142, 1487–1498 (2016). https://doi.org/10.1007/s00432-016-2171-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-016-2171-y