Abstract
Purpose
Inhibitors of monoamine oxidase A (MAOA), a mitochondrial enzyme that degrades neurotransmitters including serotonin and norepinephrine, are commonly used to treat neurological conditions including depression. Recently, we and others identified high expression of MAOA in normal basal prostatic epithelium and high-grade primary prostate cancer (PCa). In contrast, MAOA is low in normal secretory prostatic epithelium and low-grade PCa. An irreversible inhibitor of MAOA, clorgyline, induced secretory differentiation in primary cultures of normal basal epithelial cells and high-grade PCa. Furthermore, clorgyline inhibited several oncogenic pathways in PCa cells, suggesting clinical value of MAOA inhibitors as a pro-differentiation and anti-oncogenic therapy for high-risk PCa. Here, we extended our studies to a model of advanced PCa, VCaP cells, which were derived from castration-resistant metastatic PCa and express a high level of MAOA.
Methods
Growth of VCaP cells in the presence or absence of clorgyline was evaluated in vitro and in vivo. Gene expression changes in response to clorgyline were determined by microarray and validated by quantitative real-time polymerase chain reaction.
Results
Treatment with clorgyline in vitro inhibited growth and altered the transcriptional pattern of VCaP cells in a manner consistent with the pro-differentiation and anti-oncogenic effects seen in treated primary PCa cells. Src, beta-catenin, and MAPK oncogenic pathways, implicated in androgen-independent growth and metastasis, were significantly downregulated. Clorgyline treatment of mice bearing VCaP xenografts slowed tumor growth and induced transcriptome changes similar to those noted in vitro.
Conclusion
Our results support the possibility that anti-depressant drugs that target MAOA might find a new application in treating PCa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa) screening has led to more frequent diagnosis of lower stage disease (Ilic et al. 2007); however, 3.5% of patients will present with locally advanced or metastatic cancer. Among patients with localized PCa, one-third will fail local therapy (Han et al. 2001). The first line of treatment for these patients is androgen-deprivation therapy (ADT) (Heidenreich et al. 2008). Despite ADT, a portion of these patients will relapse to a state of androgen-independent PCa (Johansson et al. 2004). Currently, docetaxel-based chemotherapy is the standard of care for patients with symptomatic metastatic androgen-independent PCa, but the overall time of survival is less than 2 years (Heidenreich et al. 2008). Clearly, new approaches to treatment and a better understanding of this stage of advanced PCa are needed.
Monoamine oxidase A (MAOA) is a mitochondrial enzyme that degrades neurotransmitters such as serotonin and norepinephrine (Shih et al. 1999). As such, MAOA has been widely studied in the context of neurological disorders such as depression and Parkinson’s disease (Youdim et al. 2006). MAOA is also expressed in non-neuronal tissues, but its biological functions in these tissues are unknown. Recently, MAOA was found to be one of the most highly differentially overexpressed genes at the transcript level in high grade (Gleason grades 4 and 5), poorly differentiated primary PCa compared to low grade (Gleason grade 3), well-differentiated PCa, suggesting a role in the progression and/or aggressiveness of high-grade cancer (True et al. 2006). Immunohistochemistry of tissue microarrays comprising >800 benign and cancerous prostate specimens also showed a significant association between high levels of MAOA protein expression and grade 4/5 cancer (True et al. 2006). In another study, we found that men whose grade 4/5 cancers had the highest levels of MAOA protein had higher levels of preoperative serum prostate-specific antigen (PSA) and higher percent of grade 4/5 PCa, two significant prognostic factors for recurrence following radical prostatectomy (Peehl et al. 2008). MAOA, therefore, appears to be a deleterious feature of PCa.
Overall, little is known about the function of monoamine oxidases in cancer (Pietrangeli and Mondovi 2004). The findings of True et al. (2006) prompted us to investigate the biological activity of MAOA in experimental models of PCa. While confirming the elevated expression of MAOA in high-grade cancers in archival prostatectomy specimens, we also noted high levels of MAOA immunoreactivity in the basal epithelia of normal prostatic glands (Zhao et al. 2008). MAOA was absent or very low, however, in normal differentiated secretory (luminal) epithelia. Primary cultures of normal prostatic basal epithelial cells retain high expression of MAOA and treatment with clorgyline, an irreversible MAOA inhibitor, induced secretory cell-like morphology, repressed expression of the basal cell marker, cytokeratin 14, and induced mRNA and protein expression of androgen receptor (AR), the quintessential characteristic of prostatic secretory epithelial cells (Zhao et al. 2008). We confirmed that AR was active in these cells and that androgen induced the expression of AR target genes such as prostate-specific antigen (PSA) (Zhao et al. 2008). MAOA expression was decreased in these cells, as is typical of secretory cells in normal tissues (Zhao et al. 2008). These studies suggest that the function of MAOA in the normal prostatic epithelium is to maintain basal or progenitor cells in the undifferentiated state. A similar function of MAOA has been reported in neuronal stem cells, in which inhibition of MAOA promotes stem cell differentiation (Chiou et al. 2006).
Based on our findings in normal cells, we hypothesized that elevated expression of MAOA in high-grade PCa contributes to its poorly differentiated phenotype. We performed microarray analysis of primary cultures derived from high-grade cancer treated with or without clorgyline to assess global changes in gene expression in response to inhibition of MAOA. Clorgyline induced AR as well as many genes associated with secretory cell differentiation (Zhao et al. 2009). Importantly, clorgyline also inhibited many gene pathways associated with oncogenesis (Zhao et al. 2009). Taken together, this recent evidence suggests that MAOA expression in high-grade PCa may have a direct role in maintaining a dedifferentiated phenotype and promoting aggressive behavior. Drugs that target MAOA, therefore, may have utility for preventing progression of well differentiated to poorly differentiated cancer, or for treating primary adenocarcinomas at high risk for recurrence following surgery.
Our goal in this study was to extend these findings to a model of metastatic PCa and expand our understanding of the effects of MAOA inhibition. We chose the VCaP cell line as a realistic model of advanced human PCa. VCaP cells were derived from a vertebral metastatic lesion of a patient with androgen-independent PCa (Korenchuk et al. 2001). These cells possess many of the same characteristics as clinical PCa including expression of PSA and wild-type AR (Korenchuk et al. 2001). In particular, VCaP cells have the TMPRSS2-ERG gene fusion (Tomlins et al. 2005), the most common genetic change in PCa. Because our past research suggested that MAOA plays a key role in the maintenance of a less-differentiated phenotype in high-grade primary PCa, we hypothesized that inhibition of MAOA in VCaP cells might similarly promote differentiation and exert anti-tumor activity. This hypothesis was tested in both in vitro and in vivo settings and the results support the concept that MAOA may be a viable new target for treating advanced PCa.
Materials and methods
Cell culture and reagents
VCaP cells were a gift from Dr. Ken Pienta (University of Michigan). Cells were maintained in Dulbecco’s modified Eagle medium (DMEM, Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT). Primary cultures of normal human prostatic epithelial (E-PZ) and stromal (F-PZ) cells, stromal cells from benign prostatic hyperplasia (F-BPH), and cancer-derived epithelial (E-CA) and stromal (F-CA) cells were established and characterized as previously described (Peehl 1992; Peehl and Sellers 2000). Clorgyline (N-methyl-N-propargyl-3-propylamine hydrochloride, Sigma–Aldrich, St. Louis, MO) was prepared at a concentration of 100 mM in sterile water.
Enzymatic activity assay of MAOA
A bioluminescent enzyme assay of MAOA was performed using MAO-GloTM Assay kit (Promega, Madison, WI) as previously described (Zhao et al. 2008). Briefly, 3 million cultured cells were harvested and lysed in 0.2 ml of 1× Reporter Lysis Buffer. Twenty-five microliters of cell lysate were incubated with an equal volume of 2× MAO Substrate Solution at room temperature for 3 h. Fifty microliters of Luciferin Detection Reagent were then added and incubated for 20 min at room temperature. MAOA activity in each sample was normalized against the protein concentration of the same sample determined using the Bradford assay (Bio-Rad, Hercules, CA).
Cell viability and proliferation assays
Cell viability was assessed after 24 h treatment with 0, 1, 10, and 50 μM clorgyline using MTT assay. Specifically, 5 × 104 cells were seeded into each well of a 96-well plate in 100 μl of DMEM with 10% FBS. Another 100 μl of DMEM with 10% FBS and 0, 2, 20, and 100 μM clorgyline were added to the wells the next day. After 24 h of treatment, cell viability was examined using an in vitro MTT-based toxicity kit (Sigma–Aldrich) according to manufacturer’s instructions. For the proliferation assay, VCaP cells (5 × 105) were seeded into each 60-mm dish in DMEM with 10% FBS. The next day, cells were treated with 1 μM clorgyline or diluent control. Cells were fed fresh medium on days 3 and 6. On days 1, 2, 4, 7, and 10, cells from triplicate dishes were harvested and counted.
Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR)
Total RNA from control and treated cells was isolated using Trizol (Invitrogen). RNA (5 μg) was then converted to cDNA using SuperScript III Reverse Transcriptase (Invitrogen). The cDNA product was mixed with SYBR® GreenER™ qPCR SuperMix (Invitrogen) and primers of choice (Supplementary Table S1) in the subsequent PCR using a MxPro3000 real-time PCR Detection System (Stratagene, La Jolla, CA). Each reaction was done in triplicate to minimize experimental variation (standard deviation was calculated for each reaction). Transcript levels of TBP or GAPDH were assayed simultaneously as an internal control to normalize transcript levels in control and treated cells.
Microarray hybridization
Fluorescently labeled cDNA probes were prepared from 50 μg of total RNA by reverse transcription using an Oligo dT primer 5′-TTTTTTTTTTTTTTTT-3′ (Qiagen, Valencia, CA) and indirect amino-allyl labeling as described previously (Zhao and Peehl 2009). Cy5-labeled probes from control or clorgyline-treated cells for each time point were mixed with Cy3-labeled probes from Universal Human Reference RNA (Stratagene) and hybridized overnight at 65ºC to spotted high-density oligonucleotide microarrays with 42,941 elements (Stanford Functional Genomics Facility, Stanford, CA). Microarray slides were then washed to remove unbound probe and scanned with a GenePix 4000B scanner (Axon Instruments, Inc., Union City, CA).
Microarray data processing and analysis
The acquired fluorescence intensities for each fluoroprobe were analyzed with GenePix Pro 5.0 software (Axon Instruments, Inc.). Spots of poor quality were removed from further analysis by visual inspection. Data files containing fluorescence ratios were entered into the Stanford Microarray Database (SMD) where biological data were associated with fluorescence ratios and genes were selected for further analysis (Sherlock et al. 2001). Data were retrieved only from spots with a signal intensity >150% above background in either Cy5- or Cy3-channels from SMD. Common genes among different data sets were identified using Microsoft Excel. The Chi-square test was used to determine gene enrichment. The microarray data were deposited in GEO (accession number GSE19822).
Xenograft tumor growth assay
One million VCaP cells were suspended in 50 μl of phosphate-buffered saline (PBS) and mixed with an equal volume of Matrigel ECM (Becton–Dickinson, Bedford, MA) on ice. Cells were injected subcutaneously into the dorsal-lumbar region of 22 RAG2-/-γc-/- male mice [immunodeficient mice that lack NK cell activity as well as T and B cells (Cooper et al. 2001)]. Quantification of tumor volume using calipers was performed biweekly to monitor and track tumor growth (tumor volume = π/6 × length × width2). Once the tumors reached a grossly measurable size of 200–300 mm3, 11 mice were used as control with daily intraperitoneal injection of water and 11 were injected intraperitoneally with 10 mg/kg of clorgyline daily for up to 21 days. Mice whose tumors became ≥2,000 mm3 before the end of the 21-day treatment regimen were killed. All tumors were harvested and snap frozen immediately after animals were killed. Histopathology of hematoxylin- and eosin-stained frozen sections of each tumor was evaluated. Tumor volume doubling time was calculated as the square root of the total growth period (days) divided by the fold change between the first and last measurements. Box-and-whisker plots were generated using MedCalc software (Mariakerke, Belgium). All of the animal experiments were done in accordance with NIH animal use guidelines, and the protocol was approved by the University Committee on Animal Resources at Stanford University.
Gene expression analysis of xenografts
Six mice bearing VCaP xenografts of similar size were used. A single clorgyline injection (10 mg/kg) was given to each of three mice, and three control mice were injected with water. After 24 h, all six mice were killed and xenografts were harvested. Total RNA was isolated from the xenografts using Trizol (Invitrogen), and 5 μg of RNA were used for qRT-PCR analysis of gene expression as described above.
Statistical analysis
One-way analysis of variance was used for comparison between multiple groups, and student’s t-test was used to compare two groups. Significance was defined as P < 0.05.
Results
VCaP cells express MAOA
The transcript level of MAOA in VCaP cells was compared to that in human prostatic fibroblasts and epithelial cells cultured from normal (F-PZ and E-PZ) or malignant (F-CA and E-CA) tissues (Supplementary Fig. 1). As previously reported (Zhao et al. 2008), cultured prostatic fibroblasts express little MAOA, mimicking lack of expression of MAOA in the human prostatic stroma. Basal epithelial cells cultured from normal tissues or epithelial cells from malignant tissues, however, express significant levels of MAOA, similar to levels of expression of MAOA in the normal prostatic basal epithelium and in high-grade primary adenocarcinomas of the prostate (Peehl et al. 2008; Zhao et al. 2008, 2009). The level of MAOA mRNA in VCaP cells was comparable to the level in primary cultures of epithelial cells and 5- to 100-fold higher than the level in stromal cells (Supplementary Fig. 1a). In addition, Western blot analysis showed that the MAOA protein level in VCaP cells was similar to that in E-CA cells, whereas no MAOA protein was detected in stromal cells (Supplementary Fig. 1b). These results confirm that the VCaP cell line is a suitable model to study MAOA function in advanced metastatic PCa.
Inhibition of MAOA enzymatic activity and VCaP cell growth by 1 μM clorgyline. a MAOA activity was decreased by 98% in VCaP cells treated for 24 h with 1 μM clorgyline. b Cell viability was similar between control and clorgyline-treated cells after 24 h. c Growth was decreased at all time points in response to MAOA inhibition by clorgyline when compared to control. Asterisk indicates statistical significance by t-test
Clorgyline slows in vitro growth of VCaP cells
We determined the effect of clorgyline, an irreversible inhibitor of MAOA (Ma et al. 2004), on MAOA enzymatic activity in VCaP cells. In the cell lysate of VCaP cells treated with 1 μM of clorgyline for 24 h, MAOA activity was decreased by 98% compared to that in control VCaP cells (Fig. 1a). MTT-based cell viability assay detected no toxicity of clorgyline to VCaP cells after 24 h even at a concentration of 50 μM (Fig. 1b). To determine the effect of inhibition of MAOA on cell growth, VCaP cells were cultured in DMEM with 10% FBS over a 10-day period with or without clorgyline. In the presence of 1 μM clorgyline, cell growth was inhibited consistently throughout the course of the experiment (Fig. 1c). The maximum difference between control cells and cells treated with clorgyline was seen 10 days after initiation of treatment, at which time the number of treated cells was 47% that of control cells. Therefore, inhibition of MAOA by clorgyline slowed the proliferation of VCaP cells in vitro.
Transcriptome changes in response to clorgyline
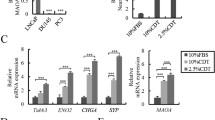
We previously examined the effects of clorgyline on the transcriptional program of E-CA cells cultured from high-grade primary adenocarcinomas of the prostate (Zhao et al. 2009). A similar analysis was performed with VCaP cells. At time zero, semi-confluent VCaP cells were fed DMEM with 10% FBS and diluent (control) or 1 μM clorgyline (treated). Total RNA was isolated at 24 h and subjected to cDNA microarray analysis. In clorgyline-treated VCaP cells, 2,944 genes were upregulated at least twofold and 778 genes were downregulated at least twofold compared to control (Supplementary Table S2). The number of downregulated genes was much lower than that of upregulated genes, consistent with our previous study using E-CA cells, in which expression of 4,026, 5,606 and 2,299 genes was increased and 3,576, 2,486, and 597 genes decreased by at least twofold in response to clorgyline at 6-, 24-, or 96-h time points, respectively. In E-CA cells, expression of 156 genes was significantly increased in response to clorgyline as determined by significance analysis of microarrays (SAM) (Tusher et al. 2001). Seventy of the 156 genes in the SAM list of genes upregulated in E-CA were increased by at least twofold in clorgyline-treated VCaP cells (Table 1). By chance, only 18 genes were expected to overlap between the two gene lists; therefore, these findings represent a significant enrichment (P < 10−33). In addition, only 1 of the 156 SAM genes upregulated in E-CA cells was found in the list of genes downregulated in VCaP cells by clorgyline (the expected number of genes by chance was 5). Expression in treated VCaP cells of the top 10 genes selected by SAM as upregulated in clorgyline-treated E-CA cells was measured by qRT-PCR. All ten genes showed significantly increased RNA levels in VCaP cells treated with clorgyline compared to control (Fig. 2). These results suggest that inhibition of MAOA by clorgyline induced similar effects on the transcriptome of VCaP cells to those that we previously observed in epithelial cells cultured from high-grade primary adenocarcinomas.
Gene expression changes of the top 10 genes upregulated in clorgyline-treated E-CA cells selected by SAM in clorgyline-treated VCaP cells in vitro by qRT-PCR. The relative expression levels of the top 10 genes in MAOA-inhibited VCaP cells at 24-h time point compared to control. Asterisk indicates statistical significance by t-test
Inhibition of MAOA counteracts oncogenic pathways in VCaP
The SAM list generated from clorgyline-treated E-CA cells is enriched with genes repressed in 7 of 12 oncogenic pathway signatures compiled from the literature (Creighton 2008). Its overlap with the list of genes regulated by clorgyline in VCaP cells suggested that inhibition of MAOA by clorgyline induced anti-oncogenic effects in VCaP cells similar to those we observed in E-CA cells. To gain a comprehensive understanding of the effects of inhibition of MAOA on oncogenic pathways, we compared genes whose expression changed at least twofold in response to clorgyline in VCaP cells with 12 oncogenic pathway signatures (Creighton 2008). Three of the pathways showed statistically meaningful changes in gene expression in clorgyline-treated VCaP cells compared to control, namely the Src, beta-catenin, and MAPK pathways. Specifically, of the 1,944 genes downregulated by Src (Creighton 2008), 518 were upregulated in VCaP cells due to clorgyline treatment, which is significantly enriched (P < 10−84), while 76 of the 1,507 Src-induced genes were downregulated by clorgyline in VCaP cells (P < 10−4) (Fig. 3a). Similarly, clorgyline significantly upregulated genes suppressed by beta-catenin (Creighton 2008), including APC, as well as genes suppressed by MAPK with P < 10−61 and P < 10−3, respectively (Fig. 3b, c), although genes induced by beta-catenin and MAPK were not enriched in the list of genes downregulated in VCaP cells by clorgyline. These results suggest that clorgyline treatment induces a transcriptional program that is inversely correlated with Src, beta-catenin, and MAPK pathway signatures, indicating that MAOA inhibition may, at least partially, reverse these oncogenic pathways.
Attenuation of Src, beta-catenin, and MAPK pathways by MAOA inhibition in VCaP cells as determined by microarray analysis. a Number of genes overlapping between those whose expression changed ≥2-fold after MAOA inhibition and the Src pathway signature. b Number of genes overlapping between those whose expression changed ≥2-fold after MAOA inhibition and the beta-catenin pathway signature. c Number of genes overlapping between those whose expression changed ≥2-fold after MAOA inhibition and the MAPK pathway signature. d Number of genes overlapping between those whose expression changed ≥2-fold after MAOA inhibition and the androgen pathway signature. For a–d, asterisk indicates statistical significance by Chi-square test
Inhibition of MAOA regulates androgen signaling in VCaP Cells
We previously reported that clorgyline induced AR signaling in cultures of prostatic epithelial cells derived from both normal tissues as well as from primary adenocarcinomas (Zhao et al. 2008, 2009). When compared with a list generated by DePrimo et al. (DePrimo et al. 2002) of 258 genes upregulated by androgen in LNCaP cells (a PCa cell line derived from a lymph node metastasis that has a mutated AR), 59 of these genes were also upregulated in VCaP cells treated with clorgyline, representing a highly significant enrichment (P < 10−6) (Fig. 3d; Table 2). The list of 59 genes includes a number of well-known androgen-induced genes such as ELL2, NDRG1, and TPD52 (O’Malley et al. 2009; Pflueger et al. 2009; Rubin et al. 2004). Interestingly, the canonical AR target gene, PSA, is not on the list of genes identified as regulated by androgen in LNCaP cells by Deprimo et al., and the expression of PSA was surprisingly decreased by 3.3-fold in the microarray analysis of clorgyline-treated VCaP cells despite the slight upregulation of AR by 1.5-fold. Consistent with the microarray results, PSA expression as determined by qRT-PCR in clorgyline-treated cells was decreased by 60% compared to control, whereas AR expression was upregulated by 3.7-fold (Fig. 4). The TMPRSS2-ERG fusion transcript, typically induced by AR, was also downregulated by 45% (Fig. 4). The 23 genes downregulated in LNCaP cells by androgen that were identified by DePrimo et al. were not enriched in the list of genes downregulated by clorgyline in VCaP cells. These results suggest that clorgyline influenced androgen signaling in VCaP cells in a complex manner in that it upregulated AR and a subset of androgen-regulated genes yet unexpectedly downregulated other targets that are classically induced by AR. Clearly, further investigations are needed to understand the mechanisms and consequences of such effects.
Clorgyline induces differentiation-related genes
In normal and malignant primary cultures of prostatic epithelial cells, clorgyline induced the expression of genes associated with secretory differentiation (Zhao et al. 2008, 2009). Genes specifically expressed in secretory prostatic epithelial cells as identified by Liu and True (2002) were significantly enriched in clorgyline-induced genes in VCaP cells (Table 3). In addition, the classic secretory cell marker cytokeratin 18 was also upregulated 2.8-fold, which is consistent with our previous conclusion that inhibition of MAOA promotes secretory differentiation in normal and malignant prostatic epithelial cells. Moreover, clorgyline treatment of VCaP cells induced genes normally repressed by EZH2 (Yu et al. 2007) (Table 3). The enrichment of EZH2 target genes in the list of genes upregulated by clorgyline is statistically significant (P < 0.05). EZH2 is a critical component of a multiprotein complex that represses the expression of genes involved in differentiation (Sellers and Loda 2002). We determined the expression of EZH2 by qRT-PCR and found that it was downregulated by 36% in clorgyline-treated cells compared to control (Fig. 4). Taken together, these results suggest that clorgyline promotes differentiation in VCaP cells, possibly by restoring gene expression suppressed by EZH2.
Clorgyline inhibits xenograft tumor growth
To investigate the effect of clorgyline treatment on tumor growth in vivo, we treated VCaP xenograft-bearing mice with daily clorgyline injections. Xenografts were generated from VCaP cells injected subcutaneously into 22 immunodeficient RAG2-/-γc-/- male mice (Cooper et al. 2001). Once the tumors reached a grossly measurable size of 200–300 mm3, 11 mice were intraperitoneally injected with water as control and 11 were injected with 10 mg/kg of clorgyline daily for up to 21 days. The mice were randomized and the tumor volume was equivalent between the treated and control groups. Specifically, the mean tumor volume of the treated group was 275 ± 77 mm3 at the start of the treatment, whereas the control group was 278 ± 66 mm3. We chose the concentration of 10 mg/kg because similar doses of clorgyline have been used extensively in neurological studies in mice (Fox et al. 2007; Kitanaka et al. 2006; Thomas et al. 2008) and were reported to be effective. The clorgyline-treated group had a longer tumor volume doubling time than the control group (Fig. 5a). The mean doubling time was 12.3 versus 9.1 days for the treated and control groups, respectively (P = 0.02). The mean tumor volume in the clorgyline-treated group was consistently lower compared to control over the entire course of treatment with the biggest difference observed at the end of the experiment (Fig. 5b). H&E-stained sections of the harvested tumors showed similar histopathology between treated and control xenografts, with no evidence of dramatically increased cell death or necrosis in the treated tumors (Fig. 5c). In conjunction with the in vitro findings, these results suggest that inhibition of MAOA slows tumor growth in vivo mainly by slowing proliferation rather than by inducing apoptosis.
Clorgyline inhibits VCaP xenograft growth and alters gene expression. a Tumor volume doubling time was increased in clorgyline-treated mice compared to control; N = 11 for both groups. b Mean tumor volumes for treated and control groups at each time point demonstrated a consistently lower size in the treated group; N = 11 for both groups. c H&E staining showed similar histology of control and treated xenografts. d The relative expression levels of the top 10 SAM genes in MAOA-inhibited VCaP xenografts after 24 h of treatment with clorgyline compared to control. Asterisk indicates statistical significance by t-test
Clorgyline induces changes in gene expression in xenografts
In a separate experiment, we evaluated the expression levels of the top 10 SAM genes upregulated by clorgyline in E-CA and VCaP cells in vitro in xenografts from mice with or without clorgyline treatment. VCaP xenografts from three mice were harvested 24 h after a single clorgyline injection into each mouse. Another three xenografts from mice injected with water were harvested at the same time and used as control. Six of the 10 genes were significantly upregulated in VCaP xenografts from mice treated with clorgyline as determined by qRT-PCR (Fig. 5d), suggesting that clorgyline had similar effects on the transcriptional program of VCaP cells in vivo and in vitro.
We also performed qRT-PCR to determine expression levels of AR, PSA, EZH2, APC, and TMPRSS2-ERG in clorgyline-treated xenografts compared to control. None of these were statistically significantly different between the treated and control groups, possibly due to small sample size (N = 3 for each group). Since expression changes of these genes were not particularly large even in the in vitro setting, it perhaps would not be unanticipated that changes would be difficult to demonstrate in the more complex in vivo environment.
Discussion
Using VCaP cells as a model, we demonstrated that clorgyline, an MAOA inhibitor, slowed cell growth both in vitro and in vivo. Toxicity assays in vitro and the absence of notable necrosis or cell death in treated xenografts suggest that this effect is mainly due to inhibition of proliferation rather than to induction of apoptosis. Gene expression profiling revealed changes in transcriptional programs in clorgyline-treated VCaP cells affecting similar pathways and biological processes to those in treated epithelial cells cultured from high-grade primary PCa (Zhao et al. 2009). Specifically, clorgyline upregulated genes that are repressed by the oncogenes Src, beta-catenin, and MAPK, and downregulated genes that are activated by Src. Moreover, clorgyline induced gene expression associated with secretory differentiation in VCaP cells. The anti-oncogenic and pro-differentiation effects of clorgyline on cells cultured from both high-grade primary adenocarcinomas as well as androgen-independent metastases point to the possibility of MAOA inhibitors as therapeutic agents for several stages of PCa treatment.
The effects of clorgyline in VCaP cells showed both similarities and differences to those in primary cultures of prostatic epithelial cells. First, clorgyline inhibited VCaP cell growth but not that of primary cultures. This may be due to differences in composition of culture medium or to biological differences between the cells. Second, clorgyline counteracted a larger number of oncogenic pathways in cells cultured from high-grade PCa than in VCaP cells (7 vs. 3). However, two of the three pathways affected in VCaP cells were among the seven pathways affected in primary E-CA cells, indicating a common mechanism of action of clorgyline between these cells. This premise is supported by the fact that the top 10 SAM genes upregulated by clorgyline in primary E-CA cells were also upregulated in VCaP cells as determined by qRT-PCR. Finally, one-third of the secretory cell-specific genes upregulated in VCaP by clorgyline was also upregulated in primary E-CA cells, suggesting a similar differentiation-promoting effect of clorgyline in these cells.
Similar to primary E-CA cells, VCaP cells treated with clorgyline showed increased expression of AR and a subset of genes previously reported to be upregulated by androgen in another PCa cell line, LNCaP (DePrimo et al. 2002). Nine of the 59 androgen-induced genes upregulated by clorgyline in VCaP cells were also induced in clorgyline-treated primary E-CA cells (Zhao et al. 2009). However, several of the classic AR target genes, notably PSA, were not upregulated by clorgyline in VCaP cells, whereas PSA was induced in clorgyline-treated E-CA cells (Zhao et al. 2009). AR signaling is complex and model-dependent, and recent studies suggest that there are major differences in androgen-mediated signaling pathways in hormone-dependent versus castration-resistant PCa cells (Wang et al. 2009). The role of androgen-mediated changes in the anti-tumor effects of clorgyline remains to be fully elucidated. Inhibition of oncogenic pathways or promotion of differentiation, perhaps through downregulation of EZH2 and re-expression of genes repressed by the polycomb group complex containing EZH2, may be the more dominant activities leading to tumor repression by clorgyline.
Perhaps the most promising finding from our study is that clorgyline not only inhibited VCaP xenograft growth in vivo, but also induced similar gene expression changes to those that we observed in vitro in both E-CA and VCaP cells. Specifically, six of the ten SAM genes upregulated by clorgyline in E-CA and VCaP cells in vitro were also upregulated in clorgyline-treated xenografts. Notably, these six genes are among those most highly induced by clorgyline in cultured VCaP cells (Figs. 2 and 5d), which may explain why induction of these genes was observed in vivo, while induction of the ones whose upregulation was modest in cultured VCaP cells was not. Some of these genes have been implicated in other types of cancers. For example, PHF3, whose expression is the most highly upregulated in clorgyline-treated VCaP xenografts, is frequently lost in gliomas (Fischer et al. 2001). Additional studies are needed to investigate the role of these genes in PCa. Regardless, these genes may serve as valuable biomarkers to monitor patient response to clorgyline in clinical settings.
In conclusion, we have shown that clorgyline exerts anti-oncogenic and pro-differentiation effects in a model of advanced PCa, and decreases the proliferative ability of PCa both in vitro and in vivo. The ability of clorgyline to counteract oncogenic pathways and promote differentiation suggests that MAOA inhibitors, which are already used in clinical practice for treating neurological disorders, could be therapeutic options for advanced stages of PCa.
References
Chiou SH, Ku HH, Tsai TH, Lin HL, Chen LH, Chien CS, Ho LL, Lee CH, Chang YL (2006) Moclobemide upregulated Bcl-2 expression and induced neural stem cell differentiation into serotoninergic neuron via extracellular-regulated kinase pathway. Br J Pharmacol 148:587–598
Cooper RN, Irintchev A, Di Santo JP, Zweyer M, Morgan JE, Partridge TA, Butler-Browne GS, Mouly V, Wernig A (2001) A new immunodeficient mouse model for human myoblast transplantation. Hum Gene Ther 12:823–831
Creighton CJ (2008) Multiple oncogenic pathway signatures show coordinate expression patterns in human prostate tumors. PLoS One 3:e1816
DePrimo SE, Diehn M, Nelson JB, Reiter RE, Matese J, Fero M, Tibshirani R, Brown PO, Brooks JD (2002) Transcriptional programs activated by exposure of human prostate cancer cells to androgen. Genome Biol 3:RESEARCH0032
Fischer U, Struss AK, Hemmer D, Michel A, Henn W, Steudel WI, Meese E (2001) PHF3 expression is frequently reduced in glioma. Cytogenet Cell Genet 94:131–136
Fox MA, Jensen CL, Gallagher PS, Murphy DL (2007) Receptor mediation of exaggerated responses to serotonin-enhancing drugs in serotonin transporter (SERT)-deficient mice. Neuropharmacology 53:643–656
Han M, Partin AW, Pound CR, Epstein JI, Walsh PC (2001) Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am 28:555–565
Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, Schmid HP, Zattoni F (2008) EAU guidelines on prostate cancer. Eur Urol 53:68–80
Ilic D, O’Connor D, Green S, Wilt T (2007) Screening for prostate cancer:a Cochrane systematic review. Cancer Causes Control 18:279–285
Johansson JE, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, Adami HO (2004) Natural history of early, localized prostate cancer. JAMA 291:2713–2719
Kitanaka N, Kitanaka J, Takemura M (2006) Modification of morphine-induced hyperlocomotion and antinociception in mice by clorgyline, a monoamine oxidase-A inhibitor. Neurochem Res 31:829–837
Korenchuk S, Lehr JE, MClean L, Lee YG, Whitney S, Vessella R, Lin DL, Pienta KJ (2001) VCaP, a cell-based model system of human prostate cancer. In Vivo 15:163–168
Liu AY, True LD (2002) Characterization of prostate cell types by CD cell surface molecules. Am J Pathol 160:37–43
Ma J, Yoshimura M, Yamashita E, Nakagawa A, Ito A, Tsukihara T (2004) Structure of rat monoamine oxidase A and its specific recognitions for substrates and inhibitors. J Mol Biol 338:103–114
O’Malley KJ, Dhir R, Nelson JB, Bost J, Lin Y, Wang Z (2009) The expression of androgen-responsive genes is up-regulated in the epithelia of benign prostatic hyperplasia. Prostate 69:1716–1723
Peehl D (1992) Culture of human prostatic epithelial cells. In: Freshney R (ed) Culture of epithelial cells. New York, Wiley-Liss
Peehl DM, Sellers RG (2000) Cultured stromal cells:an in vitro model of prostatic mesenchymal biology. Prostate 45:115–123
Peehl DM, Coram M, Khine H, Reese S, Nolley R, Zhao H (2008) The significance of monoamine oxidase-A expression in high grade prostate cancer. J Urol 180:2206–2211
Pflueger D, Rickman DS, Sboner A, Perner S, LaFargue CJ, Svensson MA, Moss BJ, Kitabayashi N, Pan Y, de la Taille A et al (2009) N-myc downstream regulated gene 1 (NDRG1) is fused to ERG in prostate cancer. Neoplasia 11:804–811
Pietrangeli P, Mondovi B (2004) Amine oxidases and tumors. Neurotoxicology 25:317–324
Rubin MA, Varambally S, Beroukhim R, Tomlins SA, Rhodes DR, Paris PL, Hofer MD, Storz-Schweizer M, Kuefer R, Fletcher JA et al (2004) Overexpression, amplification, and androgen regulation of TPD52 in prostate cancer. Cancer Res 64:3814–3822
Sellers WR, Loda M (2002) The EZH2 polycomb transcriptional repressor–a marker or mover of metastatic prostate cancer? Cancer Cell 2:349–350
Sherlock G, Hernandez-Boussard T, Kasarskis A, Binkley G, Matese JC, Dwight SS, Kaloper M, Weng S, Jin H, Ball CA et al (2001) The Stanford microarray database. Nucleic Acids Res 29:152–155
Shih JC, Chen K, Ridd MJ (1999) Monoamine oxidase:from genes to behavior. Annu Rev Neurosci 22:197–217
Thomas DM, Francescutti-Verbeem DM, Kuhn DM (2008) The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J Neurochem 105:605–616
Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehr R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R et al (2005) Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310:644–648
True L, Coleman I, Hawley S, Huang CY, Gifford D, Coleman R, Beer TM, Gelmann E, Datta M, Mostaghel E et al (2006) A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc Natl Acad Sci USA 103:10991–10996
Tusher VG, Tibshirani R, Chu G (2001) Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98:5116–5121
Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, Chen Z, Beroukhim R, Wang H, Lupien M et al (2009) Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 138:245–256
Youdim MB, Edmondson D, Tipton KF (2006) The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci 7:295–309
Yu J, Cao Q, Mehra R, Laxman B, Tomlins SA, Creighton CJ, Dhanasekaran SM, Shen R, Chen G, Morris DS et al (2007) Integrative genomics analysis reveals silencing of beta-adrenergic signaling by polycomb in prostate cancer. Cancer Cell 12:419–431
Zhao H, Peehl DM (2009) Tumor-promoting phenotype of CD90(hi) prostate cancer-associated fibroblasts. Prostate 69:991–1000
Zhao H, Nolley R, Chen Z, Reese SW, Peehl DM (2008) Inhibition of monoamine oxidase A promotes secretory differentiation in basal prostatic epithelial cells. Differentiation 76:820–830
Zhao H, Flamand V, Peehl DM (2009) Anti-oncogenic and pro-differentiation effects of clorgyline, a monoamine oxidase A inhibitor, on high grade prostate cancer cells. BMC Med Genomics 2:55–69
Acknowledgments
Dr. Flamand was supported by an Association Francaise d’Urologie Fellowship. Dr. Zhao is supported by the National Cancer Institute (Grant number 1 K01 CA123532). Dr. Peehl is supported by the National Cancer Institute (1 R01 CA121460) and a Challenge Award from the Prostate Cancer Foundation. The authors thank Sarah Young and Zuxiong Chen for technical assistance.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Flamand, V., Zhao, H. & Peehl, D.M. Targeting monoamine oxidase A in advanced prostate cancer. J Cancer Res Clin Oncol 136, 1761–1771 (2010). https://doi.org/10.1007/s00432-010-0835-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-010-0835-6