Abstract
Purpose
A small glutamine-rich tetratricopeptide repeat-containing protein alpha (SGTA) is a 35 kDa protein involved in a number of biological processes. However, the role of SGTA in non-small-cell lung cancer (NSCLC) tumorigenesis has never been elucidated. The purpose of this study was to determine whether SGTA could serve as a biomarker for stratification and prediction of prognosis in NSCLC.
Methods
Small glutamine-rich tetratricopeptide repeat-containing protein alpha expression was evaluated by Western blot in 8 paired fresh lung cancer tissues and immunohistochemistry on 83 paraffin-embedded sections. The effect of SGTA was assessed by RNA interference in A549 cells. Serum starvation and refeeding, flow cytometry, CCK-8, and tunnel assays were performed.
Results
Small glutamine-rich tetratricopeptide repeat-containing protein alpha was highly expressed in NSCLC and significantly correlated with NSCLC histological differentiation, clinical stage, and Ki-67. Multivariate analysis indicated that SGTA was an independent prognostic factor for NSCLC patients’ survival. The present investigation demonstrated that suppression of SGTA expression resulted in a significant decline of proliferation in A549 cells. Besides, SGTA could abolish the toxicity of cisplatin in A549 cells.
Conclusions
These findings suggested that SGTA might play an important role in promoting the tumorigenesis of NSCLC, and thus be a promising therapeutic target to prevent NSCLC progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer, predominantly non-small-cell lung cancer (NSCLC), is the leading cause of cancer-related death in the world. NSCLC, composed of three types according to histologic types: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma, is the most common form of lung cancer (Ji et al. 2011). Patients’ 5-year survival rate with NSCLC is terrifically <15 % (Jemal et al. 2010; Siegel et al. 2011), owing to the poor diagnostic rate in the early stage, high rates of recurrence, and difficulty for therapy. A better understanding of the underlying molecular mechanisms of progression of NSCLC would significantly benefit the clinical outcome. Besides, it is certainly of great interest to identify novel biomarkers and therapeutic targets for NSCLC.
SGT, small glutamine-rich tetratricopeptide repeat (TPR)-containing protein, also referred as viral U-binding protein (UBP), is a 35 kDa protein involved in a number of biological processes, including participation in endocytosis, interaction with growth hormone receptors (GHR), control of viral replication (Callahan et al. 1998; Cziepluch et al. 2000; Fielding et al. 2006), mitosis, apoptosis (Ommen et al. 2010; Yin et al. 2006), and cell division (Dutta et al. 2008; Worrall et al. 2008). SGT comprises three structural units: an N-terminal self-association domain, a tetratricopeptide repeat (TPR) domain, and a C-terminal glutamine-rich domain (Dutta et al. 2008; Liou and Wang 2005). Owing to its TPR domain, SGT is a regulator of protein–protein interaction. SGT was identified as a protein interacting with non-structural protein NS1 of parvovirus H-1, thus inhibited the efficiency of viral particle release (Dutta et al. 2008; Wang et al. 2003). Previous reports indicated that SGT/UBP was a co-chaperone that negatively regulated heat shock protein 70 (Hsp70) via heat shock cognate protein (Hsc70)-mediated hydrolysis of ATP (Angeletti et al. 2002). Thus, the protein chaperon complex might be involved in Vpu function, HIV particle exit, and parvovirus DNA replication (Angeletti et al. 2002; Fielding et al. 2006; Tobaben et al. 2003). Previous study supported the emerging view that SGT and Bag-6 were essential components of a protein network intricately regulating Hsp70 functions which were required for a wide variety of cellular processes, including cell division (Winnefeld et al. 2006). According to previous studies, cells depleted of SGT were arrested in mitosis, defected in cytokinesis, and occured in M-phase-specific cell death (Winnefeld et al. 2006). It was also found that SGT was a pro-apoptotic factor, through binding to Hsp90b in the cytoplasm (Yin et al. 2006). Over-expression of SGT could promote apoptosis in 7,721 cells (Wang et al. 2005). During cell apoptosis, SGT was dissociated with Hsp90b and accumulated in the nucleus, which suggested that the nuclear accumulation of SGT was closely related to and played an important role in apoptosis (Yin et al. 2006). Given the importance of its function in cell cycle and apoptosis, it is conceivable that SGT may be a crucial regulator of tumorigenesis.
Small glutamine-rich tetratricopeptide repeat-containing protein alpha (SGTA), an important family member of SGT, was reported to be implicated in various cellular processes (Handley et al. 2001; Schantl et al. 2003; Angeletti et al. 2002). Albrecht Moritz and colleagues found that phosphorylation of SGTA at Ser305 was essential for PDGFRα stabilization and cell survival in PDGFRα-dependent cancer cells (Moritz et al. 2010). Known also as a GHR-interacting protein, binding independently with the ubiquitin-conjugating system, SGTA acted as a modulator of ubiquitin system controlling the down-regulation of GHR by competing with its UbE motif. SGTA might participate in a regulatory loop acting to enhance cancer cell sensitivity to chaperone inhibitors (Moritz et al. 2010). As a putative member of the androgen receptor-chaperone cochaperone complex, SGTA might play an essential role in androgen signaling pathway. It was also found that SGTA might provide a connection between multiple pathways in polycystic ovary syndrome (PCOS), affecting PCOS risk via modulating apoptosis, as well as androgen signaling. Furthermore, the SGTA gene might affect insulin resistance, and the apparent association with beta cell function represented compensatory insulin hypersecretion. Impressively, SGTA could promote apoptosis by enhancing DNA fragmentation and nuclear breakdown. It was also found that SGTA knockdown led to apoptosis via misaligned chromosomes and mitotic arrest (Goodarzi et al. 2008). Since SGTA was expressed ubiquitously in various tissues, this protein was likely to serve a housekeeping function. SGT displayed a mitosis-specific phosphorylation pattern pointing to a possible regulation of this protein during mitosis. Given the importance of SGTA in cell mitosis and apoptosis, we conjectured that SGTA might play a role in cell cycle.
In this study, we aimed to perform a comprehensive analysis of SGTA in a large cohort of surgically resected samples with NSCLC, in order to receive more detailed insights into the importance of each individual variances and their complicated interactions with patients’ outcome. We found that SGTA was highly expressed in NSCLC tissues and related to NSCLC histological differentiation and clinical stage. Further, we confirmed that SGTA had a role in regulating cell proliferation in A549 cells. Our research indicated that SGTA might function as a cell cycle regulator in NSCLC.
Materials and methods
Patients and tissue samples
The NSCLC fresh samples were processed immediately after surgical removal. Protein was analyzed in eight snap-frozen tumorous and adjacent non-tumorous tissue samples that were stored at −80 °C. For histologic examination, tissues were obtained from 83 patients who underwent lung resection without preoperative systemic chemotherapy at the Surgery Department of the Affiliated Hospital of Nantong University from 2005 to 2007. All the specimens were fixed in formalin and embedded in paraffin. After obtaining informed consent, patients were interviewed to obtain information on demographic characteristics, and clinical data were collected. The follow-up time was 5 years, with a range from 1 to 57 months. All patients were deceased by 57 months after diagnosis. The main clinical and pathologic variables were shown in Table 1. Forty-seven patients were men while 36 were women, and their average age was 60 years (range 26–77). Tumors were classified as well (grade I; n = 27), moderately (grade II; n = 30), or poorly (grade III; n = 26) differentiated. All human tissues were collected using protocols approved by the Ethics Committee of Affiliated Hospital of Nantong University.
Antibodies
The antibodies used for Western blotting and immunohistochemistry in this study included: mouse anti-human SGTA antibody, mouse anti-human Ki-67 antibody, mouse anti-human PCNA antibody, rabbit anti-human cyclin A antibody, rabbit anti-human cyclin D1 antibody, and rabbit anti-human GAPDH antibody; all the antibodies were obtained from Santa Cruz Biotechnology, USA.
Western blot
Tissue and cell protein were promptly homogenized in a homogenization buffer containing 50 mM Tirs-HCl, PH 7.5, 0.1 % NP-40, 60 mM β-glycerophosphate, 150 mM NaCl, 5 mM EDTA, 0.1 mM NaF, 0.1 mM sodium orthovanadate, and complete protease inhibitor cocktail (Roche Diagnostics), and then centrifuged at 13,000 g for 20 min to collect the supernatant. Protein concentrations were determined with a Bio-Rad protein assay (BioRad, Hercules, CA, USA). Proteins were separated with SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to polyvinylidene difluoride filter (PVDF) membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5 % fat-free milk in TBST (20 mM Tris, 150 mM NaCl, 0.05 % Tween-20) for 2 h at room temperature, and then, the filters were washed with TBST for three times and incubated overnight with polyclonal antibody against using the primary antibodies described later and horseradish peroxidase-linked IgG as the secondary antibodies. Immunoreactive bands were visualized by chemiluminescence detection system (Pierce). After the chemiluminescence was exposed to X-ray films, the films were scanned with a Molecular Dynamics densitometer (Imaging Technology, Ontario, Canada). The band density was measured with a computer-assisted image analysis system (Adobe Systems, San Jose, CA, USA) and normalized against GAPDH level. Band densities were measured for at least three independent reactions.
Immunohistochemistry (IHC)
Tissue sections were 4 μm thick, deparaffinized, rehydrated through graded alcohol, and quenched in 3 % hydrogen peroxide to block endogenous peroxidase activity, and thereafter, the sections were processed in 10 mM citrate buffer (pH 6.0) and heated to 121 °C in an autoclave for 20 min to retrieve the antigen. After rinsing in PBS (pH 7.2), the sections were then incubated 2 h at room temperature with mouse anti-human SGTA antibody (diluted 1:400) and mouse anti-human Ki-67 antibody (diluted 1:400). Negative control slides were processed in parallel using a non-specific immunoglobulin IgG (Sigma Chemical Co, St. Louis, MO, USA) at the same concentration as the primary antibody. All slides were processed using the peroxidase–anti-peroxidase method (DAKO, Hamburg, Germany). After rinsing in PBS, the peroxidase reaction was visualized by incubating the sections with the liquid mixture DAB (0.1 % phosphate buffer solution, 0.02 % diaminobenzidine tetrahydrochloride, and 3 % H2O2). After rinsing in water, the sections were counterstained with hematoxylin, dehydrated, and cover slipped.
Immunohistochemical evaluation
All the immunostained sections were evaluated in a blinded manner without knowledge of the clinical and pathological parameters of the NSCLC patients. SGTA-positive cells were counted by monitoring at least 1,000 cells from at least 5 randomly selected fields. To allow statistical analyses, we divided patients into three groups according to the expression ratio: high expression group (>75 %, score of 3), middle expression group (50–75 %, score of 2), and low expression group (<50 %, score of 1). For density evaluation, a score of 1 was for low staining, 2 was for middle staining, and 3 was for intense staining. Then, we multiplied the two scores and classified them into two groups: score of 1 (0–4.5) and score of 2 (4.5–9) (Wang et al. 2012). When evaluating the Ki-67 protein immunoreaction, staining was scored in a semi-quantitative fashion. A cut-off value of 50 % or more positively stained nuclei in 5 high-power fields was used to identify Ki-67 staining: high expression group (≥50 %) and low expression group (<50 %). In half of the samples, staining was repeated three times to avoid possible technical errors, and similar results were obtained in these samples. The above procedures of evaluation were performed by three independent pathologists with a multihead microscope, and a consensus was achieved.
Cell cultures and transient transfection
The human lung cancer cell line A549 was purchased from Cell library, China Academy of Science. A549 cells were cultured in high-glucose DMEM (GibCo BRL, Grand Island, NY, USA) with 10 % fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin (GibCo BRL, Grand Island, NY, USA) at 37 °C, and 5 % CO2. Cells were cultured in 6-well dishes, 24-well dishes, and 96-well dishes. The medium was replaced 24 h later with fresh medium for treatment. The cells suspended in medium-contained DMSO were used as a control. The siRNA targets were #1: 5′-CCGUGGCUUACUACAAGAA-3′ and #2: 5′-GGCUUCAUGAGCAUGGCUU-3′, While the control target was 5′-UUCUCCGAACGUGUCACGU-3′, all of which were obtained from GenePharma Co. Ltd. Cells were seeded the day before transfection using DMEM with 10 % FBS without antibiotics. Transient transfection was carried out using lipofectamine 2000 in accordance with the manufacturer’s protocol. Cells were incubated 6 h at 37 °C in high-glucose DMEM with no serum or antibiotics. FBS was then added to the cells to achieve a final concentration of 10 % in high-glucose DMEM. Transfected cells were used for succedent experiments 48 h after transfection. The experiments were repeated at least three times.
Cell cycle analyses
For cell cycle analysis, A549 cells were fixed in 70 % ethanol overnight at 4 °C and then incubated with 1 mg/ml RNase A for 20 min. Subsequently, cells were stained with propidium iodide (PI, 50 μg/ml) (Becton–Dickinson, San Jose, CA, USA) in PBS-Triton ×100 for an additional 20 min at 4 °C and analyzed by a Becton–Dickinson flow cytometer BD FACScan (San Jose, CA, USA) and Cell Quest acquisition and analysis programs. Gating was set to exclude cell debris, cell doublets, and cell clumps. The results were gained from three independent experiments.
Cell counting kit (CCK)-8 assay
Cell proliferation was measured using the commercial CCK-8 kit in accordance with the manufacturer’s protocol. Briefly, cells were seeded onto 96-well cell culture cluster plates (Corning, Corning, NY, USA) at a concentration of 2 × 104 cells/well in volumes of 100 μl and grew overnight. CCK-8 reagents (Dojindo, Kumamoto, Japan) were added to a subset of wells under different treatments. Cells were incubated for another 2 h at 37 °C, and absorbance was quantified using an automated plate reader. Absorbency was measured at a test wavelength of 490 nm and a reference wavelength of 650 nm using a microplate reader (Bio-Rad). The experiments were repeated at least three times.
Terminal deoxynucleotidyl transferase-mediated biotinylated-dUTP nick-end labeling
Terminal deoxynucleotidyl transferase-mediated biotinylated-dUTP nick-end labeling (TUNEL) staining was performed using the In Situ Cell Death Detection Kit, Fluorescence (Roche Applied Science, Mannheim, Ger-many). Frozen tissue sections were rinsed with PBS and treated with 1 % Triton-100 in PBS for 2 min on ice. Slides were rinsed in PBS and incubated for 60 min at 37 °C with 50 μl of TUNEL reaction mixture. The negative control sections incubated for 60 min at 37 °C with 50 μl of Label solution. After washing with PBS, the slides were analyzed with fluorescence microscopy (Leica, DM 5000B; Leica CTR 5000; Germany).
Statistical analysis
All computations were carried out using the SPSS13.0 statistical program. All values were expressed as mean ± SEM. The SGTA expression and clinicopathological features were analyzed using the χ 2 test. Kaplan–Meier curves were constructed, and the log-rank test was performed for analysis of survival data. Multivariate analysis was performed by Cox’s proportional hazards model, and the risk ratio and its 95 % confidence interval were recorded for every marker. P < 0.05 was required for statistical significance. Each experiment consisted of at least three replicates per condition. Standard error of the mean (SEM).
Densitometric analyses
The density of specific bands was measured with a computer-assisted image analysis system (Adobe Systems, San Jose, CA, USA) and normalized against GAPDH level. The relative differences between the control and the treatment groups were calculated and expressed as relative increases setting control as 1. Values were responsible for at least three independent reactions.
Results
SGTA was over-expressed in lung cancer tissues
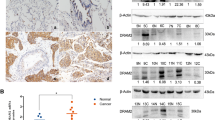
To evaluate the expression and the clinical significance of SGTA in NSCLC progression and further characterize the relations between SGTA and proliferation marker PCNA, we performed Western blot in 8 paired fresh NSCLC and non-tumorous adjacent tissues. As shown in Fig. 1, the expression of SGTA in lung cancer was similar to that of PCNA, both of which had higher expression in cancer tissues than that in adjacent tissues. Furthermore, it was found that SGTA was highly expressed in poor differentiated specimens compared with well-differentiated groups, which was correlated with Ki-67. We then investigated the expression of SGTA and Ki-67 using IHC. SGTA had a higher expression in poor differentiated specimens compared with well-differentiated groups. Representative examples of reactivity for SGTA and Ki-67 were shown in Fig. 2. SGTA was expressed both in the cytoplasm and nucleus, whereas Ki-67 was especially found in the nucleus (Fig. 2). This was consistent with the previous reports that the SGT protein was detected in the cytoplasm and the nucleus by immunofluorescence assay (Cziepluch et al. 1998).
Expressions of SGTA and PCNA in 8 paired NSCLC tumorous (T) and adjacent non-tumorous (N) tissue specimens. A representative Western blot image showed that the SGTA expression was significantly higher in tumorous tissues than in non-tumorous adjacent tissues. PCNA was used as a tumor proliferative marker. GAPDH was used as a control for protein load and integrity
Immunohistochemical analysis of SGTA and Ki67 expression in NSCLC tissues. a–l Paraffin-embedded tissue sections were stained with antibodies of SGTA and Ki-67 and counterstained with hematoxylin. a–f Both high SGTA and Ki-67 expression in lung squamous cancer cells. g–l Both high SGTA and Ki-67 expression in lung adenocarcinoma carcinoma cells. a, b, g, h, Histological differentiation grade 1. c, d, i, j, Histological differentiation grade 2. e, f, k, l, Histological differentiation grade 3. The experiment details were described in section “Materials and methods.” Magnification ×400. All the results were repeated at least three times
Correlation of SGTA expression with clinicopathologic features in NSCLC
To further explore the physiological and pathological relationship between the expression of SGTA and Ki-67 in NSCLC, the presence of SGTA in different grade and histological types is summarized in Table 1. SGTA had a higher expression in poor differentiated specimens compared with well-differentiated groups. It was found that SGTA expression was significantly associated with histological differentiation (P < 0.001) and clinical stage (P < 0.001). But there was no correlation between SGTA expression and other prognostic factors such as age, gender, smoking status, lymph node status, histological type, and tumor size. In addition, the expression of SGTA was high in carcinoma cells that expressed nuclear Ki-67 (Table 1). Besides, we found that there was a positive correlation between SGTA expression and Ki-67-based proliferative activity (r = 0.682, P < 0.01; Fig. 3).
SGTA was significantly associated with the survival of NSCLC patients
Survival information was available for all patients with follow-up data until death. To estimate the prognostic significance of SGTA, we performed the Kaplan–Meier analysis, and patients with SGTA-positive tumor were significantly correlated with poor overall survival (P < 0.001; Fig. 4). Multivariate analysis using the Cox’s proportional hazards model showed that SGTA (P = 0.039), histologic differentiation (P = 0.008), clinical stage (P = 0.003), and Ki-67 (P = 0.030) significantly influenced survival (Table 2). In a word, multivariate analysis using the Cox’s proportional hazards model suggested that SGTA (P = 0.039; Table 2) was an prognostic factor for patients’ overall survival.
The alteration of SGTA expression in proliferating A549 cells
It was reported that SGTA might play a role in the cell cycle regulation of various human cancers. The Western blot and IHC results in NSCLC suggested that high SGTA expression might correlate with oncogenesis of NSCLC. We further detected the expression of SGTA during cell cycle progression in A549 cells. Expectedly, we found that after refeeding from serum starvation, SGTA content was significantly increased and reached maximal at 24 h upon serum addition by Western blot (Fig. 5). The expression of cell cycle markers such as cyclin A and cyclin D1 was also up-regulated after serum refeeding (Fig. 5). Further, the distribution of cell cycle was analyzed using flow cytometry analysis. A549 cells were arrested in the G1 phase by serum deprivation for 72 h according to flow cytometry. And then upon serum addition, the cells at S phase were increased gradually from 16.51 to 30.28 % (Supplementary Fig). Thus, these results indicated that SGTA might play a crucial role in the regulation of A549 cell proliferation.
The expression of SGTA, cyclin A, and cyclin D1 in proliferating A549 cells. a A representative Western blot image showed that the expression of SGTA, cyclin A, and cyclin D1 in A549 cells that were subjected to serum starvation (S) for 72 h and refeeding (R) for 0, 4, 8, 12, 24, and 36 h. GAPDH was used as a control for protein load and integrity. b A bar chart demonstrated the relative protein expression of SGTA, cyclin A, and cyclin D1 in A549 cells at different time points, as measured by Western blot analysis. Data are presented as mean ± SEM of 3 independent measurements (*,#,^ P < 0.05 vs. control at S72 h)
Depletion of SGTA inhibited the proliferation of A549 cells
To further study the biological significance of SGTA on A549 cellular proliferation, A549 cells were transiently transfected with SGTA siRNA or control siRNA constructs. Forty-eight hours after transfection, the efficiency of SGTA siRNA-mediated down-regulation was assessed by Western blot analysis. It was found that the expression of SGTA was significantly reduced in SGTA siRNA cells compared with control siRNA group (Fig. 6a). The expression of cell cycle protein such as cyclin A and cyclin D1 was correlated positively with SGTA expression (Fig. 6a, b). At the same time, to assess the effects of SGTA knocked down on A549 cell proliferation, flow cytometry, and CCK-8 assays were performed. To determine whether the cell cycle distribution was changed after knockdown of SGTA in A549 cells, we performed flow cytometry 48 h after the SGTA siRNA transient transfection. Cell cycle analysis revealed an accumulation of cells in the G0/G1 phase, a decreased population at the S phase from 18.62 to 11.39 % and G2/M phase from 24.13 to 10.66 % after transfection of SGTA siRNA#2 into the A549 cell line (Fig. 6c), suggesting that SGTA could promote the accumulation of the percent of cells in S and G2/M phases and thus the cell growth. The CCK-8 assay of A549 cells treated with siRNA exhibited a significant deceleration of cell proliferation rate compared with the control siRNA (Fig. 6d). Taken together, these data demonstrated that siRNA targeting SGTA exhibited inhibition of S-phase and M-phase entry, as well as a specific inhibitory effect on A549 cell line growth.
Down-expression of SGTA inhibited the cell proliferation and the expression of cell cycle-related molecules in A549 cells. a A representative Western blot image showed that SGTA, cyclin A, and cyclin D1 protein expression in the cells were decreased by treatment of SGTA siRNA and control siRNA. b A bar chart demonstrated the relative protein expression of SGTA, cyclin A, and cyclin D1 in A549 cells by control siRNA or SGTA siRNA as measured by Western blot analysis. Data are presented as mean ± SEM of 3 independent measurements. GAPDH was used as internal control for Western blot analysis (*,#,^ P < 0.05). c DNA content between SGTA siRNA and control siRNA-treated cells were compared by flow cytometry. After transfection, A549 cells were starved for 24 h and then cultured using DMEM with 10 % FBS until 24 h later for collection. d The comparison in growth curves between the cells treated with SGTA siRNA and control siRNA, as measured by CCK-8 assay. The experiments were repeated at least three times
SGTA abolished the cytotoxicity of cisplatin in A549 cells
According to the previous studies, among all patients underwent chemotherapy with cisplatin, the prognosis was still different. A549 cells were sensitive to cisplatin, and our results showed that SGTA might promote the progression of NSCLC, so we hypothesized that SGTA might be involved in chemotherapy resistance and abolish the toxicity of cisplatin in A549 cells. A549 cells were treated with various doses of cisplatin for 48 h, and the effect of cisplatin on survival was determined by CCK-8 and tunnel assays. As shown in Fig. 7a, b, the cell proliferation was deceased while the apoptotic rate of A549 cells was enhanced in a dose-dependent manner. To further investigate the role of SGTA in A549 cells sensitive to cisplatin, the proliferation and apoptosis in A549 cells with SGTA knockdown were determined. When SGTA was knocked down in A549 cells, growth arrest, and apoptosis were significantly increased compared with the control siRNA A549 cells treated with 20 μg/ml cisplatin. Figure 7c, d shows that cisplatin inhibited the survival of A549 cells, and cells treated with cisplatin and SGTA siRNA#2 had the highest growth arrest and apoptosis rate. As we have already known, cisplatin can induce cell apoptosis, and we have also determined cell apoptosis after being treated with cisplatin and SGTA siRNA#2 48 h later by Western blot. As we expected, active caspase 3 expression in SGTA siRNA cells treated with cisplatin was higher than that of control siRNA cells (Fig. 7e, f). These results indicate that SGTA can down-regulate the sensitivity of A549 cells for cisplatin.
SGTA could abolish A549 cells sensitivity to cisplatin. a A549 cells were treated with different concentrations of cisplatin (0, 2.5, 5, 10, 20, 40 μg/ml) for 48 h, and CCK-8 regents were added. The absorbance values were measured to show the cells’ viability. b Tunnel assay showed cell apoptosis of A549 cells treated with different concentrations of cisplatin. c CCK-8 assay showed the cell viability changes of A549 cells, in which SGTA was knocked down. Data are mean ± SEM. Statistical differences compared with the controls were given as *P < 0.05, and statistical differences compared with cisplatin-treated cells were given as # P < 0.05. d Tunnel assay showing cell apoptosis of A549 cells. Data are mean ± SEM. Statistical differences compared with the controls were given as *P < 0.05, and statistical differences compared with cisplatin-treated cells were given as # P < 0.05. e Western blot analysis determined the expression of active caspase 3 with cisplatin, SGTA siRNA#2, or control siRNA treated. GAPDH expression was used as a reference. f A bar chart demonstrated the relative protein expression of active caspase 3 in A549 cells under different treatments. Statistical differences compared with the controls were given as *P < 0.05, and statistical differences compared with cisplatin-treated cells were given as # P < 0.05. All the results were responsible for at least three independent experiments
Discussion
Non-small-cell lung cancer is one of the most common cancers and a leading cause of cancer-related death in most countries, especially in Asia. Although the rates of morbidity and mortality have decreased in recent years in NSCLC patients with surgically treated, the prognosis of NSCLC remains unsatisfactory and the 5-year survival rate is limited to 15 % (Holgersson et al. 2013), even after comprehensive therapies like surgical excision, ethanol injection, chemotherapy, and radiofrequency. What is more, this tumor shows a high percentage of recurrence and metastasis. Thus, a deeper understanding of the molecular events associated with NSCLC is of great necessity. In this study, we first identified SGTA to be a protein highly expressed in NSCLC specimens and correlated with histological differentiation and clinical stage of NSCLC. Multivariate analysis with the Cox’s proportional hazards model indicated that SGTA could be an independent prognostic factor for the survival of NSCLC patients. Furthermore, depletion of SGTA would significantly reduce the expression of cyclin A and cyclin D1, and hence the proliferation of A549 NSCLC cell line. Finally, our result indicated that SGTA might also confer the chemoresistance of NSCLC to cisplatin through suppressing cisplatin-induced apoptosis. Taken together, these findings supported the hypothesis that SGTA could be an important regulator and prognostic factor for NSCLC.
Dysregulation of various oncogenic and tumor-suppressing signaling might be key factors for normal bronchus epithelial cell oncogenesis. The link between SGTA and cancer-related signaling has been discovered in recent years. The change in AR:SGTA ratio was reported to have an influence in the cellular and molecular response to maintain androgenic signals in prostate cancer cells, which may influence the progression of prostate cancer (Trotta et al. 2012; Buchanan et al. 2007). SGTA was also found to be involved in the regulation of ER-associated degradation (Xu et al. 2012) and HSP70 function (Angeletti et al. 2002). However, a detailed mechanism describing how up-regulation of SGTA contributed to NSCLC carcinogenesis remained largely unidentified and need to be determined in future studies. Our present study suggested that the alteration of SGTA expression might enhance the malignancy of NSCLC. To date, there is no study about the clinical significance of SGTA in NSCLC, and the present study is the first to analyze SGTA expression in NSCLC, with respect to possible associations with clinicopathological parameters as well as patients’ prognosis. The results of this study provided a new insight into the activity and the regulation of SGTA in NSCLC, which would facilitate our understanding of the development and the treatment of this malignancy. Therefore, these results suggested that SGTA, as a positive regulator of cell cycle, might contribute to the progression of NSCLC.
In summary, we found SGTA to be frequently expressed in NSCLC tissues and positively correlated with A549 cellular proliferation. Our preliminary survival analysis indicated that high SGTA expression might be associated with inferior overall survival, but this early finding need to be confirmed with a larger group of patients. Besides, alteration in SGTA expression levels might contribute to deregulation of the cell cycle and was involved in the pathogenesis of NSCLC. Furthermore, SGTA could abolish the toxicity of cisplatin in A549 cells. All this indicates that SGTA may play an important role in NSCLC, and its evaluation provides important prognostic information for NSCLC. However, further studies are necessary to elucidate the molecular mechanisms of SGTA in NSCLC pathogenesis.
References
Angeletti PC, Walker D, Panganiban AT (2002) Small glutamine-rich protein/viral protein U-binding protein is a novel cochaperone that affects heat shock protein 70 activity. Cell Stress Chaperones 7:258–268
Buchanan G, Ricciardelli C, Harris JM, Prescott J, Yu ZC, Jia L, Butler LM, Marshall VR, Scher HI, Gerald WL, Coetzee GA, Tilley WD (2007) Control of androgen receptor signaling in prostate cancer by the cochaperone small glutamine rich tetratricopeptide repeat containing protein alpha. Cancer Res 67:10087–10096
Callahan MA, Handley MA, Lee YH, Talbot KJ, Harper JW, Panganiban AT (1998) Functional interaction of human immunodeficiency virus type 1 Vpu and Gag with a novel member of the tetratricopeptide repeat protein family. J Virol 72:8461
Cziepluch C, Kordes E, Poirey R, Grewenig A, Rommelaere J, Jauniaux JC (1998) Identification of a novel cellular TPR-containing protein, SGT, that interacts with the nonstructural protein NS1 of parvovirus H-1. J Virol 72:4149–4156
Cziepluch C, Lampel S, Grewenig A, Grund C, Lichter P, Rommelaere J (2000) H-1 parvovirus-associated replication bodies: a distinct virus-induced nuclear structure. J Virol 74:4807–4815
Dutta S, Kotaka M, Tan YJ (2008) Expression, purification and preliminary crystallographic analysis of recombinant human small glutamine-rich tetratricopeptide-repeat protein. Acta Crystallogr Sect F Struct Biol Cryst Commun 64:602–604
Fielding BC, Gunalan V, Tan TH, Chou CF, Shen S, Khan S, Lim SG, Hong W, Tan YJ (2006) Severe acute respiratory syndrome coronavirus protein 7a interacts with hSGT. Biochem Biophys Res Commun 343:1201–1208
Goodarzi MO, Xu N, Cui J, Guo X, Chen YI, Azziz R (2008) Small glutamine-rich tetratricopeptide repeat-containing protein alpha (SGTA), a candidate gene for polycystic ovary syndrome. Hum Reprod 23:1214–1219
Handley MA, Paddock S, Dall A, Panganiban AT (2001) Association of Vpu-binding protein with microtubules and Vpu-dependent redistribution of HIV-1 Gag protein. Virology 291:198–207
Holgersson G, Bergqvist M, Nyman J, Hoye E, Helsing M, Friesland S, Holgersson M, Ekberg L, Morth C, Ekman S, Blystad T, Ewers SB, Loden B, Henriksson R, Bergstrom S (2013) The impact of hyperfractionated radiotherapy regimen in patients with non-small cell lung cancer. Med Oncol 30:320
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA Cancer J Clin 60:277–300
Ji XD, Li G, Feng YX, Zhao JS, Li JJ, Sun ZJ, Shi S, Deng YZ, Xu JF, Zhu YQ, Koeffler HP, Tong XJ, Xie D (2011) EphB3 is overexpressed in non-small-cell lung cancer and promotes tumor metastasis by enhancing cell survival and migration. Cancer Res 71:1156–1166
Liou ST, Wang C (2005) Small glutamine-rich tetratricopeptide repeat-containing protein is composed of three structural units with distinct functions. Arch Biochem Biophys 435:253–263
Moritz A, Li Y, Guo A, Villen J, Wang Y, MacNeill J, Kornhauser J, Sprott K, Zhou J, Possemato A, Ren JM, Hornbeck P, Cantley LC, Gygi SP, Rush J, Comb MJ (2010) Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci Signal 3:ra64
Ommen G, Chrobak M, Clos J (2010) The co-chaperone SGT of Leishmania donovani is essential for the parasite’s viability. Cell Stress Chaperones 15:443–455
Schantl JA, Roza M, De Jong AP, Strous GJ (2003) Small glutamine-rich tetratricopeptide repeat-containing protein (SGT) interacts with the ubiquitin-dependent endocytosis (UbE) motif of the growth hormone receptor. Biochem J 373:855–863
Siegel R, Ward E, Brawley O, Jemal A (2011) Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin 61:212–236
Tobaben S, Varoqueaux F, Brose N, Stahl B, Meyer G (2003) A brain-specific isoform of small glutamine-rich tetratricopeptide repeat-containing protein binds to Hsc70 and the cysteine string protein. J Biol Chem 278:38376–38383
Trotta AP, Need EF, Butler LM, Selth LA, O’Loughlin MA, Coetzee GA, Tilley WD, Buchanan G (2012) Subdomain structure of the co-chaperone SGTA and activity of its androgen receptor client. J Mol Endocrinol 49:57–68
Wang H, Zhang Q, Zhu D (2003) hSGT interacts with the N-terminal region of myostatin. Biochem Biophys Res Commun 311:877–883
Wang H, Shen H, Wang Y, Li Z, Yin H, Zong H, Jiang J, Gu J (2005) Overexpression of small glutamine-rich TPR-containing protein promotes apoptosis in 7721 cells. FEBS Lett 579:1279–1284
Wang Y, Yang S, Ni Q, He S, Zhao Y, Yuan Q, Li C, Chen H, Zhang L, Zou L, Shen A, Cheng C (2012) Overexpression of forkhead box J2 can decrease the migration of breast cancer cells. J Cell Biochem 113:2729–2737
Winnefeld M, Grewenig A, Schnolzer M, Spring H, Knoch TA, Gan EC, Rommelaere J, Cziepluch C (2006) Human SGT interacts with Bag-6/Bat-3/Scythe and cells with reduced levels of either protein display persistence of few misaligned chromosomes and mitotic arrest. Exp Cell Res 312:2500–2514
Worrall LJ, Wear MA, Page AP, Walkinshaw MD (2008) Cloning, purification and characterization of the Caenorhabditis elegans small glutamine-rich tetratricopeptide repeat-containing protein. Biochim Biophys Acta 1784:496–503
Xu Y, Cai M, Yang Y, Huang L, Ye Y (2012) SGTA recognizes a noncanonical ubiquitin-like domain in the Bag6-Ubl4A-Trc35 complex to promote endoplasmic reticulum-associated degradation. Cell Rep 2:1633–1644
Yin H, Wang H, Zong H, Chen X, Wang Y, Yun X, Wu Y, Wang J, Gu J (2006) SGT, a Hsp90beta binding partner, is accumulated in the nucleus during cell apoptosis. Biochem Biophys Res Commun 343:1153–1158
Acknowledgments
This work was supported by the Natural Science Foundation of China (No. 81272708).
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Qun Xue and Liting Lv contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
432_2013_1474_MOESM1_ESM.tif
Flow cytometry was used to analyze the cell cycle progression of A549 cells that were subjected to serum starvation (S) for 72 h and refeeding (R) for 0, 4, 8, 12, 24, and 36 h
Rights and permissions
About this article
Cite this article
Xue, Q., Lv, L., Wan, C. et al. Expression and clinical role of small glutamine-rich tetratricopeptide repeat (TPR)-containing protein alpha (SGTA) as a novel cell cycle protein in NSCLC. J Cancer Res Clin Oncol 139, 1539–1549 (2013). https://doi.org/10.1007/s00432-013-1474-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-013-1474-5