Abstract
Post-transplant cyclophosphamide plus calcineurin inhibitor (CNI)(tacrolimus or cyclosporine A) plus mycophenolate mofetil (PTCy/TAC or CSA/MMF) and anti-thymocyte globulin plus CNI (tacrolimus or cyclosporine A) plus methotrexate (ATG/TAC or CSA/MTX) are common graft-versus-host disease (GVHD) prophylaxis regimens. We compared the two regimens in patients with acute myeloid leukemia (AML) undergoing allogeneic transplantation from matched siblings or unrelated donors. 402 received PTCy/TAC or CSA/MMF and 5648 received ATG/TAC or CSA/MTX. Patients in the PTCy-based group were younger (48.7 vs. 51.5 years, p = 0.024) and there was a higher frequency of patient cytomegalovirus seropositivity and female donor to male patient combination in this group (77.8% vs. 71.8%, p = 0.009 and 18.4% vs. 14.4%, p = 0.029, respectively). More patients in the PTCy-based group received reduced-intensity conditioning (51.5% vs. 41%, p < 0.0001). No differences were observed in the incidence of acute GVHD grade II–IV and III–IV (21.2% vs. 20.4%, p = 0.92 and 8.1% vs. 6%, p = 0.1) or 2-year total and extensive chronic GVHD (33.7% vs. 30%, p = 0.09 and 10.7% vs. 11.2%, p = 0.81) between the groups. In the multivariate analysis, all transplant outcomes did not differ between the groups. PTCy/CNI/MMF and ATG/CNI/MTX are alternative regimens for GVHD prophylaxis in AML patients.
Similar content being viewed by others
Introduction
Graft-versus-host disease (GVHD) is a major obstacle to successful allogeneic hematopoietic stem cell transplantation (alloHSCT) and a leading cause of transplant-related mortality [1,2,3]. The gold standard for GVHD prophylaxis includes calcineurin inhibitors (CNIs), cyclosporine A (CSA), or tacrolimus (TAC) in combination with methotrexate (MTX) or mycophenolate mofetil (MMF) [4]. Anti-thymocyte globulin (ATG) has been demonstrated in several well-designed randomized studies to reduce the incidence of GVHD and is currently recommended for GVHD prophylaxis in patients undergoing alloHSCT from both matched unrelated donors (MUD) and mismatched unrelated donors (MMUD) as well as matched sibling donors (MSD) [4,5,6,7,8,9]. In parallel, post-transplant cyclophosphamide (PTCy), which was originally used in the haploidentical setting and led to a significant reduction in chronic (c) GVHD and non-relapse mortality (NRM), is being increasingly incorporated as GVHD prophylaxis in alloHSCT from other donor types, including MUD, MMUD and MSD [10,11,12,13,14]. In several, mostly registry-based European studies, PTCy-based GVHD prophylaxis was compared to non-PTCy, mostly ATG-containing GVHD prophylaxis regimens post alloHSCT from MUD, MMUD, or MSD in patients with acute myeloid leukemia (AML) demonstrating in the vast majority of them a significant reduction of NRM and cGVHD with PTCy [13,14,15,16,17]. The Center for International Blood and Marrow Transplant Research (CIBMTR) compared in a prospective multicentre phase 2 trial, the combination of PTCy, TAC, and MMF to TAC/MMF with bortezomib or TAC/ MTX and maraviroc in patients undergoing alloHSCT from MUD, MMUD and MSD, demonstrating that PTCy was the most effective regimen, yielding the best GVHD-free, relapse-free survival (GRFS) [18]. Subsequently, the CIBMTR performed a randomized multicenter phase III trial (CTN 1703) comparing PTCy plus TAC and MMF (experimental arm) to TAC plus MTX (control arm) in patients undergoing alloHSCT from MUD, MMUD, or MSD demonstrating lower incidence of severe acute and cGVHD and better GFRS in the PTCy arm [19]. However, the caveat of this practice-changing seminal study is that ATG was not included in the control arm. Taking advantage of the European Society for Blood and Marrow Transplantation (EBMT)/ Acute Leukemia Working Party (ALWP) registry, and that ATG in combination with CSA is the most frequent GVHD prophylaxis regimen for sibling and unrelated transplants in European centers [4], we aimed to compare PTCy in combination with CNI (TAC or CSA) and MMF (PTCy, TAC or CSA/MMF) to ATG, in combination with CNI (TAC or CSA) and MTX (ATG, TAC or CSA/MTX) as GVHD prophylaxis in patients with AML in first complete remission (CR1) transplanted from siblings or unrelated donors.
Subjects and methods
Study design and data collection
This was a retrospective, multicenter study. Data were provided by the registry of ALWP of the EBMT. The EBMT is a non-profit, scientific society representing more than 600 transplant centers, mainly located in Europe, which are required to report all consecutive stem cell transplantations and follow-ups once a year. Data are entered, managed, and maintained in a central database. Since the 1st of January 2003, all transplantation centers have been required to obtain written informed consent prior to data registration with the EBMT, as per the Declaration of Helsinki of 1975. Data accuracy is assured by the individual transplant centers and by quality control measures that include verification of the computer print-out of the entered data, cross-checking with the national registries, on-site visits to selected teams, and regular internal and external audits. The study was approved by the ALWP of the EBMT institutional review board and conducted per the Declaration of Helsinki and Good Clinical Practice guidelines. The results of disease assessments at HSCT were also submitted and form the basis of this report.
Criteria for selection
Eligibility criteria for this analysis included adult patients ≥18 years of age with AML who underwent their first alloHSCT from MSD, MUD, or MMUD (9/10) in CR between 2010 and 2020. Only peripheral blood stem cells (PBSC) as a graft source were allowed. Eligible GVHD prophylaxis included PTCy/CNI (TAC or CSA)/MMF or ATG/CNI (TAC or CSA)/MTX. The exclusion criteria were alloHSCT from haploidentical or cord blood donors; previous history of HSCT, disease status >CR1 or active disease, bone marrow (BM), and T cell-depleted hematopoietic cell graft. Pre-transplantation preparative regimens included both reduced-intensity conditioning (RIC) and myeloablative conditioning (MAC).
Data collected included recipient and donor characteristics (age, gender, and cytomegalovirus [CMV] serostatus), Karnofsky performance status (KPS), and hematopoietic cell transplantation-specific comorbidity index (HCT-CI), type of AML (de novo vs. secondary AML (sAML)), disease characteristics including disease status, cytogenetic risk, year of transplant, type of conditioning regimen, and GVHD prophylaxis, based on the reports from individual transplant centers as per previously established criteria [20]. The conditioning regimen was defined as MAC when containing total body irradiation (TBI) with a dose >6 Gray or a total dose of busulfan (Bu) > 8 mg/kg or >6.4 mg/kg when administered orally or intravenously, respectively. All other regimens were defined as RIC [20]. Grading of acute (a) GVHD was performed using established criteria [21]. cGVHD was classified as limited or extensive according to published criteria [22]. For this study, all necessary data were collected according to the EBMT guidelines, using the EBMT minimum essential data forms. The list of institutions contributing data to this study is provided in the Supplementary Appendix.
Statistical analysis
The median, interquartile range (IQR), and range were used for quantitative variables, and frequency and percentage for categorical variables. The study endpoints were the incidence of aGVHD and cGVHD, overall survival (OS) leukemia-free survival (LFS), relapse incidence (RI), non-relapse mortality (NRM), and GRFS. All endpoints were measured from the time of the transplantation. Engraftment was defined as achieving an absolute neutrophil count (ANC) of 0.5×109/L for three consecutive days. OS was defined as time to death from any cause. LFS was defined as survival with no evidence of relapse or progression. NRM was defined as death from any cause without previous relapse or progression. We used modified GRFS criteria. GRFS events were defined as the first event among grade III–IV aGVHD, extensive cGVHD, relapse, or death from any other cause [23]. Patient, disease, and transplant-related characteristics for the two cohorts were compared using the Mann–Whitney U test for numerical variables, and the χ2 or Fisher’s exact test for categorical variables. Median follow-up was calculated by the reverse Kaplan-Meier method. All outcomes were censored at 2 years post-transplantation to take into account the difference in follow-up periods between groups. The probabilities of OS, LFS, and GRFS were calculated using the Kaplan–Meier method. aGVHD, cGVHD, RI, and NRM were estimated using cumulative incidence (CI) curves in a competing risk setting. The RI and NRM were calculated using CI functions in a competing risk setting, with death in remission being treated as a competing event for relapse. Death was considered as a competing event for engraftment. To estimate the CI of acute or cGVHD, relapse and death were considered as competing events. Univariate analyses were performed using the log-rank test for LFS, OS, and GRFS whereas Gray’s test was used to compare CI estimates. Multivariate analyses (MVA) were performed using the Cox proportional-hazards regression model [24]. All variables differing significantly between the two groups, and potential risk factors were included in the model. To take into account the heterogeneity in the effect of a characteristic or a treatment across centers, we introduce a random effect (or frailty) into the Cox multivariate models [25]. To minimize the effect of confounding factors, a propensity score matching analysis was also performed. For each patient receiving PTCy/CNI/MMF, two separate matched controls receiving ATG/CNI/MTX were identified using exact and propensity-score matched criteria. Propensity score was estimated using logistic regression model. Exact matching was used for donor type (MSD/MUD/MMUD), type of AML (secondary/de novo), cytogenetic risk group (adverse/other), conditioning intensity (RIC/MAC), and nearest neighbor for recipient age, year of transplant, time from diagnosis to transplant, KPS (≥90 vs <90), sex matching (female to male/other), patient and donor CMV serology. Cluster-robust standard errors were used to account for dependence between observations within matched pairs. The patients were well matched, with standardized mean difference estimates of less than 10% for all matched parameters [26]. Results were expressed as the hazard ratio (HR) with a 95% confidence interval (95% CI). All p values were two-sided with a type 1 error rate fixed at 0.05. Statistical analyses were performed with SPSS 25.0 (SPSS Inc., Chicago, IL, USA) and R 4.0.2 (R Core Team Fifty (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/) [27].
Results
In total, 6050 patients met the inclusion criteria, 402 received PTCy/TAC or CSA/MMF and 5648 received ATG/TAC or CSA/MTX as GVHD prophylaxis. Table 1 shows the baseline demographic and clinical characteristics. Median follow-up was 23.4 and 41.8 months, respectively (p < 0.0001). Patients in the PTCy/TAC or CSA/MMF group were younger, with a median age of 48.7 (range 18–75.6) vs. 51.5 (18–77.8) years in the ATG/TAC or CSA/MTX group (p = 0.024), while gender did not differ. In both groups, 55.7% and 52.1% of the patients were male (p = 0.17), respectively. The median year of transplant was 2018 (range 2010–2020) and 2016 (2007–2020), respectively (p < 0.0001). Donors were MMUD (35.6% vs. 16%), MUD (45.3% vs. 61.4%), and MSD (19.2% vs. 22.6%) respectively, (p < 0.0001) (Table 1). De novo and sAML were diagnosed in 84.1% and 15.9% in the PTCy/TAC or CSA/MMF group and 85.3% and 14.7% in the ATG/TAC or CSA/MTX group, respectively (p = 0.49). In total, 276 (7.2%) patients were categorized as having a favorable cytogenetic risk, while intermediate and adverse risk were represented by 2585 (67.4%) and 974 (25.4%) patients, respectively, with no difference between the two prophylaxis groups (Table 1). KPS as well as HCT-CI did not differ between the groups. The PTCy/TAC or CSA/MMF group was characterized by a longer time from diagnosis to alloHSCT (5.7 vs. 5.3 months, p < 0.0001), higher frequency of female donor to male recipient combination (18.4% vs. 14.4%%, p = 0.029) and higher frequency of patients who were seropositive for CMV when compared to the ATG/TAC or CSA/MTX group (77.8% vs. 71.8%, p = 0.009). More patients in the PTCy/TAC or CSA/MMF group received RIC (51.5% vs. 41.0% in the ATG/TAC or CSA/MTX group, p < 0001). The most frequent conditioning regimen for both groups was Bu/fludarabine (Flu) in 55.2% and 41.3%, followed by thiotepa/Bu/Flu at 16.4% and 6.2%, and Bu/Cy in 3.2% and 21.5% of patients in the PTCy/TAC or CSA/MMF and ATG/TAC or CSA/MTX groups, respectively (Supplementary Table S1).
Transplantation outcome
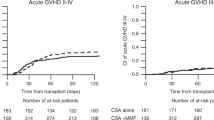
Engraftment and GVHD incidence did not differ between patients who received PTCy/TAC or CSA/MMF and ATG/TAC or CSA/MTX as GVHD prophylaxis, as depicted in Table 2. Neutrophil recovery (ANC > 0.5 × 109/L) was achieved in 98.7% in the PTCy/TAC or CSA/MMF group vs. 98.6% in the ATG/TAC or CSA/MTX group, respectively (p = 0.84) (Table 2). On day +180, the incidence of aGVHD grades II–IV and III–IV was (21.2% vs. 20.4%, p = 0.92 and 8.1% vs. 6.0%, p = 0.1, in PTCy/TAC or CSA/MMF and ATG/TAC or CSA/MTX groups, respectively. Two-year incidence of total and extensive cGVHD were 33.7% vs. 30% (p = 0.09) and 10.7% vs. 11.2% (p = 0.81), respectively (Table 2, Fig. 1). Similarly, 2-year NRM and RI as well as LFS, OS, and GRFS did not differ between patients who received PTCy/TAC or CSA/MMF and ATG/TAC or CSA/MTX. There were no significant differences between the groups with respect to 2-year NRM and RI [9.7% (6.9–13%) vs. 13.3% (12.4–14.3%) (p = 0.11) and 25% (20.4–29.8%) vs. 27.1% (25.9–28.4%) (p = 0.52), respectively (Table 2)]. Similarly, there were no significant differences between the groups with respect to the 2-year LFS, OS, and GRFS [65.3% (59.9–70.2%) vs. 59.6% (58.2–60.9%) (p = 0.12), 70.8% (65.3–75.5%) vs. 67.4% (66.1–68.7%) (p = 0.27) and 54.1% (48.6–59.3%) vs. 51% (49.6–52.4%) (p = 0.71), respectively (Table 2, Figs. 1, 2).
Post-transplant cyclophosphamide, tacrolimus, or cyclosporine A and mycophenolate mofetil (PTCy CSA/Tacro MMF) compared to anti-thymocyte globulin, tacrolimus, or cyclosporine A and methotrexate (ATG CSA/Tacro MTX) combinations as graft-versus-host disease prophylaxis (GVHD) post-allogeneic stem cell transplantation from siblings and unrelated donors in patients with acute myeloid leukemia: acute GVHD II–IV, acute GVHD III–IV, chronic GVHD, and GVHD-free and relapse-free survival (GRFS).
Post-transplant cyclophosphamide, tacrolimus or cyclosporine A and mycophenolate, mofetil (PTCy CSA/Tacro MMF) compared to anti-thymocyte globulin, tacrolimus or cyclosporine A and methotrexate (ATG CSA/Tacro MTX) combinations as graft-versus-host disease prophylaxis (GVHD) post allogeneic stem cell transplantation from siblings and unrelated donors in patients with acute myeloid leukemia: Non-relapse mortality (NRM), relapse incidence (RI), leukemia-free survival (LFS) and overall survival (OS).
Multivariate analysis
In the MVA, the incidence of aGVHD II–IV (HR = 0.95; 95% CI: 0.71–1.27; p = 0.71), aGVHD III–IV (HR = 1.38; 95% CI: 0.88–2.16; p = 0.16), cGVHD all grades (HR = 1.2; 95% CI: 0.91–1.56; p = 0.19), and extensive cGVHD (HR = 0.66; 95% CI: 0.41–1.04; p = 0.074) did not differ significantly between the PTCy/TAC or CSA/MMF and ATG/TAC or CSA/MTX groups (Table 3). Similarly, no difference was observed in risk of NRM, RI, LFS or OS between the two groups with HRs of 0.79 (95% CI: 0.53–1.17; p = 0.32),1.03 (95% CI: 0.8–1.33; p = 0.79), 0.94 (95% CI: 0.76–1.16; p = 0.56), 0.99 (95% CI: 0.78–1.26; p = 0.93) and 0.95 (95% CI: 0.78–1.15; p = 0.60), respectively (Table 4). MMUD was a prognostic factor for increased risk of aGVHD, cGVHD, NRM, GRFS, LFS, and OS while MUD was associated with a higher risk of NRM and aGVHD grade II–IV. Older patient age (per 10 years) was a prognostic factor for NRM, LFS, OS, and GRFS while sAML was associated with a higher risk of NRM and lower LFS. Risk factors for RI were adverse cytogenetics, shorter time from diagnosis to alloHSCT, KPS < 90, RIC, and CMV seropositivity in patients. In addition, prognostic factors for NRM were KPS < 90, MAC, patient CMV seropositivity, and less recent year of transplantation. Adverse cytogenetics and KPS < 90 were prognostic factors for LFS, OS, and GRFS while a shorter time from diagnosis to alloHSCT was a prognostic factor for LFS and GRFS. RIC was associated with a lower risk of aGVHD grade II–IV as well as a lower risk of total and extensive cGVHD. Female donor to male patient transplant combination was a risk factor for total and extensive cGVHD. LFS, OS, and GRFS were affected by patient CMV seropositivity and earlier years of transplantation. Additionally, CMV seropositivity in patients was a risk factor for aGVHD (both grades II–IV and grades III–IV) as well as total and extensive cGVHD.
Matched-pair analysis
Using the criteria mentioned above, 350 PTCy/TAC or CSA/MMF were matched with 663 ATG/TAC or CSA/MTX (Table 5). The results of the matched-pair analysis were consistent with previous results. Incidence of both acute and cGVHD was similar between patients receiving PTCy/TAC or CSA/MMF group and those receiving ATG/TAC or CSA/MTX: aGVHD grades II–IV 21.6% (17.4–26.1%) vs. 19% (16–22.1%), HR = 1.11 (95% CI 0.84–1.49, p = 0.46), aGVHD grades III–IV 8% (5.4–11.2%) vs. 5.2% (3.7–7.2%), HR = 1.52 (95% CI 0.92–2.5, p = 0.099), total cGVHD 31.7% (26.3–37.2%) vs 30.7% (26.8–34.7%), HR = 1.07 (95% CI 0.83–1.38, p = 0.62) and extensive cGVHD 9.3% (6.1–13.2%) vs 12.8% (10.1–15.8%), HR = 0.69 (0.44–1.1, p = 0.12), respectively (Table 5B). Two-year NRM and RI did not differ as well 9.3% (6.4–12.9%) vs 13.5% (10.9–16.3%), HR = 0.7 (95% CI 0.47–1.07,p = 0.097) and 27.3% (22.2–32.6%) vs. 24.9% (21.4–28.6%), HR = 1.1 (0.83–1.46, p = 0.5), respectively (Table 5C). There were also no differences in LFS, OS, and GRFS between the two groups 63.4% (57.5–68.7%) vs 61.6% (57.5–65.5%), HR = 0.96 (0.76–1.21, p = 0.71); 69.7% (63.8–74.8%) vs 67.9% (63.9–71.6%), HR = 0.93 (0.72–1.2, p = 0.59) and 53.3% (47.4–58.9%) vs 52.9% (48.7–57%), HR = 1.03 (0.84–1.26), p = 0.76), respectively (Table 5C).
Cause of death
A total of 1716 patients died within 2 years post-transplantation, 97 of those receiving PTCy/TAC or CSA/MMF, and 1619 of those receiving ATG/TAC or CSA/MTX. The original disease was the main cause of death accounting for 57.9% and 51.5% of deaths, respectively. The second cause of death was infection with 16.8% and 20.2%, followed by GVHD with 11.6% and 13.9% of deaths, in patients receiving PTCy/TAC or CSA/MMF, and ATG/TAC or CSA/MTX, respectively. Multi-organ failure accounted for 3.2% and 1% of the deaths, respectively. Other causes of death were infrequent (<2%) and included veno-occlusive disease of the liver, cardiac toxicity, hemorrhage, graft failure, central nervous system toxicity, interstitial pneumonitis, and second malignancies including lymphoproliferative disorders, with no difference between the prophylaxis groups (Table 6).
Discussion
In this real-life study, we evaluated in the European setting the two regimens used for GVHD prophylaxis in the CIBMTR CTN 1703 phase III study, namely PTCy plus TAC and MMF and TAC plus MTX in AML patients undergoing alloHSCT from MUD, MMUD, or MSD including ATG in the comparative arm and CSA in both arms as both are included in the most frequent GVHD prophylaxis regimens in European centers. The studied regimens thus included CNI, CSA, or TAC, with MMF or MTX in combination with ATG or PTCy which is being increasingly used as GVHD prophylaxis, not just in the haploidentical setting but also in alloHSCT from unrelated and even matched sibling donors [28,29,30]. We did not observe any differences in aGVHD grades II–IV or III–IV or cGVHD all grades and extensive between patients treated with PTCy/CSA or TAC/MMF and ATG/CSA or TAC/MTX.These results were also confirmed by matched pair analysis. Moreover, there were no differences, including by matched pair analysis, between the two GVHD prophylaxis regimens in other transplantation outcomes including NRM, RI, LFS, OS, and GRFS in agreement with some of the previous publications in regard including a recent publication by Brissot et al. on behalf of the ALWP comparing GVHD prophylaxis using either PTCY or ATG in patients with AML who underwent alloHSCT in CR1 from 10/10 unrelated donors, with no statistical differences between the two groups with respect to NRM, RI, OS, LFS, and GRFS [16].Similar results were obtained by Bailin et al. in 132 consecutive patients undergoing a matched or 9/10 mismatched unrelated donor transplantation in Spain comparing PTCy-based to ATG-based prophylaxis with no significant differences in 2-year OS, EFS, RI, and NRM [17].In another study comparing reduced doses of PTCy (40 mg/Kg) to ATG-based anti-GVHD prophylaxis in 9/10 mismatched unrelated donor transplantation, the authors observed significantly longer 1-year OS in the PTCy group with a similar 1-year PFS, RI, and NRM [18]. As for GVHD, PTCy in combination with TAC or CSA and MMF or MTX, was shown to be very effective in reducing both GVHD and NRM [13,14,15,16,17]. The Seattle group evaluated the incidence of GVHD with PTCy/CSA GVHD prophylaxis in patients with various hematological malignancies undergoing alloHSCT from MSD or MUD with PBSC grafts demonstrating no severe aGVHD and a 1-year cumulative incidence of cGvHD as low as 16% [31]. Similarly, in a previous study from the ALWP of the EBMT analyzing 423 patients with acute leukemia undergoing alloHSCT from MSD or MUD who received PTCy-based GVHD prophylaxis including in combination with CSA, MTX, or MMF the authors demonstrated reduced risk of severe cGVHD and mortality leading to improved survival [32]. The combination of PTCy with TAC and MMF was the subject of a study by Carnevale-Schianca et al. in which the authors showed encouraging results in terms of GVHD-event-free survival (GVHD-EFS) (53% at 2 years) and very low NRM (4% at 1 year) [33]. Mehta et al. compared 964 patients with various hematological malignancies undergoing MSD (n = 412) and MUD (n = 552) transplants with PTCy- based (n = 386) versus TAC/MTX (n = 578) GVHD prophylaxis. The MUD group also received ATG. In the MVA, the risk of grade III–IV aGVHD was similar in the PTCy and TAC/MTX groups in both MSD and MUD cohorts, while the risk of cGVHD was significantly lower with PTCy in the MSD cohort. Moreover, PTCy was associated with a significantly lower risk of NRM and better PFS in MUD patients and with an improved GRFS in both the MSD and MUD groups [34].These encouraging data were subsequently confirmed by randomized studies. The HOVON-96 trial prospectively randomized PTCy/CSA vs. CSA / MMF GVHD prophylaxis in 160 patients with diverse hematological diseases undergoing MSD or MUD alloHSCT.The cumulative incidence of grade II–IV aGVHD at 6 months was 30% vs. 48%, and the 2-year CI of extensive cGVHD was 16% vs. 48%, respectively. The 1-year estimated GRFS was 45% vs. 21% [35]. Another multicenter phase III trial compared PTCy with BM grafts to TAC/MTX with BM grafts or CD34-selected PBSCs in patients with acute leukemia or MDS and an HLA-matched donor. PTCy was associated with comparable cGVHD and survival to the control arms [36]. The results of the CIBMTR randomized multicenter phase III trial (CTN 1703) comparing PTCy / TAC /MMF / to TAC / MTX in patients undergoing alloHSCT from MUD, MMUD, and MSD were recently published, demonstrating lower incidence of severe acute and cGVHD and better GFRS in the PTCy arm [19]. However, all of these single-center, registry-based, and even randomized controlled studies demonstrating (in the vast majority), the superiority of PTCy in conjunction with two IS drugs over historically IS-based anti-GVHD prophylaxis, did not include ATG, a cornerstone of anti-GVHD prophylaxis in Europe [4,5,6,7, 9], in the comparator arm. In the current study, we compared PTCy combined with CSA or TAC and MMF vs. ATG combined with CSA or TAC and MTX and demonstrated no differences in incidence of GVHD both acute and cGVHD as well as in other transplant outcome parameters, including MSD as well as 9–10/10 MUD alloHSCT in a relatively large cohort of AML patients transplanted in CR1. Several previous mostly single center studies have compared PTCy-based to ATG-based anti-GVHD prophylaxis with somewhat variable results [16, 34, 37,38,39,40,41,42]. Modi et al. compared PTCy-based to thymoglobulin-based anti-GVHD prophylaxis in patients with AML and MDS undergoing alloHSCT from MMUD demonstrating reduced rates of both aGVHD and cGVHD as well as TRM with PTCy [37]. Berro and colleagues compared PTCy to ATG in patients receiving unrelated alloHSCT for various hematological malignancies with PTCy resulting in lower risk of grade II–IV aGVHD and TRM, while incidence of grade III–IV aGVHD and cGVHD were comparable [38]. More recently, Dachy et al. compared PTCy to ATG in patients undergoing MUD, reporting lower incidence of day 100 grade II–IV aGVHD, 1 year cGvHD (not confirmed by MVA) with comparable 1 year TRM with PTCy [39]. Notably, Moissev et al. reported on 87 patients with AML or acute lymphocytic leukemia at various disease stages that underwent 8-10/10 unrelated PBSC alloHSCT with received PTCy/TAC/MMF anti-GVHD prophylaxis in a single center that was compared to 125 patients (historical controls) that received ATG/TAC and MTX or MMF. They demonstrated the superiority of the PTCy-based GVHD prophylaxis in terms of both acute and cGVHD and NRM but also a better EFS, OS, and GRFS [40]. In contrast, Massoud and colleagues compared PTCy-based to anti-T-cell lymphocyte globulin anti-GVHD prophylaxis in a very heterogeneous group of patients with a broad range of hematological malignancies and donors including haploidentical donors, showing similar overall incidence of GVHD but an increased incidence of moderate/severe cGVHD with PTCy (44% vs. 38%, p = 0.005) [41].It is conceivable that the variable results are due to major differences in the various studies comparing PTCy to ATG including in the type and status of the basic disease, the type of donor, conditioning regimens, grafts and stem cell sources, the IS combined with either PTCy or ATG as GVHD prophylaxis and other factors. In an attempt to consolidate the variable results Tang et al. recently performed a meta-analysis of PTCy versus ATG prophylaxis in patients undergoing unrelated alloHSCT, demonstrating lower incidence of grades II–IV and grades III–IV aGVHD, and NRM resulting in a better OS with PTCy compared to ATG-based regimen, with comparable incidence of cGVHD [42]. Finally, as mentioned above, Brissot et al. compared GVHD prophylaxis using either PTCY (n = 174) or ATG (n = 1452) with various (n = 13) IS combinations including ATG/CSA or TAC/MTX in 45% and PTCy CSA or TAC/MMF in 40%, in patients with AML who underwent alloHSCT in CR1 from a 10/10 HLA-MUD between 2010-2017, showing no difference in the incidence of acute or cGVHD including in the severe and extensive forms, and no difference in any other transplant outcomes [16]. Our current similar findings are based on a real-life European based study in a large cohort of a homogenous group of AML patients undergoing alloHSCT in a more recent period with a defined IS regimen (TAC or CSA with MMF vs MTX) combined with PTCy vs. ATG, respectively.These results are very different from those of the randomized prospective study (CTN1703) that compared the same anti-GVHD prophylaxis regimens but omitting ATG from the control arm and allowing only TAC, demonstrating a significant superiority of PTCy reducing severe acute and cGVHD and improving GRFS [19]. With the limitations and inferiority of a registry-based non-randomized study, it is still conceivable, based on our current study and previous literature, that including ATG in the control arm may have changed at least to some degree, the CTN 1703 results. The field is still waiting for a well-designed, prospective randomized controlled, multicenter study comparing PTCy to ATG with two defined IS drugs in each arm, as GVHD prophylaxis post unrelated alloHSCT in patients with AML.
The other factors observed to be associated with an increased risk of acute and or cGVHD (including alloHSCT from unrelated and mismatched donors, year of transplant, MAC, female donor-to-male patient combination, and patient CMV seropositivity) are in agreement with previous publications defining risk factors for GVHD post alloHSCT [1,2,3,4,5,6,7,8,9]. In this regard, a previously reported large EBMT mega-file study assessed GVHD incidence and outcome over different periods (1990–2015) in 102,557 patients with hematological malignancies after the first allogeneic sibling or unrelated donor transplant [43]. In multivariate analysis, transplantation from an unrelated donor was associated with an increased risk of acute GVHD and mortality [43]. CMV seropostivity being a risk factor also for relapse is somewhat intriguing in light of previous reports that suggest a potential antileukemic effect associated with cytomegalovirus reactivation [44]. One possible explanation is that the immunosuppression and GVHD abrogated the protective effect of CMV reactivation. In agreement with this possibility is the recent study by Turki AT demonstrating that in AML patients in CR1 who had received ATG for in vivo T cell depletion, CMV reactivation was associated with a significantly increased relapse rate [44]. Being retrospective and registry-based, this transplantation study has several limitations including the risk of selection bias, including certain indications to select one GVHD prophylaxis versus another, and the possibility of unavailable data that could not have been considered, such as type and dose of ATG, dose and schedule of PTCy, frontline therapies as well as mutation profiling and measurable residual disease that is missing in approximately half of the patients.
In conclusion, in this relatively large, real-world multi-center, registry-based retrospective analysis of AML patients transplanted from MMUD, MUD, or MSD in CR1, we have demonstrated that the use of PTCy/CNI (TAC or CSA)/MMF and ATG/CNI (TAC or CSA)/MTX as GVHD prophylaxis has resulted in similar incidence of both acute and cGVHD and the main transplant outcomes.
Data availability
AN, ML, FC, and MM had full access to all study data (available upon data-specific request).
References
Zeiser R, Blazar BR. Acute graft-versus-host disease—biologic process, prevention, and therapy. N. Engl J Med. 2017;377:2167–79.
Malard F, Holler E, Sandmaier BM, Huang H, Mohty M. Acute graft-versus-host disease. Nat Rev Dis Prim. 2023;9:27.
Zeiser R, Blazar BR. Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N. Engl J Med. 2017;377:2565–79.
Penack O, Marchetti M, Aljurf M, Arat M, Bonifazi F, Duarte RF, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2024;11:e147–e159.
Finke J, Bethge WA, Schmoor C, Ottinger HD, Stelljes M, Zander AR, et al. ATG-Fresenius Trial Group. Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol. 2009;10:855–64.
Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood. 2001;98:2942–7.
Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M, et al. Cell Therapy Transplant Canada. Addition of anti-thymocyte globulin to standard graft-versus-host disease prophylaxis versus standard treatment alone in patients with haematological malignancies undergoing transplantation from unrelated donors: final analysis of a randomised, open-label, multicentre, phase 3 trial. Lancet Haematol. 2020;7:e100–e111.
Soiffer RJ, Kim HT, McGuirk J, Horwitz ME, Johnston L, Patnaik MM, et al. Prospective, randomized, double-blind, phase III clinical trial of anti-T-lymphocyte globulin to assess impact on chronic graft-versus-host disease-free survival in patients undergoing HLA-matched unrelated myeloablative hematopoietic cell transplantation. J Clin Oncol. 2017;35:4003–11.
Kröger N, Solano C, Wolschke C, Bandini G, Patriarca F, Pini M, et al. Anti-lymphocyte globulin for prevention of chronic graft-versus-host disease. N. Engl J Med. 2016;374:43–53.
Luznik L, O’Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39:683–93.
Nagler A, Labopin M, Blaise D, Raiola AM, Corral LL, Bramanti S, et al. Non-T-depleted haploidentical transplantation with post-transplant cyclophosphamide in patients with secondary versus de novo AML in first complete remission: a study from the ALWP/EBMT. J Hematol Oncol. 2023;16:58.
Sanz J, Galimard JE, Labopin M, Afanasyev B, Angelucci E, Ciceri F, et al. Post-transplant cyclophosphamide after matched sibling, unrelated and haploidentical donor transplants in patients with acute myeloid leukemia: a comparative study of the ALWP EBMT. J Hematol Oncol. 2020;13:46.
Battipaglia G, Labopin M, Kröger N, Vitek A, Afanasyev B, Hilgendorf I, et al. Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood. 2019;134:892–9.
Lorentino F, Labopin M, Ciceri F, Vago L, Fleischhauer K, Afanasyev B, et al. Post-transplantation cyclophosphamide GvHD prophylaxis after hematopoietic stem cell transplantation from 9/10 or 10/10 HLA-matched unrelated donors for acute leukemia. Leukemia. 2021;35:585–94.
Bailén R, Kwon M, Pascual-Cascón MJ, Ferrà C, Sanz J, Gallardo-Morillo A, et al. Post-transplant cyclophosphamide for GVHD prophylaxis compared to ATG-based prophylaxis in unrelated donor transplantation. Ann Hematol. 2021;100:541–53.
Brissot E, Labopin M, Moiseev I, Cornelissen JJ, Meijer E, Van Gorkom G, et al. Post-transplant cyclophosphamide versus anti-thymocyte globulin in patients with acute myeloid leukemia in first complete remission undergoing allogeneic stem cell transplantation from 10/10 HLA-matched unrelated donors. J Hematol Oncol. 2020;13:87.
Soltermann Y, Heim D, Medinger M, Baldomero H, Halter JP, Gerull S, et al. Reduced dose of post-transplantation cyclophosphamide compared to ATG for graft-versus-host disease prophylaxis in recipients of mismatched unrelated donor hematopoietic cell transplantation: a single-center study. Ann Hematol. 2019;98:1485–93.
Bolaños-Meade J, Reshef R, Fraser R, Fei M, Abhyankar S, Al-Kadhimi Z, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomized phase 2 trial with a non-randomized contemporaneous control group (BMT CTN 1203). Lancet Haematol. 2019;6:e132–e143.
Bolaños-Meade J, Hamadani M, Wu J, Al Malki MM, Martens MJ, Runaas L, et al. Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N. Engl J Med. 2023;388:2338–48.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl. 2009;15:1628–33.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transpl. 1995;15:825–8.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17.
Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transpl. 2016;51:610–1.
Kanate AS, Nagler A, Savani B. Summary of scientific and statistical methods, study endpoints and definitions for observational and registry-based studies in hematopoietic cell transplantation. Clin Hematol Int. 2019;2:2–4.
Andersen PK, Klein JP, Zhang MJ. Testing for center effects in multi-center survival studies: a Monte Carlo comparison of fixed and random effects tests. Stat Med. 1999;18:1489–500.
McCafrey DF, Griffin BA, Almirall D, Slaughter ME, Ramchand R, Burgette LF. A tutorial on propensity score estimation for multiple treatments using generalized boosted models. Stat Med. 2013;32:3388–414.
R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2020. https://www.R-project.org/.
Al Malki MM, Tsai NC, Palmer J, Mokhtari S, Tsai W, Cao T, et al. Posttransplant cyclophosphamide as GVHD prophylaxis for peripheral blood stem cell HLA-mismatched unrelated donor transplant. Blood Adv. 2021;5:2650–9.
Bacigalupo A, Jones R. PTCy the “new” standard for GVHD prophylaxis. Blood Rev. 2023;62:101096.
Leick M, Bin Chen Y. A glimpse into what happens after PTCy. Blood. 2022;139:479–81.
Mielcarek M, Furlong T, O’Donnell PV, Storer BE, McCune JS, Storb R, et al. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–8.
Ruggeri A, Labopin M, Bacigalupo A, Afanasyev B, Cornelissen JJ, Elmaagacli A, et al. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Onco. 2018;11:40.
Carnevale-Schianca F, Caravelli D, Gallo S, Becco P, Paruzzo L, Poletto S, et al. Post-transplant cyclophosphamide and tacrolimus–mycophenolate mofetil combination governs GVHD and immunosuppression need, reducing late toxicities in allogeneic peripheral blood hematopoietic cell transplantation from HLA-matched donors. J Clin Med. 2021;10:1173.
Mehta RS, Saliba, et al. Post-transplantation cyclophosphamide versus tacrolimus and methotrexate graft-versus-host disease prophylaxis for HLA-matched donor transplantation. Transpl Cell Ther. 2022;28:695.e1–695.e10.
Broers AEC, de Jong CN, Bakunina K, Hazenberg MD, van Marwijk Kooy M, de Groot MR, et al. Posttransplant cyclophosphamide for prevention of graft-versus-host disease: results of the prospective randomized HOVON-96 trial. Blood Adv. 2022;6:3378–85.
Luznik L, Pasquini MC, Logan B, Soiffer RJ, Wu, et al. Randomized phase III BMT CTN trial of calcineurin inhibitor-free chronic graft-versus-host disease interventions in myeloablative hematopoietic cell transplantation for hematologic malignancies. J Clin Oncol. 2022;40:356–68.
Modi D, Kondrat K, Kim S, Deol A, Ayash L, Ratanatharathorn V, et al. Post-transplant cyclophosphamide versus thymoglobulin in HLA-mismatched unrelated donor transplant for acute myelogenous leukemia and myelodysplastic syndrome. Transpl Cell Ther. 2021;27:760–7.
Berro M, Rivas M, Trucco J, Paganini I, Ravchina I, Kusminsky G. Post-transplant cyclophosphamide demonstrates lower non-relapse mortality and better graft-versus-host disease/relapse-free survival compared with antithymocyte globulin in unrelated donor hematopoietic stem cell transplantation. A single-center experience. Bone Marrow Transpl. 2021;56:986–8.
Dachy F, Furst S, Calmels B, Pagliardini T, Harbi S, Bouchacourt B, et al. GVHD prophylaxis with post-transplant cyclophosphamide results in lower incidence of GVHD and allows faster immunosuppressive treatment reduction compared to antithymocyte globulin in 10/10 HLA-matched unrelated allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2023;58:1179–81.
Moiseev IS, Pirogova OV, Alyanski AL, Babenko EV, Gindina TL, Darskaya EI, et al. Graft-versus-host disease prophylaxis in unrelated peripheral blood stem cell transplantation with post-transplantation cyclophosphamide, tacrolimus, and mycophenolate mofetil. Biol Blood Marrow Transpl. 2016;22:1037–42.
Massoud R, Gagelmann N, Fritzsche-Friedland U, Zeck G, Heidenreich S, Wolschke C, et al. Comparison of immune reconstitution between anti-T-lymphocyte globulin and posttransplant cyclophosphamide as acute graft-versus-host disease prophylaxis in allogeneic myeloablative peripheral blood stem cell transplantation. Haematologica. 2022;107:857–67.
Tang, Liu L, Li Z, Dong T, Wu T, Niu Q, et al. Post-transplant cyclophosphamide versus anti-thymocyte globulin in allogeneic hematopoietic stem cell transplantation from unrelated donors: a systematic review and meta-analysis. Front Oncol. 2023;13:1071268.
Greinix HT, Eikema DJ, Koster L, Penack O, Yakoub-Agha I, Montoto S, et al. Improved outcome of patients with graft-versus-host disease after allogeneic hematopoietic cell transplantation for hematologic malignancies over time: an EBMT mega-file study. Haematologica. 2022;107:1054–63.
Turki AT, Tsachakis-Mück N, Leserer S, Crivello P, Liebregts T, Betke L, et al. Impact of CMV reactivation on relapse of acute myeloid leukemia after HCT is dependent on disease stage and ATG. Blood Adv. 2022;6:28–36.
Acknowledgements
We thank all the EBMT centers and national registries for contributing patients to this study (Supplementary appendix material). We also thank the data managers for their excellent work.
Author information
Authors and Affiliations
Contributions
AN wrote the manuscript, designed the study, and interpreted the data. ML and MM designed the study, performed the statistical analyses, interpreted the data, and edited the manuscript. RS helped with writing the first draft of the manuscript. TS, RMH, LG, US, AR, SM, AK, JP, PD, TGD, EF, GH, MS, CCL, AS, EB, and FC reviewed the manuscript and provided clinical data. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The scientific boards of the ALWP of the EBMT approved this study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nagler, A., Labopin, M., Swoboda, R. et al. Post-transplant cyclophosphamide, calcineurin inhibitor, and mycophenolate mofetil compared to anti-thymocyte globulin, calcineurin inhibitor, and methotrexate combinations as graft-versus-host disease prophylaxis post allogeneic stem cell transplantation from sibling and unrelated donors in patients with acute myeloid leukemia: a study on behalf of the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant 59, 1012–1021 (2024). https://doi.org/10.1038/s41409-024-02284-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-024-02284-5
- Springer Nature Limited