Abstract
Purpose
The outcome of cyclosporin A (CSA) alone (n = 19) as graft-versus-host disease (GVHD) prophylaxis was compared to that of CSA combined with methotrexate (MTX) (n = 43) in children with acute leukemia who underwent hematopoietic stem cell transplantation.
Methods
All respective donors were HLA-identical siblings. All patients received CSA at a dose of 3 mg/kg/day starting on day −1. A CSA level of 80–130 ng/ml was aimed for. The 43 patients in the historical control were given an additional 10 mg/m2 dosage of MTX on days 1, 3, 6, and 11.
Results
Patients who received CSA alone had a significantly reduced cumulative incidence of relapse (5 vs. 40 %; p = 0.002), a significantly increased 5-year event-free survival (84 vs. 35 %; p = 0.001), and a significantly increased 5-year overall survival (84 vs. 42 %; p = 0.004). The incidence of acute GVHD grade II–IV and chronic GVHD in patients in the CSA group was equivalent to the CSA+MTX group (26 vs. 19 %; p = 0.440, and 32 vs. 23 %; p = 0.428).
Conclusions
In conclusion, post-transplant immunosuppression consisting of CSA alone is well tolerated and may contribute to a superior outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Graft-versus-host disease (GVHD) is a major complication and one of the main causes of death after hematopoietic stem cell transplantation (HSCT) with an allogeneic donor. The GVHD prophylaxis influences the incidence of GVHD (Hoyt et al. 2008; Kanda et al. 2006; Kohno et al. 2006), relapse rate, and patient’s survival (Inamoto et al. 2011; Lee et al. 2004; Locatelli et al. 2000a, b). GVHD is associated with graft-versus-leukemia effect, which is mediated by donor T lymphocytes and decreases the risk of relapse (Weiden et al. 1979; Feinstein and Storb 2001; Kanda et al. 2004). Therefore, a reduced post-transplant immunosuppression might have a positive impact on patient’s long-term survival. The outcome of different immunosuppressive prophylaxis regimen for adult patients has been studied extensively (Piñana et al. 2010; Lai et al. 2009; Holler 2007), whereas the effect on children has yet to be determined. The search for the optimal GVHD prophylaxis has been part of many studies, but most of them include only adult patients. So far, very few studies have provided information about the outcome of immunosuppressive prophylaxis after HSCT in children and its respective incidence of acute and chronic GVHD, relapse rate, and long-term survival (Koga et al. 2003; Locatelli et al. 2000a, b). In most cases, children are only a small part of larger studies and are not analyzed separately. Considering that the still growing organism of young children is vulnerable to the consequences of GVHD itself, the study of the impact of different GVHD prophylaxis on children is important.
In 2000, the European Group for Blood and Marrow Transplantation Working Party Pediatric Diseases (EBMT WP-PD) and the International BFM Study Group–Subcommittee Bone Marrow Transplantation (IBFM-SG) presented a survey which indicated that the majority of the pediatric oncology centers in Europe use the combination of cyclosporine A (CSA) and methotrexate (MTX) for the GVHD prophylaxis in children (Peters et al. 2000). The prophylaxis for children with a human leukocyte antigen (HLA)-matched sibling donor usually consisted of intravenously administered CSA 3 mg/kg/day. In addition, they received MTX at a dose of 10 mg/m2 on days +1, +3, and +6 after HSCT and leucovorin at a dose of 15 mg/m2 given 24 h after MTX administration.
Due to the sparse information about the efficacy of GVHD prophylaxis on children, we performed a retrospective study analyzing 62 children with acute lymphoblastic leukemia (ALL) or acute myeloid leukemia (AML) who underwent HSCT at our pediatric oncology center. In this report, we describe the outcome of GVHD prophylaxis regimen in childhood. We present our results regarding the incidence of acute and chronic GVHD, relapse rate, long-term survival, event-free survival, and transplant-related mortality by administrating the combination of CSA and MTX or CSA alone in a pediatric population.
Methods
We analyzed the data of the collected records of all patients under the age of 18 years and one patient of the age of 22 years who underwent sibling hematopoietic stem cell transplantation at the Department of Pediatrics at the University Hospital of Jena, Germany, between January 1984 and December 2008. During this period, 62 patients with acute lymphoblastic leukemia (n = 35) or acute myeloid leukemia (n = 27) received a transplant from their respective HLA-identical sibling. The patient received either a bone marrow (n = 56), peripheral blood stem cell (n = 4), or umbilical cord blood (n = 2) transplantation. Details of the conditioning regimens and supportive care techniques used for transplantation have been reported previously (Gruhn et al. 1998). From 1984 to 1997, the patients were administered an immunosuppressive prophylaxis consisting of CSA plus MTX (n = 43). Thereafter, we changed the GVHD prophylaxis regimen. From 1998 to 2008, the patients were given cyclosporin A alone (n = 19). The day of transplantation was designated as day 0. Patients in the CSA+MTX group received CSA at a dosage of 3 mg/kg/day starting on day −1. Additionally, a 10 mg/m2 dosage of MTX was given on days 1, 3, 6, and 11. Leucovorin was given at a dose of 15 mg/m2 24 h after MTX administration. The 19 patients in the CSA group received CSA as a single agent at a total dose of 3 mg/kg/day starting on day −1. Regarding the CSA level, we aimed for 80–130 ng/ml in all patients. Characteristics of the patients are shown in Table 1. According to the clinical data, the symptoms of acute GVHD were graded by standard clinical criteria and staged according to the Glucksberg et al.’s criteria including the extent of rash, daily diarrhea volume, and serum bilirubin (Glucksberg et al. 1974). A chronic GVHD was diagnosed and staged according to the Seattle clinical grading and staging system (Shulman et al. 1980).
Transplant-related mortality was defined as any transplant-related cause of death after HSCT except relapse of leukemia. Events that censored the event-free survival were death of the patient, a relapse, or secondary malignancy.
Statistical analysis comparing the differences concerning patient’s gender, age, diagnosis, and state of remission prior to transplantation as well as the donor’s gender and age in both groups was performed using the χ2 test and Mann–Whitney U test. The cumulative incidence of acute and chronic GVHD, relapse rate, and transplant-related mortality as well as the cumulative probability of overall survival and event-free survival were described according to the Kaplan–Meier method (Kaplan and Meier 1958). The groups were compared using the log-rank test (Mantel and Haenszel 1958). A multivariate Cox regression analysis was performed including the following variables: type of GVHD prophylaxis, age, sex, grade of acute GVHD, diagnosis (AML vs. ALL), year of transplantation, and remission status prior to transplantation. We determined p < 0.05 as statistically significant. All statistical analyses were calculated using SPSS statistics program.
Results
Sixty two patients with a median age of 11 years (range 6 months–22 years) entered the study. Concerning the patient’s gender, age, diagnosis, and state of remission prior to transplantation, the two groups did not differ significantly. Of the patients in the CSA+MTX arm, 86.0 % were in complete remission at transplantation compared to 73.7 % in the CSA arm (Table 1).
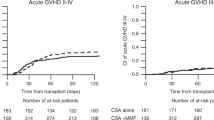
In all, 21 (33.9 %) of the 62 assessable patients showed symptoms of acute GVHD. Thirteen patients (30.2 %) in the CSA+MTX arm and 8 patients (42.1 %) in the CSA arm developed acute GVHD after a median of 38 days (range 14–60 days) and 36 days (range 15–83 days) after HSCT (see Table 2). The Kaplan–Meier estimate of developing grade II to grade IV acute GVHD showed no significant difference (p = 0.440) (Fig. 1).
Chronic GVHD was observed in 16 (25.8 %) of the 62 patients after a median of 188 days (range 101–1411 days) after HSCT. Three of those patients, all in the CSA+MTX group, showed signs of a limited chronic GVHD and 13 patients, 7 in the CSA+MTX group and 6 in the CSA group, those of an extensive chronic GVHD (see also Table 2). In 7 cases, chronic GVHD followed acute GVHD, and in 9 cases, chronic GVHD was observed as de novo disease. The cumulative incidence of chronic GVHD occurrence was not significantly different (p = 0.428) (Fig. 2). Chronic GVHD developed in patients in the CSA+MTX group and CSA group after a median of 209 days (range 101–1,411 days) and 177 days (range 108–293 days) after HSCT, respectively.
Of 62 patients with acute leukemia, 18 patients (29.0 %), 17 in the CSA+MTX group and 1 in the CSA group, relapsed after a median of 140 days (range 19–322 days) after HSCT (Table 2). Patients who received CSA alone as post-transplant immunosuppression had a significantly reduced cumulative incidence of relapse (5 vs. 40 %; p = 0.002) as seen in Fig. 3.
A total of 28 patients (45.2 %), 25 in the CSA+MTX arm and 3 in the CSA arm, died. Eleven patients died due to their relapse of ALL or AML, therefore illustrating ALL or AML as the primary cause of death. Acute GVHD was the cause of death for 4 patients. There is a significantly increased 5-year overall survival for the patients who received CSA alone (84 vs. 42 %; p = 0.004; Fig. 4).
The findings show a significantly increased 5-year event-free survival in the group with CSA alone as post-transplant immunosuppression (84 vs. 35 %; p = 0.001) (Fig. 5).
In total, 12 patients, 10 in the CSA+MTX arm and 2 in the CSA arm, died without evidence of relapsed leukemia or having a second HSCT after a median of 68 days (range, 18-331 days) after HSCT. The cumulative rates of transplant-related mortality were 23.3 % in the CSA+MTX group and 10.5 % in the CSA group (p = 0.165).
We performed a multivariate Cox regression analysis including the following variables: type of GVHD prophylaxis, age, sex, grade of acute GVHD, diagnosis (AML vs. ALL), year of transplantation, and remission status prior to transplantation. The year of transplantation was included in order to analyze whether factors independent from the alteration of GVHD prophylaxis contributed significantly to the reduction in incidence of relapse over the time period of this study. The Cox regression analysis showed that only the type of GVHD prophylaxis (p = 0.002) and the remission status prior to transplantation (p < 0.001) remained as independent prognostic factors for cumulative incidence of relapse.
Discussion
This retrospective study shows that CSA alone as GVHD prophylaxis offers a safe alternative to the combination of CSA+MTX in children who receive a stem cell transplant from an HLA-identical sibling. Regarding the incidence of acute GVHD, the data of this study showed no significant difference comparing the CSA+MTX arm to the CSA arm. Since CSA alone is a less intensive GVHD prophylaxis, a higher incidence and severity of GVHD could be expected for the patients in this group. However, as other studies showed, children tend to have a lower incidence and a lower grade of GVHD than adult patients (Weisdorf et al. 1991; Stockschlaeder et al. 1992; Flowers et al. 2011). Therefore, a reduced GVHD prophylaxis might have a better outcome for children with ALL or AML. Acute GVHD grade III was reported in 21.1 and 2.3 % of the patients in the CSA group and CSA+MTX group, respectively. However, none of the patients given CSA alone developed acute GVHD grade IV, compared to 7.0 % in the CSA+MTX arm. Hence, we cannot report a reduced severity of acute GVHD when given the combination of CSA and MTX. As reported by Frassoni et al. (1996), patients with a donor of a different gender have a higher risk of developing acute GVHD. Male patients with a female donor are especially at a higher risk (Gratwohl et al. 1998). In our study, there was no significant difference regarding the incidence of acute GVHD between patients with a donor of the same gender and patients with a donor of a different gender. In 22 cases, a male patient had a female donor and 7 of them developed acute GVHD. A higher risk for male patients with female donor could not be determined.
A high relapse rate is one of the main complications after HSCT in children (Schrappe et al. 2000; Möricke et al. 2010; Schrappe et al. 2012). In this study, 18 patients suffered from relapse; 17 of them received CSA in combination with MTX as GVHD prophylaxis. The data demonstrate a significant decrease in relapse of patients following administration of post-transplant immunosuppression with CSA alone (5.3 %) compared to those receiving CSA+MTX (39.5 %, p = 0.002). It is important to highlight that the CSA group was compared to a historical control and that therefore other improvements may have contributed to the reduced relapse rate. However, the proportion of patients who were not in remission was even higher in the CSA group (26.3 vs.14.0 %). The incidence of GVHD is associated with a graft-versus-leukemia (GVL) effect (Weiden et al. 1979) which is important to reduce the relapse rate (Xia et al. 2006; Fowler and Gress 2000; Li et al. 2009). The study of Kanda et al. (2006) reports a significant reduction in relapse in patients who developed acute or chronic GVHD. In our study, 7 of the 18 patients who suffered from relapse had developed acute or chronic GVHD; however, most of them had had an acute GVHD grade I, which is associated with a lower GVL effect. These results suggest a better outcome regarding the relapse rate for patients with ALL or AML given CSA alone as post-transplant immunosuppression.
Another interesting finding in this study is the significantly higher overall survival for patients given CSA alone (84.2 %) compared to those given CSA in combination with MTX (41.9 % p = 0.004). In total, 28 of the 62 patients died. The main cause of death was the relapse of ALL or AML (39.3 %). GVHD as cause of death was with 14.3 % of lesser importance. An intensive GVHD prophylaxis lowers the incidence of GVHD and therefore limits death caused by that. Then again, an intensive GVHD prophylaxis increases the risk of relapse and thereby the possible death caused by their relapse of ALL or AML disease. Given the fact that patients’ death was caused more often by their relapse of ALL or AML than by GVHD, the relapse rate has a stronger influence on the overall survival than the incidence of GVHD. Only 3 of the 18 patients who suffered from relapse survived, whereas 26 of the 30 patients who developed a GVHD without relapse did not die. Considering that children seem to develop GVHD less frequently and of lesser severity, GVHD has a lower impact on the overall survival of children.
Therefore, the higher relapse rate and number of deaths in the CSA+MTX group lead to a significantly higher event-free survival in the CSA group (p = 0.001).
The administration of CSA+MTX compared to CSA alone as post-transplant immunosuppression resulted in no significant difference for the transplant-related mortality (p = 0.165). Since relapse rate and therefore death caused by ALL or AML have no influence on transplant-related mortality, the difference between the CSA+MTX arm (23.3 %) and CSA arm (10.5 %) is notably smaller.
In conclusion, this study demonstrates that in children with ALL or AML undergoing HSCT with an HLA-identical sibling as donor, CSA alone as post-transplant immunosuppression is well tolerated and may contribute to a lower relapse rate and higher overall survival and event-free survival in comparison to patients treated with CSA and MTX. There was no significant difference regarding the incidence of acute and chronic GVHD. These findings suggest that CSA alone as GVHD prophylaxis may result in a better outcome for children with acute leukemia.
References
Feinstein L, Storb R (2001) Reducing transplant toxicity. Curr Opin Hematol 8:342–348
Flowers ME, Inamoto Y, Carpenter PA et al (2011) Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 117:3214–3219
Fowler DH, Gress RE (2000) Th2 and Tc2 cells in the regulation of GVHD, GVL, and graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma. Leuk Lymphoma 38:221–234
Frassoni F, Labopin M, Gluckman E, Prentice HG, Vernant JP, Zwaan F et al (1996) Results of allogeneic bone marrow transplantation for acute leukemia have improved in Europe with time—a report of the acute leukemia working party of the European group for blood and marrow transplantation (EBMT). Bone Marrow Transplant 17:13–18
Glucksberg H, Storb R, Fefer A et al (1974) Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA matched sibling donors. Transplantation 18:295–304
Gratwohl A, Hermans J, Goldman JM et al (1998) Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet 352:1087–1092
Gruhn B, Häfer R, Kosmehl H, Fuchs D, Zintl F (1998) Cyclosporin A-induced graft-versus-host disease following autologous bone marrow and stem cell transplantation in hematological malignancies of childhood. Bone Marrow Transplant 21:901–907
Holler E (2007) Risk assessment in haematopoietic stem cell transplantation: GvHD prevention and treatment. Best Pract Res Clin Haematol 20:281–294
Hoyt R, Ritchie DS, Roberts AW et al (2008) Cyclosporin, methotrexate and prednisolone for graft-versus-host disease prophylaxis in allogeneic peripheral blood progenitor cell transplants. Bone Marrow Transplant 41:651–658
Inamoto Y, Flowers M, Lee S et al (2011) Influence of immunosuppressive treatment on risk of recurrent malignancy after allogeneic hematopoietic cell transplantation. Blood 118:456–463
Kanda Y, Izutsu K, Hirai H et al (2004) Effect of graft-versus-host disease on the outcome of bone marrow transplantation from an HLA-identical sibling donor using GVHD prophylaxis with cyclosporin A and methotrexate. Leukemia 18:1013–1019
Kanda Y, Hyo R, Yamashita T et al (2006) Effect of blood cyclosporine concentration on the outcome of hematopoietic stem cell transplantation from an HLA-matched sibling donor. Am J Hematol 81:838–844
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Koga Y, Nagatoshi Y, Kawano Y, Okamura J (2003) Methotrexate vs Cyclosporin A as a single agent for graft-versus-host disease prophylaxis in pediatric patients with hematological malignancies undergoing allogeneic bone marrow transplantation from HLA-identical siblings: a single-center analysis in Japan. Bone Marrow Transplant 32:171–176
Kohno A, Morishita Y, Iida H et al (2006) Low-dose cyclosporin A with short-term methotrexate for graft-versus-host disease prophylaxis in allogeneic bone marrow transplantation from human leukocyte antigen-identical siblings: a prospective phase II study in Japanese patients. Int J Hematol 84:83–89
Lai Y, Ma J, Schwarzenberger P et al (2009) Combination of CsA, MTX and low-dose, short-course mycophenolate mofetil for GVHD prophylaxis. Bone Marrow Transplant 43:61–67
Lee KH, Choi SJ, Lee JH et al (2004) Cyclosporine alone vs cyclosporine plus methotrexate for post-transplant immunosuppression after HLA-identical sibling bone marrow transplantation: a randomized prospective study. Bone Marrow Transplant 34:627–636
Li JM, Giver CR, Lu Y, Hossain MS, Akhtari M, Waller EK (2009) Separating graft-versus-leukemia from graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Immunotherapy 1:599–621
Locatelli F, Bruno B, Zecca M et al (2000a) Cyclosporin A and short-term methotrexate versus cyclosporin A as graft versus host disease prophylaxis in patients with severe aplastic anemia given allogeneic bone marrow transplantation from an HLA-identical sibling: results of a GITMO/EBMT randomized trial. Blood 96:1690–1697
Locatelli F, Zecca M, Rondelli R et al (2000b) Graft versus host disease prophylaxis with low-dose cyclosporine-A reduces the risk of relapse in children with acute leukemia given HLA-identical sibling bone marrow transplantation: results of a randomized trial. Blood 95:1572–1579
Mantel N, Haenszel W (1958) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22:719–748
Möricke A, Zimmermann M, Reiter A et al (2010) Long-term results of five consecutive trials in childhood acute lymphoblastic leukemia performed by the ALL-BFM study group from 1981 to 2000. Leukemia 24:265–284
Peters C, Minkov M, Gadner H et al (2000) Statement of current majority practices in graft-versus-host disease prophylaxis and treatment in children. Bone Marrow Transplant 26:405–411
Piñana JL, Valcárcel D, Fernández-Avilés F et al (2010) MTX or mycophenolate mofetil with CsA as GVHD prophylaxis after reduced-intensity conditioning PBSCT from HLA-identical siblings. Bone Marrow Transplant 45:1449–1456
Schrappe M, Reiter A, Zimmermann M et al (2000) Long-term results of four consecutive trials in childhood ALL performed by the ALL-BFM study group from 1981 to 1995. Leukemia 14:2205–2222
Schrappe M, Hunger SP, Pui CH et al (2012) Outcomes after induction failure in childhood acute lymphoblastic leukemia. N Engl J Med 366:1371–1381
Shulman HM, Sullivan KM, Weiden PL (1980) Chronic graft-versus-host syndrome in man: a long-term clinicopathologic study of 20 Seattle patients. Am J Med 69:204–217
Stockschlaeder M, Storb R, Pepe M et al (1992) A pilot study of low-dose cyclosporin for graft-versus-host prophylaxis in marrow transplantation. Br J Haematol 80:49–54
Weiden PL, Flournoy N, Thomas ED et al (1979) Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med 300:1068–1073
Weisdorf D, Hakke R, Blazar B et al (1991) Risk factors for acute graft-versus-host disease in histocompatible donor bone marrow transplantation. Transplantation 51:1197–1203
Xia G, Truitt RL, Johnson BD (2006) Graft-versus-leukemia and graft-versus-host reactions after donor lymphocyte infusion are initiated by host-type antigen-presenting cells and regulated by regulatory T cells in early and long-term chimeras. Biol Blood Marrow Transplant 12:397–407
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Weiss, M., Steinbach, D., Zintl, F. et al. Superior outcome using cyclosporin A alone versus cyclosporin A plus methotrexate for post-transplant immunosuppression in children with acute leukemia undergoing sibling hematopoietic stem cell transplantation. J Cancer Res Clin Oncol 141, 1089–1094 (2015). https://doi.org/10.1007/s00432-014-1885-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-014-1885-y