Abstract
There is currently a major concern regarding the optimal immunosuppression therapy to be administered after hematopoietic stem cell transplantation (HSCT) to reduce both the toxicity of GvHD and the rate of relapse. We report the outcome of high-risk leukemia children transplanted with a new way of managing cyclosporine (CsA)-based GvHD prophylaxis. A total of 110 HSCT in 109 ALL or AML children who received CsA without mycophenolate or methotrexate in matched related as well as in matched or mismatched unrelated stem cell transplantation were included. CsA dosage regimens were individualized to obtain specific trough blood concentrations values. The incidences of grade I–II and III–IV acute GvHD were 69.1% and 1.8%, respectively, and 8.4% for chronic GvHD. GvHD was neither more frequent nor severe in unrelated than in related HSCT. GvHD occurred in 87% of patients with a mean CsA trough concentration ⩽120 ng/mL versus 43% with concentration >120 ng/mL (P<0.0001). Five-year disease-free survival (DFS) and overall survival were 78% and 83.6%, respectively. DFS was 76.9% for ALL and 80.4% for AML patients. There was no difference in DFS between matched siblings and matched unrelated or mismatched unrelated HSCT. DFS in patients with minimal residual disease (MRD) ⩾10−3 and in those with MRD <10−3 before SCT was comparable. Our results indicate that a GvHD prophylaxis regimen based on CsA without mycophenolate or methotrexate is safe and effective whatever the donor compatibility is. These results suggest that GvL effect may be enhanced by this strategy of GvHD prophylaxis.
Similar content being viewed by others
Introduction
Allogenic hematopoietic stem cell transplantation (HSCT) remains the best option to treat high-risk AML and very high-risk ALL in children. Immunological conflicts induced by HSCT have been shown to reduce the risk of relapse.1, 2 GvHD is associated with the GvL effect that is mediated by donor T lymphocytes, and particularly natural killer (NK) lymphocytes, their proliferation occurring along with GvHD.3, 4 GvHD can unfortunately complicate HSCT by causing substantial morbidity and transplantation-related, non-relapse mortality (TRM). The search for an optimal strategy for GvHD prophylaxis to obtain a beneficial effect on leukemia without increasing TRM has been the object of many studies. The best results on relapse rates have been seen in patients with mild GvHD.5
Cyclosporine (CsA) is the most extensively used drug to prevent GvHD after allogeneic HSCT. CsA is generally combined to short courses of methotrexate (MTX) for GvHD prophylaxis in children despite a possible disadvantage on GvL effect.6 CsA is rarely used alone, except for patients transplanted from sibling donors.7 To date, there is no report on the use of CsA without MTX in patients transplanted from unrelated donors.
The significant intra- and interpatient variability in CsA pharmacokinetics makes the monitoring of blood concentrations and individualized dosage necessary. Early post-transplantation exposure to CsA has been related to acute GvHD (aGvHD) severity.8, 9, 10 It is now generally agreed that CsA trough blood concentration (TBC) is the pharmacokinetic parameter that best correlates with GvHD outcome.11, 12 Nevertheless, there are no data regarding specific values of CsA TBC to target to favor mild aGvHD without increasing the incidence of severe GvHD. Based on our previous experience, we have been performing therapeutic drug monitoring of CsA TBC combined with Bayesian dose adjustment, and targeting relatively low levels in leukemia patients.13
The aim of the present study was to evaluate GvHD prophylaxis based on CsA alone with specific blood monitoring, and especially without MTX or mycophenolate mofetil (MMF), even for unrelated donors, in a large cohort of children transplanted for high-risk acute leukemia. The relationships between CsA pharmacokinetics and outcome or relapse were also assessed.
Patients and methods
Patients
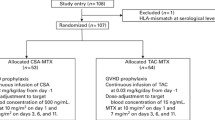
A total of 117 HSCT in 115 children (aged 0.5–19 years) with high-risk leukemia undergoing HSCT in our center between 2000 and 2013 met the following inclusion criteria: AML or ALL or biphenotypic leukemia in first or further CR before transplantation, matched sibling or matched unrelated donor (MURD) or mismatched unrelated donor (MMURD), GvHD prophylaxis consisting of CsA without MTX or MMF and patients receiving CsA in two daily 2-h infusions. Two patients underwent a second HSCT after relapse. Three patients for whom CsA had to be stopped early because of renal toxicity or thrombotic microangiopathy before engraftment were excluded from the analysis of relationships between CsA pharmacokinetics until engraftment and aGvHD occurring concomitantly (one patient died). Four patients (including one second HSCT) who died before engraftment were excluded from both the pharmacokinetic and disease-free survival (DFS) analysis, as the end point of the study was GvHD prophylaxis but not leukemia treatment.
Among the 110 remaining HSCT patients (109 patients), 52 were for AML, 52 for ALL and 6 for biphenotypic leukemia. ALL patients in their first CR (CR1) eligible for transplantation were patients with induction failure or high minimal residual disease (MRD) after induction (⩾10−2), hypodiploidy <40 chromosomes, mixed lineage leukemia rearrangement and until 2005, patients with T-cell ALL, poor prednisone response and WBC count >100 g/L, and patients with bcr-abl rearrangement.14 For infants and patients with bcr-abl rearrangement after 2005, indications of HSCT were as described in the INTERFANT and ESPHALL trials.15, 16 Patients with relapsed ALL were treated according to the risk stratification of BFM relapses protocols.17 For AML patients in CR1, HSCT was recommended for all patients with monosomy 5 or 7, translocation t(6,9) and mixed lineage leukemia rearrangement, except for translocation t(9,11) with related or unrelated donors, and was performed in other cases with matched sibling donors (MSDs) only, except for patients with translocation t(8,21).18 All AML relapses were indications to transplant after CR2 was achieved.19
For all the patients, GvHD prophylaxis consisted of CsA. CsA was combined with 7.5 mg/kg rabbit antithymocyte globulin for unrelated donors (including cord blood), and divided into three doses of 2.5 mg/kg (TBI-based conditioning regimens: days −3, −2, −1; busulfan-based conditioning regimens: days −5, −3 and −1). Corticosteroids (1 mg/kg per day, days 1–30) were added for cord blood transplantation. No patient received neither MTX nor MMF.
Forty-seven out of the 110 HSCT were from an HLA-MSD, 16 from an MURD (10/10 for bone marrow and 6/6 for cord blood) and 47 from an MMURD (9/10 or 8/10 for bone marrow and 4/6 or 5/6 for cord blood). The preferred stem cell source was bone marrow. Peripheral stem cells or cord blood were used in 5 and 17 patients, respectively. HSCT characteristics are summarized in Table 1.
Two-thirds of AML patients (36/52) were transplanted in CR1, and the others in CR2 (16/52). Most ALL patients were transplanted in CR1 (25/52) or CR2 (24/52), and the remaining three patients in CR3. Patients with biphenotypic leukemia were transplanted in CR1, except for one patient in CR2.
The pretransplant conditioning regimen was based on TBI with either etoposide or cyclophosphamide (n=54), or a busulfan-based regimen combined with either cyclophosphamide or fludarabine (n=56). Etoposide was added to busulfan and cyclosphosphamide in young ALL patients. Thiotepa was added to busulfan and cyclophosphamide if a special risk of central nervous system relapse could be identified (n=4). Therapeutic drug monitoring of busulfan plasma concentrations was performed to individualize its doses to obtain a target area under the curve between 980 and 1250 μM.min.13
GvHD prophylaxis
Apart from in vivo T cell depletion for unrelated donors and prednisolone for cord blood stem cell source, GvHD prophylaxis consisted of CsA alone for all patients. Neither MTX nor MMF was used at any time. Administration of CsA was always started the day before transplantation by using an initial 2-h IV infusion of 1.5 mg/kg two times daily. Subsequent doses were determined individually by a Bayesian approach as described previously.13 When oral administration could be tolerated, CsA was given orally every 12 h. In the absence of GvHD, CsA dosage was tapered starting 2 months following transplantation.
CsA monitoring strategy
Measurements of CsA TBC were performed on peripheral venous blood samples by immunoenzymatic assay.20 During IV treatment, CsA TBC was measured two to three times weekly starting from the day after transplantation. Target TBC was 120 ng/mL irrespective of the donor compatibility (we had previously reported that antithymocyte globulin use was a significant factor for preventing aGvHD by reducing the consequences of HLA disparities11).
Grading of aGvHD and treatment
aGvHD was graded by standard clinical criteria and staged according to the Glucksberg et al.21 criteria including the extent of rash, daily diarrhea volume and serum bilirubin. aGvHD occurred in the early post-transplantation period, during IV treatment with CsA, and in hospitalized patients. CsA doses were increased as soon as the first signs of GvHD occurred to obtain TBC ranging from 150 ng/mL for grade I GvHD to 200 ng/mL for at least grade II GvHD. Corticosteroids were only added in patients with at least grade II aGVHD (prednisolone 1–2 mg/kg per day, depending on the stage) either immediately for severe GvHD or after 48 h of increased CsA doses with adequate CsA TBC in patients with poorly responding moderate GvHD.
MRD analysis
Monitoring for MRD was performed on bone marrow samples collected 30 days before transplantation and on day 90 after transplantation. For ALL patients, MRD was measured by quantitative PCR. In case of impossible MRD quantification or analysis failure by PCR in ALL patients, multiparameter flow cytometry-based MRD results were accepted. For AML patients, MRD was measured only by multiparameter flow cytometry.
PCR was performed with patient-specific, allele-specific oligonucleotide primers for specific DNA marker amplification. The DNA marker used could be leukemic clone-specific rearrangements of the Ig and TCR genes, or oncogenic DNA markers. Allele-specific oligonucleotide-PCR was performed and interpreted as described in up-to-date EuroMRD guidelines.22
Multiparameter flow cytometry was performed on fresh cells after erythrocyte lysis using staining with Ab panels, as described previously in ALL.23 At diagnosis, leukemia-associated immunophenotypes were determined on 10 000 to 15 000 nucleated cells. For follow-up samples, as described above, 2.5 × 105 non-gated events or more stained nucleated cells were analyzed using FACSCalibur flow cytometers (BD Bioscience, San Jose CA, USA). In AML patients, MRD was measured by multiparameter flow cytometry as described previously.24 MRD results were given as the percentage of leukemic cells in the total population of nucleated cells. The MRD positivity was defined as ⩾10−3.22, 23, 24, 25
Statistical analysis
All statistical analyses were performed using SPSS for Windows (version 11.0; SPSS, Chicago, IL, USA). Pearson's χ2 tests were used to compare possible differences in qualitative variables between groups of patients. Comparison of quantitative variables such as mean CsA trough concentration between groups of patients used Student's T-test or Mann–Whitney U-test. Individual means of CsA trough concentrations observed during two kinds of periods (during the first 2 weeks post-transplantation and during aplasia, i.e., until engraftment) were considered. The Kaplan–Meier method was used for survival or probability estimates. The log-rank test was used for comparison of survival between groups of diseases or MRD or probabilities of aGvHD between groups of CsA TBC values. All differences were considered significant when the P-value was <0.05.
Results
Incidence of GvHD and relationships with CsA pharmacokinetics
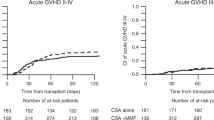
aGvHD occurred in 70.9% (78 of the 110 HSCT). Among them, 93.6% presented with only cutaneous signs. The incidence of the different grades of aGvHD was as follows: grade I, 40.9% (45/110); grade II, 28.2% (31/110); and grade III–IV, 1.8% (2/110). Chronic GvHD occurred in 8.4% of HSCT (9/107, 3 patients dying before D100). The mean of individual mean observed CsA TBC in patients with aGvHD was 114±16 ng/mL during the first 2 weeks post transplantation. It was 113±15 ng/mL until engraftment (which occurred at day 22±8). They were significantly higher in patients without aGvHD: 137±23 ng/mL (P<0.001) and 134±21 ng/mL (P<0.001), respectively. A CsA TBC of 120 ng/mL appeared to be the most relevant value to target to obtain mild or moderate GvHD. Indeed, aGvHD occurred in 87.1% of patients with a mean CsA TBC ⩽120 ng/mL during the first 2 weeks post transplantation, and only in 42.5% of patients with mean CsA TBC >120 ng/mL (P<0.0001). The difference in the incidence of GvHD was similar if mean CsA TBC observed until engraftment were considered (84.7% versus 44.7%, P<0.001). Probabilities of aGvHD associated with mean CsA TBC cutoff of 100, 110, 120 and 130 ng/mL during the first 2 weeks post-transplantation, for both type of leukemia (ALL or AML), are shown in Table 2. Overall incidence of aGvHD was comparable in patients transplanted from MSD or URD. The incidence of aGvHD was 72% (34/47) in MSD, 75% (12/16) in MURD and 68% (32/47) in MMURD (P=0.836). The incidence of severe GvHD was not different between patients transplanted from URD and those from MSD (2/63 patients with grade II–IV aGvHD in MSD and none in URD HSCT, P=0.098). CsA TBC values in patients with or without aGvHD according to donor compatibility are given in Table 3. Dose adjustments were highly variable between patients, and also for each individual. Mean daily doses allowing to reach a target TBC around 120 ng/mL were 3.53±1.10 (range 1.50–5.50) mg/kg in small children (0–8 years old, n=49) and 2.38±1.13 (range 0.83–5.70) in older children (9–19 years old, n=59) (P<0.001).
Incidence of relapse and DFS
Eighteen patients (16.4%) relapsed between 1.4 and 23.6 months post HSCT (mean±s.d.: 7.8±6.1 months). Three patients were not evaluable because of early death (<3 months post HSCT). Five-year DFS was 78.0% (95% confidence interval (CI): 70.6–86.2) (Figure 1). DFS according to CR number, leukemia phenotype, donor compatibility and use of corticosteroids are given in Table 4. None of the six patients with biphenotypic leukemia relapsed, but two of them died before 3 months after HSCT.
TRM and overall survival
TRM was low: 11 out of 115 patients (9.5%) died from causes other than relapse—chronic pulmonary GvHD (n=2), infection (n=5), pulmonary complications (n=1), veno-occlusive disease (n=2) and GvHD after donor lymphocyte infusion (n=1). Five-year overall survival was 80.0% (95% CI: 73.3–87.9) for the whole population and 83.3% (95% CI: 76.6–90.7) for the 110 HSCT included in the study.
Prognostic significance of MRD and effect of aGvHD
The MRD level could be measured in 85 patients before transplantation (47 ALL; 32 AML; 6 biphenotypic AL), and in 72 patients after transplantation (43 ALL; 26 AML; 3 biphenotypic AL). DFS in patients with MRD ⩾10−3 and in those with MRD <10−3 before HSCT were comparable: 80.0% (95% CI: 65.8–97.3) and 74.9% (95% CI: 64.6–86.7), respectively (P=0.617). Among the 60 patients with MRD <10−3 at the time of HSCT, 11 (18.3%) relapsed after HSCT. Four (16.0%) out of the 25 patients with MRD ⩾10−3 at the time of HSCT relapsed between 3.3 and 9.4 months after HSCT. A majority of those who did not relapse (71.4%) presented with grade I–II aGvHD. One of the seven patients with pretransplant MRD ⩾10−3 and without aGvHD relapsed and had higher mean CsA TBC (187 ng/mL) compared with those who did not relapse (137±25 ng/mL). Among the 19 patients with MRD ⩾10−3, 14 displayed MRD <10−3 after HSCT. Two-thirds of them developed aGvHD. However, there was no significant difference in DFS between those post-transplant MRD-negative patients and the five patients with MRD remaining ⩾10−3 after HSCT (P=0.634). Nevertheless, there was a trend toward a lower mean CsA TBC until engraftment in patients who reversed pretransplant-positive to -negative MRD after HSCT (114±15 versus 144±32 ng/mL in those who did not, P=0.056).
Among the 47 ALL patients with measurable pretransplant MRD, 38/47 had MRD <10−3 and 9/47 had MRD ⩾10−3 at the time of HSCT. Nine out of the 38 patients (19%) with pretransplant MRD <10−3 relapsed. Five out the nine patients who relapsed did not experienced aGvHD. Only one out of the nine patients with pretransplant MRD ⩾10−3 relapsed after HSCT. Six of the eight patients who did not relapse presented with grades I–II aGvHD. Among the 32 AML patients with measurable pretransplant MRD, 18 had MRD <10−3 and 14 had MRD ⩾10−3 at the time of HSCT. Two out of 18 patients with pretransplant MRD <10−3 and 3 out of 14 patients with pretransplant MRD ⩾10−3 relapsed after HSCT. Four out of the five patients who relapsed had grade I aGvHD.
Discussion
This retrospective study presents the results of our center regarding the incidence of aGvHD and chronic GvHD, relapse rate, long-term survival and TRM in a pediatric population with acute leukemia who underwent allogenic HSCT receiving CsA alone as GvHD prophylaxis. There is actually a major concern regarding the optimal immunosuppression therapy to be administered after transplantation to both reduce the toxicity of GvHD and the rate of relapse, particularly in patients with MRD ⩾10−3 before transplantation.26 Our study is the first to report the use of a GvHD prophylaxis regimen containing CsA only, without MTX or MMF in both matched siblings and matched or mismatched unrelated HSCT. The very low incidence of severe aGvHD and chronic GvHD shows the feasibility of a more gentle GvHD prophylaxis whatever the donor HLA compatibility may be. However, reaching these results required very close pharmacokinetic monitoring of CsA. Indeed, we previously reported a strong relationship between CsA TBC during the first 2 weeks post-transplantation, and the severity of aGVHD occurring at engraftment.8 In the present study, CsA TBC were maintained around 120 ng/mL, even if the intraindividual variability of CsA pharmacokinetics might have led to transient lower or higher concentrations. Our results confirm that the cutoff value 120 ng/mL seems the most relevant to target to trigger off a mild aGvHD in both ALL and AML patients. This study is the first one proposing a CsA TBC value allowing the increase of the incidence of mild but not severe aGvHD. The incidence of severe aGvHD reported in most studies on transplanted children for leukemia is generally much higher (8–19%), although GvHD prophylaxis regimens also include short courses of MTX or MMF.27, 28, 29, 30, 31, 32, 33 However, observed values of CsA TBC are almost never reported, making difficult any comparison of CsA monitoring. The incidence of chronic GvHD reported in previous similar cohorts varies between 14 and 46%,31, 34 with a majority between 20 and 30%.30, 32, 33, 34, 35, 36, 37 The incidence of chronic GvHD we report is lower (8.2%). The incidence of GvHD was not different between matched siblings or unrelated HSCT in our cohort, despite targeting similar CsA TBC. This confirms the major role of antithymocyte globulin as part of GvHD prophylaxis in patients transplanted from unrelated donors.11
We also report a low rate of TRM compared with other series where average TRM reaches 20–25%,25, 29, 30, 34 even though a large variability (7–45%) is possible.27, 35, 38, 39 Our results suggest that close monitoring of CsA therapy may permit a substantial reduction of GvHD-related mortality. Moreover, reduced immunosuppression may contribute to the low incidence of severe infections and related deaths. Other TRM causes such as severe liver veno-occlusive disease might have been limited by using Bayesian individualization of busulfan dosage regimens.13
Five-year DFS was particularly high in our cohort. DFS usually reported in children transplanted for high-risk leukemia from sibling or unrelated donors vary between 50 and 70%.25, 27, 28, 29, 30, 31, 32, 33, 40 Weiss et al.37 report a similar DFS (84%) using CsA alone as GVHD prophylaxis but in patients transplanted only from MSDs.37 They also used CsA while targeting a lower and narrower TBC range (80–130 ng/mL) compared with those usually recommended (100–200 ng/mL). This is consistent with our good results obtained with reduced immunosuppression. However, the DFS they reported in their control patients was much lower (42%). The GvL effect might have been altered by the MTX courses they added to CsA.
The low incidence of leukemia relapse observed in our cohort may result from an enhanced GvL effect favored by the use of CsA alone, without MTX or MMF. Indeed, CsA pharmacodynamic effect on NK cells is distinct and allows NK cell cytotoxicity,41 whereas it is impaired by MMF42, 43, 44 or MTX.45, 46, 47 Moreover, calcineurin inhibitors are the only immunosuppressive agents exerting their action by inhibiting interleukin-2 production. As CsA presents a concentration-dependent inhibitory effect on interleukin-2 production,48 we hypothesize that low TBC may lead to an interleukin-2 level sufficient to permit NK cells expansion. Unfortunately, most studies on leukemia outcome after HSCT in children give no details about target CsA TBC.
The role of corticosteroids on the GvL effect remains unclear. We did not find any significant difference in DFS between patients treated or not by prophylactic methylprednisolone, despite a trend to higher DFS in patients who had some (cord blood HSCT). However, cord blood as a stem cell source may also have an intrinsically enhanced GvL effect.49 Methylprednisolone has been shown to induce not only NK cell maturation but also to impair their cytotoxicity in vitro.50, 51 In vivo, prophylactic corticosteroids seemed to alter NK cell recovery after HSCT.52 The similar results between related and unrelated HSCT, which we observed, suggest that antithymocyte globulin has a minor role in GvL effect, confirming previous reports.53, 54
Some additional explanations for the GvL effect and the high DFS in our cohort might also be invoked. First, a majority of mild to moderate aGvHD grades were observed in our patients, exempting us to add other immunosuppressive agents other than methylprednisolone. Second, as this study is monocentric, patients in this study did not receive various combinations of immunosuppressive agents as usually reported.25, 28, 30, 35, 54
MRD before HSCT had no prognostic value in our cohort. The DFS of patients with negative or positive MRD before HSCT was similar. This contrasts with previous studies where DFS was about two times lower in patients with pretransplant detectable MRD.25, 27, 55, 56, 57 However, MRD could not be measured in all patients in our study. Thus, for those patients for whom data were available, only a few had positive MRD. This could also be related to the relatively short delay between CR achievement and transplantation in our center: 5.7±4.4 and 4.3±3.1 months for patients in CR1 and CR2, respectively. Nevertheless, the gentle immunosuppression given to our patients may have contributed to maintaining MRD <10−3, or to reversing MRD ⩾10−3 after HSCT. Actually, all previously published studies that described a worse outcome of pre-HSCT-positive MRD in ALL children reported the use of MTX or MMF as part of the GvHD prophylaxis regimen given.25, 27, 56 Among them, Balduzzi et al.27 obtained a relatively high rate of aGvHD (80%), which could have favored a GVL effect. However, their patients with grade II–IV GvHD received a median of three additional lines of immunosuppression, and the positive effect of GvHD on MRD might have been counterbalanced. The results obtained in our patients regarding the influence of pre-HSCT MRD on relapse are in accordance with those of Fagioli et al.30 They did not find any prognostic value of MRD in ALL either, and their cohort consisted of a majority of HSCT from MSDs with CsA alone as GvHD prophylaxis.
No data are available about the involvement of aGvHD in reducing MRD. GvHD has been shown to have a favorable effect on survival in patients with MRD ⩾10−3 as well as in patients with MRD <10−3.58 However, no relationships have been established between GvHD occurrence and the evolution of MRD. Our study suggests that GvHD may have a role in decreasing MRD. However, our cohort has too few patients with evaluable MRD before and after HSCT to draw any solid conclusions. Moreover, subclinical GvHD is not considered, although NK cell production may be sufficient to affect MRD. It is possible that some of our patients without clinical signs of GvHD might have benefit of a GvL effect just by maintaining relatively low CsA TBC. This is consistent with studies reporting no effect of GvHD on survival, while using MMF or MTX with a negative impact on NK cells.57 Further studies are mandatory to evaluate the possible link between CsA pharmacokinetics and NK cell production after HSCT.
Conclusions
Our results indicate that a GvHD prophylaxis regimen based on CsA alone, without either MTX or MMF, in children with ALL or AML is safe and effective, with similar GvHD and relapse rates in patients transplanted from MSDs and from unrelated donors. These results may be obtained provided that close monitoring of CsA TBC with specific target concentrations is performed. Further improvement may probably be achieved with better knowledge of post-transplant immunoregulation.
References
Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic marrow grafts. N Engl J Med 1979; 300: 1068–1073.
Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood 1990; 75: 555–562.
Jiang YZ, Kanfer EJ, Macdonald D, Cullis JO, Goldman JM, Barrett AJ . Graft-versus-leukaemia following allogeneic bone marrow transplantation: emergence of cytotoxic T lymphocytes reacting to host leukaemia cells. Bone Marrow Transplant 1991; 8: 253–258.
Keever CA, Klein J, Leong N, Copelan EA, Avalos BR, Kapoor N et al. Effect of GVHD on the recovery of NK cell activity and LAK precursors following BMT. Bone Marrow Transplant 1993; 12: 289–295.
Kataoka I, Kami M, Takahashi S, Kodera Y, Miyawaki S, Hirabayashi N et al. Clinical impact of graft-versus-host disease against leukemias not in remission at the time of allogeneic hematopoietic stem cell transplantation from related donors. Bone Marrow Transplant 2004; 34: 711–719.
Peters C, Minkov M, Gadner H, Klingebiel T, Vossen J, Locatelli F et al. Statement of current majority practices in graft-versus-host disease prophylaxis and treatment in children. Bone Marrow Transplant 2000; 26: 405–411.
Lee KH, Choi SJ, Lee JH, Kim S, Seol M, Lee YS et al. Cyclosporine alone versus cyclosporine plus methotrexate for post-transplant immunosuppression after HLA-identical sibling bone marrow transplantation: a randomized prospective study. Bone Marrow Transplant 2004; 34: 627–636.
Martin P, Bleyzac N, Souillet G, Galambrun C, Bertrand Y, Maire PH et al. Relationship between CsA trough blood concentration and severity of acute graft-versus-host disease after paediatric stem cell transplantation from matched-sibling or unrelated donors. Bone Marrow Transplant 2003; 32: 777–784.
Ram R, Storer B, Mielcarek M, Sandmaier BM, Maloney DG, Martin PJ et al. Association between calcineurin inhibitor blood concentrations and outcomes after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18: 414–422.
Gérard C, Bleyzac N, Girard P, Freyer G, Bertrand Y, Tod M . Links between cyclosporin exposure in tissues and graft-versus-host disease in pediatric bone marrow transplantation: analysis by a PBPK model. Pharm Res 2011; 28: 531–539.
Martin P, Bleyzac N, Souillet G, Galambrun C, Bertrand Y, Maire PH et al. Clinical and pharmacological risk factors for acute graft-versus-host disease after paediatric bone marrow transplantation from matched-sibling or unrelated donors. Bone Marrow Transplant 2003; 32: 881–887.
Willemze AJ, Press RR, Lankester AC, Egeler RM, den Hartigh J, Vossen JM . CsA exposure is associated with acute GVHD and relapse in children after SCT. Bone Marrow Transplant 2010; 45: 1056–1061.
Bleyzac N . The use of pharmacokinetic models in paediatric onco-haematology: effects on clinical outcome through the examples of busulfan and cyclosporine. Fundam Clin Pharmacol 2008; 22: 605–608.
Domenech C, Suciu S, De Moerloose B, Mazingue F, Plat G, Ferster A et al. Dexamethasone (6 mg/m2/day) and prednisolone (60 mg/m2/day) were equally effective as induction therapy for childhood acute lymphoblastic leukemia in the EORTC CLG 58951 randomized trial. Haematologica 2014; 99: 1220–1227.
Biondi A, Schrappe M, De Lorenzo P, Castor A, Lucchini G, Gandemer V et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol 2012; 13: 936–945.
Mann G, Attarbaschi A, Schrappe M, De Lorenzo P, Peters C, Hann I et al. Improved outcome with hematopoietic stem cell transplantation in a poor prognostic subgroup of infants with mixed-lineage-leukemia (MLL)-rearranged acute lymphoblastic leukemia: results from the Interfant-99 Study. Blood 2010; 116: 2644–2650.
Tallen G, Ratei R, Mann G, Kaspers G, Niggli F, Karachunsky A et al. Long-term outcome in children with relapsed acute lymphoblastic leukemia after time-point and site-of-relapse stratification and intensified short-course multidrug chemotherapy: results of trial ALL-REZ BFM 90. J Clin Oncol 2010; 28: 2339–2347.
Petit A, Ducassou S, Leblanc T, Pasquet M, Rousseau A, Ragu C et al. Relevance of a one-year maintenance therapy with interleukin-2 in the treatment of childhood acute myeloid leukemia: results from the French Multicenter, Phase III, Randomized Controlled SFCE Trial, ELAM02. Blood 2014; 124: 378.
Kaspers GJ, Zimmermann M, Reinhardt D, Gibson BE, Tamminga RY, Aleinikova O et al. Improved outcome in pediatric relapsed acute myeloid leukemia: results of a randomized trial on liposomal daunorubicin by the International BFM Study Group. J Clin Oncol 2013; 31: 599–607.
Dessars B, Cotton F, Thiry P, Gulbis B . Comparison of automated ACMIA and EMIT immunoassays for whole blood cyclosporin monitoring. Clin Lab 2003; 49: 135–140.
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation 1974; 18: 295–304.
van der Velden VH, van der Sluijs-Geling A, Gibson BE, te Marvelde JG, Hoogeveen PG, Hop WC et al. Clinical significance of flow cytometric minimal residual disease detection in pediatric acute myeloid leukemia patients treated according to the DCOG ANLL97/MRC AML12 protocol. Leukemia 2010; 24: 1599–1606.
Garand R, Beldjord K, Cavé H, Fossat C, Arnoux I, Asnafi V et al. Flow cytometry and IG/TCR quantitative PCR for minimal residual disease quantitation in acute lymphoblastic leukemia: a French multicenter prospective study on behalf of the FRALLE, EORTC and GRAALL. Leukemia 2013; 27: 370–376.
Rubnitz JE, Inaba H, Dahl G, Ribeiro RC, Bowman WP, Taub J et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol 2010; 11: 543–552.
Gandemer V, Pochon C, Oger E, Dalle JH, Michel G, Schmitt C et al. Clinical value of pre-transplant minimal residual disease in childhood lymphoblastic leukaemia: the results of the French minimal residual disease-guided protocol. Br J Haematol 2014; 165: 392–401.
Zhang P, Chen BJ, Chao NJ . Prevention of GVHD without losing GVL effect: windows of opportunity. Immunol Res 2011; 49: 49–55.
Balduzzi A, Di Maio L, Silvestri D, Songia S, Bonanomi S, Rovelli A et al. Minimal residual disease before and after transplantation for childhood acute lymphoblastic leukaemia: is there any room for intervention? Br J Haematol 2014; 164: 396–408.
Copelan EA, Hamilton BK, Avalos B, Ahn KW, Bolwell BJ, Zhu X et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood 2013; 122: 3863–3870.
Dini G, Zecca M, Balduzzi A, Messina C, Masetti R, Fagioli F et al. No difference in outcome between children and adolescents transplanted for acute lymphoblasticleukemia in second remission. Blood 2011; 118: 6683–6690.
Fagioli F, Quarello P, Zecca M, Lanino E, Rognoni C, Balduzzi A et al. Hematopoietic stem cell transplantation for children with high-risk acute lymphoblastic leukemia in first complete remission: a report from the AIEOP registry. Haematologica 2013; 98: 1273–1281.
Locatelli F, Masetti R, Rondelli R, Zecca M, Fagioli F, Rovelli A et al. Outcome of children with high-risk acute myeloid leukemia given autologous or allogeneic hematopoietic cell transplantation in the AIEOP AML-2002/01 study. Bone Marrow Transplant 2015; 50: 181–188 Erratum in: Bone Marrow Transplant 2015; 50: 320.
Peters C, Schrappe M, von Stackelberg A, Schrauder A, Bader P, Ebell W et al. Stem-cell transplantation in children with acute lymphoblastic leukemia: a prospective international multicenter trial comparing sibling donors with matched unrelated donors—the ALL-SCT-BFM-2003 Trial. J Clin Oncol 2015; 33: 1265–1274.
Zheng C, Tang B, Tong J, Liu H, Geng L, Wang X et al. Unrelated cord blood transplantation for central nervous system relapse in high-risk childhood acute lymphoblastic leukemia. Ann Hematol 2013; 92: 1665–1673.
Koh KN, Park M, Kim BE, Bae KW, Im HJ, Seo JJ . Favorable outcomes after allogeneic hematopoietic stem cell transplantation in children with high-risk or advanced acute myeloid leukemia. J Pediatr Hematol Oncol 2011; 33: 281–288.
Nemecek ER, Ellis K, He W, Bunin NJ, Bajwa RS, Cheerva A et al. Outcome of myeloablative conditioning and unrelated donor hematopoietic cell transplantation for childhood acute lymphoblastic leukemia in third remission. Biol Blood Marrow Transplant 2011; 17: 1833–1840.
Thiel E, Zhang MJ, Davies SM, Logan B, Tiedemann K, Eapen M et al. Comparison of outcomes after HLA-matched sibling and unrelated donor transplantation for children with high-risk acute lymphoblastic leukemia. Biol Blood Marrow Transplant 2012; 18: 1204–1210.
Weiss M, Steinbach D, Zintl F, Beck J, Gruhn B . Superior outcome using cyclosporin A alone versus cyclosporin A plus methotrexate for post-transplant immunosuppression in children with acute leukemia undergoing sibling hematopoietic stem cell transplantation. J Cancer Res Clin Oncol 2015; 141: 1089–1094.
Conter V, Valsecchi MG, Parasole R, Putti MC, Locatelli F, Barisone E et al. Childhood high-risk acute lymphoblastic leukemia in first remission: results after chemotherapy or transplant from the AIEOP ALL 2000 study. Blood 2014; 123: 1470–1478.
Sutton R, Shaw PJ, Venn NC, Law T, Dissanayake A, Kilo T et al. Persistent MRD before and after allogeneic BMT predicts relapse in children with acute lymphoblastic leukaemia. Br J Haematol 2015; 168: 395–404.
Pession A, Masetti R, Rizzari C, Putti MC, Casale F, Fagioli F et al. Results of the AIEOP AML 2002/01 multicenter prospective trial for the treatment of children with acute myeloid leukemia. Blood 2013; 122: 170–178.
Wang H, Grzywacz B, Sukovich D, McCullar V, Cao Q, Lee AB et al. The unexpected effect of cyclosporin A on CD56+CD16− and CD56+CD16+ natural killer cell subpopulations. Blood 2007; 110: 1530–1539.
Eissens DN, Van Der Meer A, Van Cranenbroek B, Preijers FW, Joosten I . Rapamycin and MPA, but not CsA, impair human NK cell cytotoxicity due to differential effects onNK cell phenotype. Am J Transplant 2010; 10: 1981–1990.
Ohata K, Espinoza JL, Lu X, Kondo Y, Nakao S . Mycophenolic acid inhibits natural killer cell proliferation and cytotoxic function: a possible disadvantage of including mycophenolate mofetil in the graft-versus-host disease prophylaxis regimen. Biol Blood Marrow Transplant 2011; 17: 205–213.
Meehan AC, Mifsud NA, Nguyen TH, Levvey BJ, Snell GI, Kotsimbos TC et al. Impact of commonly used transplant immunosuppressive drugs on human NK cell function is dependent upon stimulation condition. PLoS One 2013; 8: e60144.
Brenner BG, Friedman G, Margolese RG . The relationship of clinical status and therapeutic modality to natural killer cell activity in human breast cancer. Cancer 1985; 56: 1543–1548.
Rosenthal GJ, Germolec DR, Lamm KR, Ackermann MF, Luster MI . Comparative effects on the immune system of methotrexate and trimetrexate. Int J Immunopharmacol 1987; 9: 793–801.
Matheson DS, Green B, Hoar DI . The influence of methotrexate and thymidine on the human natural killer cell function in vitro. J Immunol 1983; 131: 1619–1621.
Stein CM, Murray JJ, Wood AJ . Inhibition of stimulated interleukin-2 production in whole blood: a practical measure of cyclosporine effect. Clin Chem 1999; 45: 1477–1484.
van Rood JJ, Scaradavou A, Stevens CE . Indirect evidence that maternal microchimerism in cord blood mediates a graft-versus-leukemia effect in cord blood transplantation. Proc Natl Acad Sci U S A 2012; 109: 2509–2514.
Vitale C, Cottalasso F, Montaldo E, Moretta L, Mingari MC . Methylprednisolone induces preferential and rapid differentiation of CD34+ cord blood precursors toward NK cells. Int Immunol 2008; 20: 565–575.
Vitale C, Chiossone L, Cantoni C, Morreale G, Cottalasso F, Moretti S et al. The corticosteroid-induced inhibitory effect on NK cell function reflects down-regulation and/or dysfunction of triggering receptors involved in natural cytotoxicity. Eur J Immunol 2004; 34: 3028–3038.
Giebel S, Dziaczkowska J, Wojnar J, Wojnar J, Krawczyk-Kulis M, Markiewicz M et al. The impact of immunosuppressive therapy on an early quantitative NK cell reconstitution after allogeneic haematopoietic cell transplantation. Ann Transplant 2005; 10: 29–33.
Veys P, Wynn RF, Ahn KW, Samarasinghe S, He W, Bonney D et al. Impact of immune modulation with in vivo T-cell depletion and myleoablative total body irradiation conditioning on outcomes after unrelated donor transplantation for childhood acute lymphoblastic leukemia. Blood 2012; 119: 6155–6161.
Bitan M, He W, Zhang MJ, Abdel-Azim H, Ayas MF, Bielorai B et al. Transplantation for children with acute myeloid leukemia: a comparison of outcomes with reduced intensity and myeloablative regimens. Blood 2014; 123: 1615–1620.
Leung W, Pui CH, Coustan-Smith E, Yang J, Pei D, Gan K et al. Detectable minimal residual disease before hematopoietic cell transplantation is prognostic but does not preclude cure for children with very-high-risk leukemia. Blood 2012; 120: 468–472.
Bachanova V, Burke MJ, Yohe S, Cao Q, Sandhu K, Singleton TP et al. Unrelated cord blood transplantation in adult and pediatric acute lymphoblastic leukemia: effect of minimal residual disease on relapse and survival. Biol Blood Marrow Transplant 2012; 18: 963–968.
Walter RB, Buckley SA, Pagel JM, Wood BL, Storer BE, Sandmaier BM et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood 2013; 122: 1813–1821.
Pulsipher MA, Langholz B, Wall DA, Schultz KR, Bunin N, Carroll W et al. Risk factors and timing of relapse after allogeneic transplantation in pediatric ALL: for whom and when should interventions be tested? Bone Marrow Transplant 2015; 50: 1173–1179.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bleyzac, N., Cuzzubbo, D., Rénard, C. et al. Improved outcome of children transplanted for high-risk leukemia by using a new strategy of cyclosporine-based GVHD prophylaxis. Bone Marrow Transplant 51, 698–704 (2016). https://doi.org/10.1038/bmt.2015.350
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.350
- Springer Nature Limited
This article is cited by
-
Impact of measurable residual disease by decentralized flow cytometry: a PETHEMA real-world study in 1076 patients with acute myeloid leukemia
Leukemia (2021)
-
A decision support tool to find the best cyclosporine dose when switching from intravenous to oral route in pediatric stem cell transplant patients
European Journal of Clinical Pharmacology (2020)
-
Bayesian Networks: A New Approach to Predict Therapeutic Range Achievement of Initial Cyclosporine Blood Concentration After Pediatric Hematopoietic Stem Cell Transplantation
Drugs in R&D (2018)