Abstract
Monoamine oxidase (MAO) catalyzes the oxidative deamination of monoamine neurotransmitters and dietary amines. Two pharmacological types with different substrate and inhibitor specificities were reported. Molecular cloning revealed that the two types of MAO were different genes expressed as different proteins with different functions. MAO A and B have identical intron–exon organization derived by duplication of a common ancestral gene thus they are termed isoenzymes. MAO A knockout mice exhibited aggression, the first clear evidence linking genes to behavior. MAO A KO mice exhibited autistic-like behaviors which could be prevented by reducing serotonin levels at an early developmental age (P1–P7) providing potential therapy. MAO B KO mice were non-aggressive and resistant to Parkinsongenic neurotoxin. More recently it was found that MAO A is overexpressed in prostate cancer and correlates with degree of malignancy. The oncogenic mechanism involves a ROS-activated AKT/FOXO1/TWIST1 signaling pathway. Deletion of MAO A reduced prostate cancer stem cells and suppressed invasive adenocarcinoma. MAO A was also overexpressed in classical Hodgkin lymphoma and glioma brain tumors. MAO B was overexpressed in glioma and non-small cell lung cancer. MAO A inhibitors reduce the growth of prostate cancer, drug sensitive and resistant gliomas and classical Hodgkin lymphoma, and enhance standard chemotherapy. Currently, we are developing NIR dye-conjugated clorgyline (MAO A inhibitor) as a novel dual therapeutic/diagnostic agent for cancer. A phase II clinical trial of MAO inhibitor for biochemical recurrent prostate cancer is ongoing. The role of MAO A and B in several cancer types opens new avenues for cancer therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Monoamine oxidase (MAO) A and B isoenzymes located at the mitochondrial outer membrane, catalyze the oxidative deamination of monoamine neurotransmitters in both the brain and peripheral tissues, and produce hydrogen peroxide (H2O2) as one of the products. The role of MAO in the homeostasis of neurotransmitters critical for synaptic transmission has long been recognized (Hare 1928). Two pharmacological forms, MAO A and B, were identified in 1968 (Johnston 1968; Knoll and Magyar 1972; Shih and Eiduson 1969; Youdim et al. 1969). Yet, the molecular nature of the two types of MAO that differ in substrate specificity and inhibitor sensitivity remained enigmatic for twenty years. This enigma was eventually resolved by cloning of MAO A and B genes (Bach et al. 1988).
With the MAO A and B clones available, we studied the active sites, the promoter and the mechanism of gene regulation of MAO A and B (Chen et al. 2005; Ou et al. 2004, 2006a, b; Wong et al. 2001, 2002, 2003; Wu et al. 2009a, b; Zhang et al. 2006; Zhu et al. 1992, 1994, Zhu and Shih 1997; Shih et al. 1999a, 2011). To understand their in vivo function, we generated a series of genetic mutants to explore the biological role of MAO isoenzymes (Cases et al. 1995; Grimsby et al. 1997; Chen et al. 2004; Bortolato and Shih 2011; Bortolato et al. 2018). Abnormal levels of monoamine neurotransmitters result in mental disorders (Shih et al. 1999a) and neurological diseases (Shih and Thompson 1999c), and these KO mice served as animal models for a number of disorders.
In addition to the extensive studies on MAO A and B in brain function and disorders, recent work suggests that MAO plays important roles in tumorigenesis, diabetes, obesity and cardiovascular disease (Song et al. 2013; Kaludercic et al. 2014; Camell et al. 2017; Deshwal et al. 2017). In this review, we describe the effects of MAO A and B genes on behavior and recent discoveries of MAO function in prostate cancer, glioma and Hodgkin lymphoma, and the development of a tumor-specific MAO A inhibitor conjugate as a dual function therapy and non-invasive diagnostic for human cancers.
MAO A and B genes and behavior
Cloning showed that pharmacologically defined MAO A and B are encoded by different genes with identical intron–exon organizations and thus termed isoenzymes
MAO A prefers serotonin (5-hydroxytryptamine, 5-HT) and norepinephrine as substrates and is sensitive to the irreversible inhibitor clorgyline while MAO B prefers phenylethylamine and benzylamine as substrates and is more sensitive to the selective inhibitor deprenyl. Dopamine and tyramine are the common substrates for both isoenzymes (Shih et al. 1999a). However, the substrate selectivity and inhibitor sensitivity vary depending on the species, tissues, and other factors, so the data in the literature are controversial. The molecular basis of these two types of enzymes remained a puzzle, and it was essential to solve the controversy in the field.
In 1988, the full-length human MAO A and B genes were cloned and sequenced for the first time (Bach et al. 1988). The deduced amino acid sequence showed that MAO A and B share 70% identity in primary sequence (Fig. 1). To confirm these two clones were indeed MAO A and B, each cDNA clone was expressed in mammalian cells. Similar to the endogenous enzymes, the cDNA-expressed MAO A preferred 5-HT as a substrate and was sensitive to clorgyline inhibition whereas the cDNA-expressed MAO B preferred phenylethylamine as a substrate and was sensitive to deprenyl inhibition (Lan et al. 1989a; Fig. 2). This provided unequivocal evidence that these two forms of enzymes represent two distinct proteins with different physiological functions. Demonstrating that MAO A and B are different proteins solved the decades-old controversy regarding the molecular nature of MAO A and B.
The alignment of deduced polypeptide sequences shows that MAO A and B are encoded by different genes with 70% amino acid identity with identical intron–exon organizations, and thus termed isoenzymes. Amino acids are numbered on the left side. Identical amino acids are indicated by asterisks. Dashes mark spaces introduced for maximum alignment of identical residues. Pink or blue shade denotes amino acids in each exon. Identical or divergent amino acids at the 5′ or 3′ boundary of the introns are indicated by bold asterisks or filled circles, respectively. Triangles denote potential glycosylation sites.

(Adapted from Lan et al. 1989a)
Substrate and inhibitor profiles of human MAO A and B expressed from cDNAs. a MAO activity in transfected COS cells. The human MAO A and B cDNAs containing the full coding sequences of MAO A and B were cloned into pECE vector in either the sense or antisense orientation and expressed under the control of SV40 early promoter. The resultant plasmid was transiently transfected into COS cells and the MAO A and B activities in the lysates were determined using [14C]serotonin or [14C]phenylethylamine as the substrate for MAO A or B, respectively. b Inhibition of transiently expressed MAO A and B by clorgyline (open circle) or deprenyl (filled circle). The lysate of COS cells transfected with either human MAO A or B cDNA were assayed in the presence of various concentrations of inhibitors. The inhibition of the expressed MAO A and B by clorgyline and deprenyl was determined by preincubating the inhibitors with the whole-cell homogenates at 37 °C for 1 h before addition of the substrates.
The two MAO genes are closely linked on chromosome X (Lan et al. 1989b). Both consist of 15 exons spanning at least 60 kb. The exon–intron organization of MAO A and B genes are identical (Fig. 3), suggesting that they have evolved from the same ancestral gene by duplication (Grimsby et al. 1991). In some patients with Norrie disease, which causes blindness, progressive hearing loss and mental retardation in more than one-third of the affected, both MAO A and B genes are deleted (Lan et al. 1989b).

(Adapted from Grimsby et al. 1991)
Partial structural map of the MAO A and MAO B genes showing the location of exons and sequencing strategy. Filled bars represent coding regions, and unfilled bars represent untranslated regions of the exons. Exon numbers are presented below the bars. The horizontal arrows represent the regions sequenced. The prefix “A” denotes λ bacteriophage clones, and the prefix “p” denotes pUC19 subclones of λ bacteriophage DNA. E and H, EcoRI and HindIII restriction sites, respectively; “⇊”, equivocal assignment of an exon to either restriction fragment; “//”, intron gap.
Epigenetic modification of MAO A promoter was linked to mental and neurological disorders
Recent reports implicated epigenetic mechanisms modifying the MAO A transcription in mental diseases. The DNA methylation pattern of the MAO A promoter was studied in male patients with posttraumatic stress disorder (PTSD) due to traumatic war experiences during the 1990s. Hypermethylation of three CpGs in exon/intron 1 of MAO A significantly correlated with higher PTSD severity, which suggested that decreasing MAO A transcription may increase noradrenalin level to elevate sympathetic activity to cause the disorder (Ziegler et al. 2018). The study suggested the potential use of antiadrenergic agents to buffer increased noradrenergic signaling conferred by MAO A hypermethylation.
In another study, the epigenetic pattern of MAO A promoter for panic disorder was investigated. The results from the study of female panic disorder patients showed a negative correlation between MAO A methylation and the number of panic attacks. Intriguingly, following the cognitive behavioral therapy, a significant number of hypomethylated CpG sites became increasingly methylated, indicating the reversibility of the epigenetic pattern (Ziegler et al. 2016).
MAO A KO mice exhibit aggressive and autistic-like behaviors
MAO A KO mice display behaviors similar to Brunner syndrome and autism spectrum disorder in humans
To understand the in vivo function, MAO A KO mice were generated. MAO A KO mice (Tg8) were generated by an inadvertent insertion of an IFN-β minigene into exon 2 of the MAO A coding region. 5-HT levels were increased most significantly in pup than adult brains (Cases et al. 1995). MAO A KO mice showed behavioral alterations, which include social and communication deficits, perseverative and neophobic-like responses, sensory disturbances as well as disrupted barrel fields in the somatosensory context. Pups exhibited trembling, fearfulness and difficulty in righting whereas adults showed more aggression in males. This was the first study linking the gene and behavior. These phenotypes are similar to MAO A deficiency in human males, a complex behavioral syndrome (Brunner syndrome) associated with antisocial personality, stereotyped responses, mild cognitive impairments and impulsive aggression (Brunner et al. 1993).
Further, we developed knock-in MAO A to forebrain of MAO A KO mice to demonstrate that forebrain-specific expression of MAO A in MAO A KO mice abrogates aggressive phenotype, indicating that the lack of MAO A in the forebrain causes the aggressive behavior (Chen et al. 2007). The knock-in MAO A mice showed significant MAO A catalytic activity and reduced 5-HT to wild type level in forebrain. In the knock-in MAO A mice, the disorganized somatosensory cortex barrel field structure associated with MAO A KO mice were restored, becoming morphologically similar to wild-type mice.
Later, we discovered a novel line of mutant mice [MAO AA863T KO] harboring a spontaneous point nonsense mutation (lysine 284 → stop codon) in exon 8 of the MAO A gene. This mutation is similar to the mutation found in patients with Brunner syndrome as it maps close to the site of their point nonsense mutation (glutamine 296 → stop codon) also in exon 8 (Scott et al. 2008). The MAO AA863T KO mice displayed aggression similar to the MAO A KO mice (Tg8) generated previously, confirming the evidence linking MAO A to aggression.
Male MAO AA863T KO mice exhibit an array of behavioral and neuromorphological alterations similar to all core features of autism spectrum disorder (Bortolato et al. 2013b). Since then, this line of MAO AA863T KO mice has been used for all studies as its mutation is similar to human MAO A deficiency and without the complication of the presence of IFN-β gene in the MAO A KO mice (Cases et al. 1995). It provided an excellent model to study the role of monoamines in a variety of physiological functions in vivo, aggression, anxiety, autism and other mental disorders.
Early therapeutic intervention can abrogate autistic phenotypes of MAO A KO mice
Interestingly, the increase in 5-HT in MAO A KO mice is most pronounced during the early postnatal stage; it increased considerably in pups and returned to normal in older mice. Treatment of MAO AA863T KO mice with the tryptophan hydroxylase inhibitor, p-chloro-phenylalanine (pCPA), from P1 to P7 considerably reduced the 5-HT level in the brain; it also significantly reduced anxiety determined by the marble-burying behavior and increased spontaneous alternations in a T-maze in adult (Bortolato et al. 2013b; Fig. 4). During P1-P7, MAO A KO mice had the highest 5-HT level, which affected circuit formation critical for brain development. More importantly, these results provide a potential prevention strategy for autism spectrum disorder patients associated with hyperserotonemia, which represents approximately 30% of the affected.

(Adapted from Bortolato et al. 2013b)
pCPA treatment during P1 to P7 rescued the autistic-like behaviors in adult MAO A KO mice. Effects of pCPA treatment from postnatal day 1 through 7 on a marble-burying behavior and b spontaneous alternations in a T-maze of MAO A KO mice. pCPA, an inhibitor of trypophan hydroxylase that reduces 5-HT level, was injected (30 mg/kg/day, IP) daily from P1 to P7. Values represent mean ± SEM; *P < 0.05, ***P < 0.001 compared to untreated wild-type mice; #P < 0.05, ###P < 0.001 compared to untreated MAO A deficient mice; †P < 0.05, ††P < 0.01 compared to untreated saline-treated MAO A KO mice; δδP < 0.01, δP < 0.05 compared to saline treated wild-type mice.
Early therapeutic intervention can abrogate aggressive phenotypes of MAO A KO mice
We have shown that aggression in MAO A KO male mice is mediated by 5-HT2A receptor (Shih et al. 1999b). MAO A KO male mice treated during first week of postnatal life with the 5-HT2A receptor antagonist, ketanserin, rescued aggression in adulthood. Acute treatment with 5-HTT blocker fluoxetine to inhibit 5-HT reuptake also reduced aggressive behavior in MAO A KO mice (Godar et al. 2014). These findings point to the possibility of developing novel therapeutic agents for serotonergic behavioral disorders including autism, anxiety, and aggressive behaviors.
MAO B KO mice show reduced anxiety-like responses, no aggression
MAO B knockout mice were generated using a gene-targeting vector to disrupt the MAO B gene (Grimsby et al. 1997). The synthesis of phenylethylamine and dopamine from phenylalanine is catalyzed by aromatic l-amino acid decarboxylase in dopaminergic neurons in the striatum. In MAO B KO mice, phenylethylamine levels were higher in all brain regions with the highest phenylethylamine level in the striatum (Grimsby et al. 1997; Bortolato et al. 2009).
Phenylethylamine has been implicated in the regulation of mood and emotional responses such as exploratory activity and behavioral reinforcement. MAO B KO mice show heightened reactivity to stress (Grimsby et al. 1997) with no aggressive behavior, and significantly reduced anxiety-like responses (Bortolato et al. 2009). These results suggest that MAO B KO mice may serve as a model to study the long-term effects of elevated phenylethylamine on brain function and behavior.
MAO A/B KO mice show autistic-like behavior and with significant circuit-specific reduction of cortical inputs
The MAO A/B KO mouse model was discovered by a spontaneous mutation MAO AA863T in a MAO B KO mouse colony (Chen et al. 2004). In MAO A/B KO mice, the levels of 5-HT, norepinephrine, dopamine and phenylethylamine were increased much more than the MAO A or B single KO mice, suggesting that MAO A and B can oxidize each other’s substrate when one isoenzyme is absent (Shih 2004). MAO A/B KO mice showed cognitive abnormalities and hippocampal alterations (Singh et al. 2013). Like MAO A KO mice, MAO A/B KO mice display behavioral hallmarks of autism spectrum disorder and the neuropathological alterations were generally greater in MAO A/B KO than MAO A KO mice (Bortolato et al. 2013a).
Recent advances have allowed one to generate a comprehensive map of all neural connections by building a “connectome” to reveal the structural organization of the brain. To investigate potential connectivity disruptions, we used a connectomics approach to systematically and comprehensively characterize connectopathies in MAO A/B KO mice. We discovered that MAO A/B KO mice display a significant circuit-specific reduction of cortical inputs arising from the ventrolateral orbitofrontal area to the intermediate caudoputamen (Hintiryan et al. 2016). Further studies in this line of research are ongoing.
MAO A and B genes and cancer
The role of MAO A and the effect of MAO A inhibitors on prostate cancer progression and metastasis
MAO A overexpression is correlated with Gleason scores in prostate cancer
Prostate cancer represents the second leading cause of cancer-associated death for men in the Western world. The prognosis and treatment for prostate cancer is based on several parameters including clinical stage, serum prostate specific antigen (PSA), and the Gleason score. Gleason grading system, which is based on microscopic tumor architecture, highly correlates with the probability of clinical outcome.
It has been shown that an 86-gene model could distinguish low grade (3) from high-grade (4 and 5) prostate cancers, which included MAO A (True et al. 2006). Immunohistochemistry of tissue microarrays representing prostate cancer patient cohorts showed that MAO A protein expression was significantly elevated in cancerous epithelium relative to benign secretory epithelium. Grade 4 or 5 showed higher MAO A expression than grade 3 prostate cancers, suggesting that MAO A may be a characteristic of high-grade prostate carcinoma. In another report, MAO A expression positively correlated with preoperative levels of serum PSA and the percentage of grade 4/5 cancer in the sample, which represent significant risk factors for recurrence (Peehl et al. 2008).
Our group also found that a high-level MAO A expression is strongly associated with advanced prostate cancers (Wu et al. 2014) (Fig. 5A). The high MAO A expression strongly associated with advanced Gleason score of 8–10. The Kaplan–Meier analysis predicted a significantly reduced survival for prostate cancer patients with elevated MAO A protein. Collectively, these results indicate that MAO A may distinguish aggressive from indolent form of prostate cancer based on survival data. Additional data implicating MAO A in prostate cancer comes from the reduced risk for prostate cancer for the carrier of a MAO A allele (White et al. 2012).
Elevated expression of MAO A in various human cancers. a High Gleason grade prostate cancers associated with increased MAO A expression. Normal prostatic epithelium and Gleason grade 3 and 5 prostate cancer specimens from a tissue microarray were stained for MAO A. Magnification, × 400; bars, 20 µm. (Adapted from Wu et al. 2014) b MAO A expression is increased in human glioma. Non-malignant brain and glioma tissue specimens. Scale bars represent 100 µm (Adapted from Kushal et al. 2016). c MAO A is highly expressed by Hodgkin/Reed–Sternberg (HRS) cells of classical Hodgkin lymphoma (cHL). MAO A was detected by immunohistochemistry on formalin-fixed, paraffin-embedded (FFPE) patient material. Double staining for CD30 (red) and MAO A (brown) confirms the localization of MAO A in HRS cells (original magnification × 1000) (Adapted from Li et al. 2017)
Stable knockdown of MAO A completely eliminated growth of MPC3 cells in a xenograft animal model
The role of MAO A in prostate tumorigenesis was assessed using a highly tumorigenic murine prostate carcinoma MPC3 cell line in an immunocompetent mouse model. The MPC3 line was derived from mouse primary prostate tumors harboring double loss of Pten and p53 tumor suppressors. Stable introduction of MAO A-specific shRNAs decreased MAO A enzymatic activity by greater than 50–70%. Stable knockdown of MAO A in MPC3 cells completely eliminated their growth in vivo in contrast to the tumor growth observed in mice inoculated with the control MPC3 cells (Wu et al. 2014). A similar effect was observed with human prostate cancer cell lines LNCaP, C4-2 and ARCaPM.
MAO A inhibitors reduce the proliferation, migration and invasion of prostate cancer LNCaP, C4-2B and PC-3 cells
Clorgyline, a potent selective MAO A inhibitor, reduced the proliferation, migration and invasion of prostate cancer LNCaP, C4-2B and PC-3 cells cells in vitro (Wu et al. 2014, 2015). Clorgyline treatment led to a decline in the proliferation marker Ki-67 expression, epithelial to mesenchymal transition and hypoxic response in mice harboring xenograft tumors derived of androgen-independent and aggressive prostate cancer C4-2 cells (Wu et al. 2014). Clorgyline was also shown to enhance the cytotoxicity of docetaxel in prostate cancer cells in vitro (Gordon et al. 2014). A phase I-II clinical trial of neoadjuvant chemotherapy with docetaxel and mitoxantrone followed by prostatectomy showed a significantly increased transcript level of MAO A that associated with subsequent biochemical relapse. The treatment with docetaxel induced the MAO A expression in prostate cancer LNCaP cells. Combining clorgyline with an inhibitor of survivin, a member of the inhibitor of apoptosis protein (IAP) family, was more efficacious in suppressing the growth of prostate cancer cells than the survivin inhibitor alone (Xu et al. 2015).
We are currently conducting a phase II clinical trial to assess the activity of phenelzine (Nardil®, a irreversible MAO A/B inhibitor) in patients with biochemically recurrent prostate cancer (ClinicalTrials.gov Identifier: NCT02217709). This small open-label trial repurposes phenelzine (up to 30 mg twice daily) for castrate and non-castrate patients with elevated PSA after primary therapy. The primary endpoint is the proportion of patients who achieve a PSA decline of ≥ 50% from baseline. Preliminary data suggest that phenelzine has clinical activity (PSA reduced in ~ 40% of patients) in biochemical recurrent prostate cancer following primary therapy (Gross et al. 2016). These positive preliminary outcomes need to be reproduced in large randomized trials to determine the safety and efficacy of MAO A inhibitor in patients with recurrent prostate cancer.
MAO A generated ROS activates AKT/FOXO1/TWIST1 signaling pathway
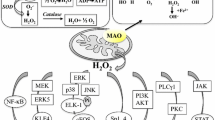
MAO A was shown to induce epithelial to mesenchymal transition of prostate cancer cells through regulating hypoxia-inducible factor 1-alpha (HIF-1α), which mediates hypoxic effects by activating downstream genes involved in tumor glycolysis, angiogenesis, invasion, migration, and metastasis. A schematic representation of the proposed mechanism is shown in Fig. 6. Reactive oxygen species (ROS) generated by MAO A inhibits prolyl hydroxylase 3 (PHD3) to increase the steady-state level of HIF-1α, resulting in enhanced expression of its target genes including vascular endothelial growth factor A (VEGFA), glucose transporter 1 (GLUT1) and Twist Family BHLH Transcription Factor 1 (TWIST1) to promote epithelial to mesenchymal transition. The interaction of VEGF with neuropilin-1 (NRP-1), in turn, leads to the activation of phosphoinositide 3-kinase (PI3K)/ protein kinase B (AKT) signal transduction pathway. Phosphorylation of Forkhead box protein O1 (FOXO1) transcriptional repressor by AKT resulted in its exclusion from the nucleus, allowing the expression of TWIST1 to promote epithelial to mesenchymal transition (Wu et al. 2014).

(Adapted from Wu et al. 2014)
A proposed mechanism that MAO A regulates prostate tumorigenesis, progression, and metastasis. MAO A generates ROS that inhibits PHD activity and stabilizes HIF1α to induce epithelial to mesenchymal transition. Generation of ROS by MAO A is elevated by hypoxic conditions that are characteristic of the tumor microenvironment. MAO A’s activation of VEGF-A/NRP1 signaling upregulates the AKT/FOXO1 pathway, resulting in the nuclear export of FOXO1 transcription repressor to increase the expression of nuclear TWIST1. Overall, increasing the MAO A expression promotes epithelial to mesenchymal transition, hypoxia and ROS production to drive prostate tumorigenesis, progression and metastasis.
MAO A is involved in neuroendocrine differentiation (NED) which is associated with hormone refractory prostate cancer (HRPC), and MAO A inhibitor prevents NED and HRPC
Current treatment modalities for prostate cancer include androgen deprivation therapy administered singularly or in combination with radiotherapy and/or chemotherapy. Despite the initial response, HRPC (also known as castration-resistant prostate cancer) may emerge in the long run and metastasize, accounting for the relatively higher rate of mortality. Hormone refractory prostate cancer is associated with NED, which refers to neuroendocrine-like prostate cancer cells displaying a high degree of chemoresistance and secreting tumor growth-sustaining cytokines.
The ROS produced by MAO A was essential for autophagy activated by androgen deprivation, and MAO A induces mitophagy. Mitophagy refers to the selective elimination of mitochondria by autophagy. Inhibiting MAO A with pargyline or phenelzine suppressed androgen deprivation-induced NED and autophagy (Lin et al. 2017), suggesting that MAO A serves as a key determinant of NED and that MAO A inhibitors may deter prostate cancer.
Loss of MAO A inhibits adenocarcinoma development, cell proliferation and cancer stem cells in prostate in Pten/MAO A KO mouse model
To ascertain the role of MAO A in prostate adenocarcinoma development in vivo, we created a prostate-specific Pten/MAO A KO mouse model. The double KO mice were generated by crossing MAO A-floxP mouse with the conditional Pten KO mouse that develops invasive prostate cancer similar to human cancer (Liao et al. 2018). Pten/MAO A KO mice compared to Pten KO mice showed a significant reduction in prostate size and nearly all Pten/MAO A KO mice developed prostatic intracellular neoplasia instead of adenocarcinoma at 4 or 6 months-old, suggesting that the deletion of MAO A decreases the incidence of invasive prostate cancer.
Immunohistochemistry showed a significant reduction in the number of epithelial cells expressing the proliferation marker Ki67 and p-AKT expression in prostate sections of 6 month-old Pten/MAO A KO mice compared to Pten KO mice (Fig. 7) (Liao et al. 2018). AKT is a serine/threonine kinase that regulates metabolism, proliferation, growth and other cellular processes in response to growth factor stimulation and requires phosphorylation to be activated. Thus, the deletion of MAO A reduces epithelial cell growth and proliferation in prostate.

(Adapted from Liao et al. 2018)
Deletion of MAO A gene suppresses the initiation of prostate cancer. Immunohistochemistry stain for the cell proliferation marker Ki67 detected high percentage of proliferative cells in Pten KO mouse prostate tissue whereas significantly less cells express Ki67 in Pten/MAO A KO mice. (Arrows point to Ki67+ cells; Bar: 100 µm)
Furthermore, the loss of MAO A also reduced OCT4- or NANOG-positive stem cell subpopulations in prostate tissue in vivo. This finding was recapitulated in vitro when MAO A expression was suppressed by RNA interference in human prostate cancer cells and confirmed with additional stem cell markers, CD44, α2β1, and CD133 in vitro. The MAO A-targeting shRNA also caused a considerable reduction in the ability to form 3D tumor spheroids, commonly used to detect the cancer stem cell phenotype (Fig. 8). The loss of MAO A reduces the stem cell subpopulation in prostate cancer. Taken together, these results suggest that MAO A expression promotes prostate cancer development by increasing cell proliferation and cancer stem cell subpopulation.

(Adapted from Liao et al. 2018)
MAO A gene knockdown in human prostate cancer cells (LNCaP) significantly reduced cultured stem cell spheroid size and number. Light microscope view of spheroid culture with prostate cancer cells expressing a scrambled shRNA (shControl, left side panel) versus a MAO A-targeting shRNA (shMAO A, right side panel). Loss of MAO A gene function reduced the size of spheroids formed after 12 days of culture in 3D Matrigel. Bar, 100 µm.
MAO A inhibitor reduces glioma growth and allows low-dose temozolomide (TMZ) chemotherapy for TMZ resistant glioma
Glioma, the most common primary brain tumor is rarely curable with poor prognosis and low survivability. Immunostaining of human glioblastoma multiforme tissues showed a significant increase in the level of MAO A expression compared to non-tumor brain tissues (Fig. 5B) (Kushal et al. 2016). Human glioma cell lines U251S and U251R expressed MAO A catalytic activity whereas normal human astrocytes displayed no detectable MAO A activity.
Clorgyline reduced the viability of TMZ-sensitive U251S cells. Clorgyline also had a similar effect on TMZ-resistant U251R cells. TMZ is an alkylating drug that has been used extensively in the current treatment as the primary chemotherapeutic agent for glioma. Further, clorgyline reduced the growth of tumor xenograft derived from intracranially injected TMZ-resistant U251R cells by decreasing invasion or angiogenesis while augmenting immune response. More significantly, combining clorgyline with the low-dose TMZ enhanced the effects of TMZ on survival of the TMZ-resistant U251R cell-derived glioma model (Fig. 9a).

(Adapted from Kushal et al. 2016)
Clorgyline, NMI or the combination with TMZ increases the survival of TMZ-resistant tumors. Athymic/nude mice were implanted intracranially with TMZ-resistant human glioma cells (U251R). Animals were then treated daily with: vehicle, TMZ (1 mg/kg), clorgyline (10 mg/kg), TMZ (1 mg/kg) + clorgyline (10 mg/kg), NMI (5 mg/kg), or TMZ (1 mg/kg) + NMI (5 mg/kg). MAO A inhibitors were administered subcutaneously daily for 21 days; TMZ treatment was given for the first 10 days only. a The Kaplan–Meier survival curve shows that clorgyline significantly prolonged survival. b The Kaplan–Meier survival curve for NMI and NMI + TMZ shows that NMI was effective alone as compared to the vehicle, and the combination of NMI and TMZ was significantly more effective (*p < 0.001).
MAO A expressed specifically in Reed-Sternberg cells in classical Hodgkin lymphomas and MAO A inhibitor enhanced the current ABVD chemotherapy
The MAO A gene expression profile in Hodgkin and non-Hodgkin lymphoma and a spectrum of reactive lymphoid tissues was examined. Immunohistochemistry on archived formalin-fixed, paraffin-embedded (FFPE) tissue sections from lymphoma patients showed MAO A expression specifically in Hodgkin Reed-Sternberg (HRS) cells for ~ 75% of classical Hodgkin lymphomas with 34.8% expressing strongly (Li et al. 2017) (Fig. 5c). Approximately 17% of primary mediastinal large B-cell lymphomas and mediastinal gray zone lymphoma showed weak MAO A expression. No expression was observed in non-neoplastic lymphoid tissues, nodular lymphocyte predominant Hodgkin lymphoma and other non-Hodgkin lymphomas. The MAO A expression was more common in Epstein-Barr virus (EBV)-negative than EBV-positive cHL and was particularly prevalent in the EBV-negative nodular sclerosing subtype. In culture, the MAO A activity was present in most cHL-derived cell lines but not in non-Hodgkin-lymphoma derived cell lines. Treatment with clorgyline reduced the growth of cHL-derived L1236 cells and U-HO1 cells, which was confirmed by RNA interference targeting MAO A. Currently, a chemotherapy regimen comprised of doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD) is used as the first line treatment for Hodgkin lymphoma. Combining ABVD with clorgyline was more effective in reducing the growth of cHL cells than either regimen alone.
MAO inhibitor dye conjugates, novel dual function agents for cancer diagnosis and therapy
The development of NIR dye–MAO A inhibitor conjugate (NMI)
Many of the currently approved MAO A inhibitors target central nervous system and other peripheral tissues where MAO A is present, thus reducing their delivery to tumors. To provide tumor-selectivity and also reduce side effects, we developed a NIR dye–MAO A inhibitor conjugate (NMI) comprised of clorgyline and a near-infrared (NIR) dye (Wu et al. 2015). The uptake of fluorescent heptamethine carbocyanine dye is mediated by tumor hypoxia and organic anion-transporting polypeptides (OATPs), which is expressed by various human cancers including prostate cancer.
NMI inhibits the proliferation, invasion and tumor formation of prostate cancer cells in vivo
NMI suppressed the proliferation, colony formation, migration and invasion of prostate cancer cells (LNCaP, C4-2B, PC-3) when dosed in the low micromolar range (IC50 of ~ 5 µM). NMI also reduced the growth of C4-2B-derived tumor xenografts in nude mice by inhibiting angiogenesis, apoptosis, and reducing serum levels of PSA. Importantly, NIR imaging showed selective accumulation of NMI at the prostate cancer xenograft formed in vivo (Fig. 10). In cell and animal models, NMI is an ideal therapeutic and diagnostic agent for both the NIR-based imaging as well as targeted ablation of prostate cancer.

(Adapted from Wu et al. 2015)
MAO A inhibitor-near infrared dye conjugate inhibits the growth of human prostate tumor xenograft in mice. The structure of NIR dye–MAO A inhibitor conjugate (NMI) comprised of clorgyline and a near-infrared (NIR) dye is shown. (upper right panel) In vivo NIR imaging of the whole body clearly shows NMI localization within the tumor. Mice bearing C4-2B tumor xenografts were intraperitoneally injected with NMI (50 nmol/mouse) and subjected to NIR imaging 24 h later. (lower right panel) NMI inhibits the growth of C4-2B tumor xenografts in mice. C4-2B cells were subcutaneously injected into male nude mice (n = 5 for each group) to establish tumor xenografts. After tumor size reached 200 mm3, mice were given intratumoral treatments (saline and NMI; 750 nmol/mouse) every other day for a 21-day period
NMI enhances the cytotoxicity of TMZ against drug-resistant glioma cells in vivo
The potential of using NMI to treat brain cancer was also explored. NIR imaging showed selective localization of NMI at xenograft tumors formed by patient-derived glioma cells in mice (Kushal et al. 2016). NMI was cytotoxic (IC50 of ~ 5 µM) against both glioma U251S as well as U251R cells that are sensitive and resistant to TMZ, respectively. Further, combining with NMI increased the cytotoxicity of TMZ against both cell lines in vitro. TMZ is an alkylating chemotherapeutic agent approved for treating newly diagnosed as well as recurrent malignant glioma. Co-treatment with NMI and TMZ significantly increased the survival of mice harboring orthotopic xenograft brain tumors derived from TMZ-resistant glioma cells through the suppression of invasion and angiogenesis, and increased immune response (Fig. 9b). Hence, the application of NMI may be extended to the treatment of glioma.
Potential use of MAO B inhibitors for brain and lung cancer therapy
MP-MUS is a MAO B-activated prodrug derived from nitrogen mustard, an antineoplastic DNA alkylating chemotherapeutic (Sharpe et al. 2015, 2018; Sharpe and Baskin 2016). The cytotoxicity of MP-MUS correlated with the MAO B expression level of treated glioma cells, providing tumor selectivity.
Danshensu, a traditional oriental medicine-derived compound, binds directly to MAO B at the typical docking site of MAO B inhibitors such as selegiline (Son et al. 2016). It inhibits MAO B activity but does not affect cell viability up to 50 µM dose. Danshensu was shown to enhance radiosensitivity in non-small cell lung cancer cells. Thus, MAO B inhibitors or MAO B-activated prodrugs may benefit cancer patients.
Conclusions
By cloning and sequencing, we were able to show that MAO exists as two isoenzymes with different structures and functions. This finding answered questions about the molecular nature of two pharmacological types of MAO that had puzzled researchers for decades and provided fundamental knowledge that propelled the field forward exponentially. The link between gene and behavior was established by showing that mice lacking MAO A display aggression. Further, it unraveled genetic interaction with environmental determinants of mental disorders, including aggression, anxiety and autism spectrum disorders, and developed novel intervention strategies. These important contributions have formed the present knowledge of MAOs in brain and peripheral functions.
More recently, an exciting new function of MAO A was discovered in cancer progression and metastasis. This, in turn, led to the development of target-specific MAO inhibitor with dual therapeutic and non-invasive diagnostic agent for prostate and drug resistant brain cancers. These findings raised more intriguing questions. It is noteworthy that there are other cancers with lower expression of MAO A. Determining the underlying molecular mechanisms will help us to better understand the role of MAO A in cancer biology and potential therapy. Combining MAO A inhibitor with current standard treatment TMZ sensitizes drug resistant glioma. Defining the mechanism through which MAO A inhibitors enhance the cytotoxicity of TMZ in glioma cells may lead to novel therapeutic targets. Likewise, combining MAO A inhibitors with androgen deprivation therapy could extend efficacy and prolong survival in patients. In the coming years, further advances are expected in the fields of autism and cancer.
A phase II clinical trial to explore activity of phenelzine (Nardil®, a irreversible MAO A/B inhibitor) in patients with biochemically recurrent prostate cancer is ongoing (ClinicalTrials.gov Identifier: NCT02217709). Our preliminary data suggests that phenelzine has clinical activity (prostate-specific antigen reduced in ~ 40% of patients) in biochemical recurrent prostate cancer following primary therapy (Gross et al. 2016). These positive preliminary studies are indicative of the potential utility of MAO A inhibitors for prostate cancer.
Decades of basic research have translated into MAO inhibitors with clinical applications for neuropsychiatric disorders (i.e. isocarboxazid, phenelzine, moclobemide, selegiline, tranylcypromine) and Parkinson’s disease (rasagiline, selegiline, safinamide). Recent translational avenues are leading to clinical studies of MAO inhibitors for novel indications. We expect further therapeutic benefits to humans in the future, which remains as the ultimate goal of our collective research efforts on MAO isoenzymes.
Abbreviations
- AKT:
-
Protein kinase B
- cHL:
-
Classical Hodgkin lymphoma
- EBV:
-
Epstein–Barr virus
- FFPE:
-
Formalin-fixed paraffin-embedded
- FOXO1:
-
Forkhead box protein O1
- GLUT1:
-
Glucose transporter 1
- H2O2 :
-
Hydrogen peroxide
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- HRPC:
-
Hormone refractory prostate cancer
- HRS:
-
Hodgkin Reed–Sternberg
- 5-HT:
-
5-Hydroxytryptamine, serotonin
- 5-HTT:
-
Serotonin transporter
- IAP:
-
Inhibitor of apoptosis protein
- MAO A:
-
Monoamine oxidase A
- MAO B:
-
Monoamine oxidase B
- NED:
-
Neuroendocrine differentiation
- NIR:
-
Near-infrared
- NMI:
-
NIR dye–MAO A inhibitor conjugate
- NRP-1:
-
Neuropilin-1
- OATPs:
-
Organic anion-transporting polypeptides
- pCPA:
-
p-Chloro-phenylalanine
- PHD3:
-
Prolyl hydroxylase 3
- PI3K:
-
Phosphoinositide 3-kinase
- PTSD:
-
Posttraumatic stress disorder
- PSA:
-
Prostate specific antigen
- ROS:
-
Reactive oxygen species
- TMZ:
-
Temozolomide
- TWIST1:
-
Twist family BHLH transcription factor 1
- VEGFA:
-
Vascular endothelial growth factor A
References
Bach AW, Lan NC, Johnson DL, Abell CW, BEmbenek ME, Kwan SW, Seeburg PH, Shih JC (1988) cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc Natl Acad Sci USA 85:4934–4938
Bortolato M, Shih JC (2011) Behavioral outcomes of monoamine oxidase deficiency: preclinical and clinical evidence. Int Rev Neurobiol 100:13–42
Bortolato M, Godar SC, Davarian S, Chen K, Shih JC (2009) Behavioral disinhibition and reduced anxiety-like behaviors in monoamine oxidase B-deficient mice. Neuropsychopharmacology 34:2746–2757
Bortolato M, Godar SC, Alzghoul L, Zhang J, Darling RD, Simpson KL, Bini V, Chen K, Wellman CL, Lin RC, Shih JC (2013a) Monoamine oxidase A and A/B knockout mice display autistic-like features. Int J Neuropsychopharmacol 16:869–888
Bortolato M, Godar SC, Tambaro S, Devoto P, Chen K, Shih JC (2013b) Early postnatal inhibition of serotonin synthesis results in long-term reductions of perseverative and anxiety-like behaviors, but not aggression, in MAO A-deficient mice. Neuropharmacology 75:223–232
Bortolato M, Floris G, Shih JC (2018) From aggression to autism: new perspectives on the behavioral sequela of monoamine oxidase deficiency. J Neural Transm (Vienna). https://doi.org/10.1007/s00702-018-1888-y
Brunner HG, Nelen M, Breakenfield XO, Ropers HH, van Oost BA (1993) Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 262:578–580
Camell CD, Sander J, Spadaro O, Lee A, Nguyen KY, Wing A, Goldberg EL, Youm YH, Brown CW, Elsworth J, Rodeheffer MS, Schultze JL, Dixit VD (2017) Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550:119–123
Cases O, Seif I, Grimsby J, Gaspar P, Chen K, Poumin S, Muller U, Aquet M, Babinet C, Shih JC (1995) Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAO A. Science 268:1763–1766
Chen K, Holschneider DP, Wu W, Rebrin I, Shih JC (2004) A spontaneous point mutation produces monoamine oxidase A/B knock-out mice with greatly elevated monoamines and anxiety-like behavior. J Biol Chem 279:39645–39652
Chen K, Ou XM, Wu W, Chen G, Shih JC (2005) R1, a novel repressor of the human monoamine oxidase A. J Biol Chem 25:11552–11559
Chen K, Rebrin I, Cases O, Seif I, Shih JC (2007) Forebrain specific expression of monoamine oxidase A reduces neurotransmitter levels, restores the brain structure and rescues aggressive behavior in monoamine oxidase A deficient mice. J Biol Chem 282:115–123
Deshwal S, Di Sante M, Di Lisa F, Kaludercic N (2017) Emerging role of monoamine oxidase as a therapeutic target for cardiovascular disease. Curr Opin Pharmacol 33:64–69
Godar SC, Bortolato M, Castelli MP, Casti A, Casu A, Chen K, Ennas MG, Tambaro S, Shih JC (2014) The aggression and behavioral abnormalities associated with monoamine oxidase A deficiency are rescued by acute inhibition of serotonin reuptake. J Psychaitr Res 56:1–9
Gordon RR, Wu M, Huang CY, Harris WP, Sim HG, Lucas JM, Coleman I, Higano CS, Gulati R, True LD, Vessella R, Lange PH, Garzotto M, Beer TM, Nelson PS (2014) Chemotherapy induced monoamine oxidase expression in prostate carcinoma functions as a cytoprotective resistance enzyme and associates with clinical outcomes. PLoS One 9:e104271
Grimsby J, Chen K, Wang LJ, lan NC, Shih JC (1991) Human monoamine oxidase A and B genes exhibit identical exon-intron organization. Proc Natl Acad Sci USA 88:3637–3641
Grimsby J, Toth M, Chen K, Kumazawa T, Klaidman L, Adams JD, Karoum F, Gal J, Shih JC (1997) Increased stress response and β B-deficient mice. Nat Genet 17:206–210
Gross M, Agus D, Dorff T, Pinski J, Quinn D, Shih JC (2016) Invited submission, a late breaking abstract “Interim analysis of phenelzine (a monoamine oxidase inhibitor) for non-metastatic recurrent prostate cancer”, October 30. Presented at Prostate Cancer Foundation Meeting, San Diego
Hare ML (1928) Tyramine oxidase: a new enzyme system in liver. Biochem J 22:968–979
Hintiryan H, Foster NN, Bowman I, Bay M, Song MY, Gou L, Yamashita S, Bienkowski MS, Zingg B, Zhu M, Yang XW, Shih JC, Toga AW, Dong HW (2016) The mouse cortico-striatal projectome. Nat Neurosci 19:1100–1114
Johnston JP (1968) Some observations upon a new inhibitor of monoamine oxidase in brain tissue. Biochem Pharmacol 17:1285–1297
Kaludercic N, Mialet-Perez J, Paolocci N, Parini A, Di Lisa F (2014) Monoamine oxidases as sources of oxidants in the heart. J Mol Cell Cardiol 73:34–42
Knoll J, Magyar K (1972) Some puzzling pharmacological effects of monoamine oxidase inhibitors. Adv Biochem Psychopharmacol 5:393–408
Kushal S, Wang W, Vaikari VP, Kota R, Chen K, Yeh TS, Jhaveri N, Groshen SL, Olenyuk BZ, Chen TC, Hofman FM, Shih JC (2016) Monoamine oxidase A (MAO A) inhibitors decrease glioma progression. Oncotarget 7:13842–13853
Lan NC, Chen CH, Shih JC (1989a) Expression of functional human monoamine oxidase A and B cDNAs in mammalian cells. J Neurochem 52:1652–1654
Lan NC, Heinzmann C, Gal A, Klisak I, Orth U, Grimsby J, Sparkes RS, Mohandas T, Shih JC (1989b) Human monoamine oxidase A and B genes map to Xp 11.23 and are deleted in a patient with Norrie disease. Genomics 4:552–559
Li PC, Siddiqi IN, Mottok A, Loo EY, Wu CH, Cozen W, Steidl C, Shih JC (2017) Monoamine oxidase A is highly expressed in classical Hodgkin lymphoma. J Pathol 243:220–229
Liao CP, Lin TP, Li PC, Geary LA, Chen K, Vaikari VP, Wu JB, Lin CH, Gross ME, Shih JC (2018) Loss of MAO A in epithelia inhibits adenocarcinoma development, cell proliferation and cancer stem cells in prostate. Oncogene 37:5175–5190
Lin YC, Chang YT, Campbell M, Lin TP, Pan CC, Lee HC, Shih JC, Chang PC (2017) MAO A-a novel decision maker of apoptosis and autophagy in hormone refractory neuroendocrine prostate cancer cells. Sci Rep 7:46338
Ou XM, Chen K, Shih JC (2004) Dual functions of transcription factors, transforming growth factor-beta-inducible early gene (TIEG)2 and Sp3, are mediated by CACCC element and Sp1 sites of human monoamine (MAO) B gene. J Biol Chem 279:21021–21028
Ou XM, Chen K, Shih JC (2006a) Glucocorticoid and androgen activation of monoamine oxidase A are regulated by R1 and SP1. J Biol Chem 281:21512–21525
Ou XM, Chen K, Shih J (2006b) Monoamine oxidase A and repressor R1 are involved in apoptotic signaling pathway. Proc Natl Acad Sci USA 103:10923–10928
Peehl DM, Coram M, Khine H, Reese S, Nolley R, Zhao H (2008) The significance of monoamine oxidase-A expression in high grade prostate cancer. J Urol 180:2206–2211
Scott AL, Bortolato M, Chen K, Shih JC (2008) Novel monoamine oxidase A knockout mice with human-like spontaneous mutation. Neuroreport 19:739–743
Sharpe MA, Baskin DS (2016) Monoamine oxidase B levels are highly expressed in human gliomas and are correlated with the expression of HiF-1α and with transcription factors Sp1 and Sp3. Oncotarget 7:3379–3393
Sharpe MA, Livingston AD, Gist TL, Ghosh P, Han J, Baskin DS (2015) Successful treatment of intracranial glioblastoma xenografts with a monoamine oxidase B-activated pro-drug. EBioMedicine 2:1122–1132
Sharpe MA, Raghavan S, Baskin DS (2018) PAM-OBG: a monoamine oxidase B specific prodrug that inhibits MGMT and generates DNA interstrand crosslinks, potentiating temozolomide and chemoradiation therapy in intracranial glioblastoma. Oncotarget 9:23923–23943
Shih JC (2004) Cloning, after cloning, knock-out mice, and physiological function of MAO A and B. Shih JC NeuroToxicology 25:21–30
Shih JC, Eiduson S (1969) Multiple forms of monoamine oxidase in developing brain. Nature 224:1309–1311
Shih JC, Thompson RF (1999c) Monoamine oxidase in neuropsychiatry and behavior. Am J Hum Genet 65:593–598
Shih JC, Chen K, Ridd MJ (1999a) Monoamine oxidase: from genes to behavior. Annu Rev Neurosci 22:197–217
Shih JC, Ridd MJ, Chen K, Meehan WP, Kung M-P, Seif I, De Mayer E (1999b) Ketanserin and tetrabenazine abolish aggression in mice lacking monoamine oxidase A. Brain Res 835:104–112
Shih JC, Wu JB, Chen K (2011) Transcriptional regulation and multiple functions of MAO genes. J Neural Transm 118:979–986
Singh C, Bortolato M, Balic N, Godard SC, Scott AL, Chen K, Thompson RF, Shih JC (2013) Cognitive abnormalities and hippocampal alterations in monoamine oxidase A and B knockout mice. Proc Natl Acad Sci 110:12816–12821
Son B, Jun SY, Seo H, Youn H, Yang HJ, Kim W, Kim HK, Kang C, Youn B (2016) Inhibitory effect of traditional oriental medicine-derived monoamine oxidase B inhibitor on radioresistance of non-small cell lung cancer. Sci Rep 6:21986
Song MS, Matveychuk D, MacKenzie EM, Duchcherer M, Mousseau DD, Baker GB (2013) An update on amine oxidase inhibitors: multifaceted drugs. Prog Neuropsychopharmacol Biol Psychiatry 44:118–124
True L, Coleman I, Hawley S, Huang CY, Gifford D, Coleman R, Beer TM, Gelmann E, Datta M, Mostaghel E, Knudsen B, Lange P, Vessella R, Lin D, Hood L, Nelson PS (2006) A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc Natl Acad Sci USA 103:10991–10996
White TA, Kwon EM, Fu R, Lucas JM, Ostrander EA, Stanford JL, Nelson PS (2012) The monoamine oxidase A gene promoter repeat and prostate cancer risk. Prostate 72:1622–7162
Wong WK, Chen K, Shih JC (2001) Regulation of human monoamine oxidase B gene by Sp1 and Sp3. Mol Pharmacol 59:852–859
Wong WK, Ou X-M, Chen K, Shih JC (2002) Activation of human monoamine oxidase B gene expression by a protein kinase C, MAPK signal transduction pathway involves c-Jun and Egr-1. J Biol Chem 277:22222–22230
Wong WK, Chen K, Shih JC (2003) Decreased methylation and transcription repressor Sp3 up-regulated human monoamine oxidase (MAO) B expression during Caco-2 differentiation. J Biol Chem 278:36227–36235
Wu JB, Chen K, Li Y, Lau YF, Shih JC (2009a) Regulation of monoamine oxidase A by the SRY gene on the Y chromosome. FASEB J 23:4029–4038
Wu JB, Chen K, Ou XM, Shih JC (2009b) Retinoic acid activates monoamine oxidase B promoter in human neuronal cells. J Biol Chem 284:16723–16735
Wu JB, Shao C, Li X, Li Q, Hu P, Shi C, Li Y, Chen YT, Yin F, Liao CP, Stiles BL, Zhau HE, Shih JC, Chung LW (2014) Monoamine oxidase A mediates prostate tumorigenesis and cancer metastasis. J Clin Invest 124:2891–2908
Wu JB, Lin TP, Gallagher JD, Kushal S, Chung LW, Zhau HE, Olenyuk BZ, Shih JC (2015) Monoamine oxidase A inhibitor-near-infrared dye conjugate reduces prostate tumor growth. J Am Chem Soc 137:2366–2374
Xu S, Adisetiyo H, Tamura S, Grande F, Garofalo A, Roy-Burman P, Neamati N (2015) Dual inhibition of survivin and MAO A synergistically impairs growth of PTEN-negative prostate cancer. Br J Cancer 113:242–251
Youdim MB, Collins GG, Sandler M (1969) Multiple forms of rat brain monoamine oxidase. Nature 223:626–628
Zhang Z, Chen K, Shih JC, Teng CT (2006) Estrogen-related receptors-stimulated monoamine oxidase B promoter activity is down-regulated by estrogen receptors. Mol Endocr 20:1547–1561
Zhu QS, Shih JC (1997) An extensive repeat structure down-regulates human monoamine oxidase A promoter activity independent of an initiator-like sequence. J Neurochem 69:1368–1373
Zhu QS, Grimsby J, Chen K, Shih JC (1992) Promoter organization and activity of human monoamine oxidase (MAO) A and B genes. J Neurosci 12:4437–4446
Zhu QS, Chen K, Shih JC (1994) Bidirectional promoter of human monoamine oxidase A (MAO A) controlled by transcription factor Sp1. J Neurosci 14:7393–7403
Ziegler C, Richter J, Mahr M, Gajewska A, Schiele MA, Gehrmann A, Schmidt B, Lesch KP, Lang T, Helbig-Lang S, Pauli P, Kircher T, Reif A, Rief W, Vossbeck-Elsebusch AN, Arolt V, Wittchen HU, Hamm AO, Deckert J, Domschke K (2016) MAO A gene hypomethylation in panic disorder-reversibility of an epigenetic risk pattern by psychotherapy. Transl Psychiatry 5(6):e773. https://doi.org/10.1038/tp.2016.41
Ziegler C, Wolf C, Schiele MA, Feric Bojic E, Kucukalic S, Sabic Dzananovic E et al (2018) Monoamine oxidase A gene methylation and its role in posttraumatic stress disorder: first evidence from the South Eastern Europe (SEE)-PTSD Study. Int J Neuropsychopharmacol 21:423–432
Acknowledgements
The work presented here was supported by NIH grants including two MERIT awards, grants from USC Trustee Tsai Family Fund, and the Boyd and Elsie Welin Professorship. The invaluable contributions of my long-time collaborator Dr. Kevin Chen are gratefully acknowledged. The contributions of international collaborators, graduate students, postdoctoral fellows, and research associates are deeply appreciated. The editing assistance of Frank Hong and Ronald Irwin is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JCS is an inventor on issued US patents 9,771,625 and 9,675,620 related to MAO inhibitor-based compounds that target prostate and brain cancers.
Rights and permissions
About this article
Cite this article
Shih, J.C. Monoamine oxidase isoenzymes: genes, functions and targets for behavior and cancer therapy. J Neural Transm 125, 1553–1566 (2018). https://doi.org/10.1007/s00702-018-1927-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-018-1927-8