Abstract
We evaluated the effects of pretreatment with clorgyline, an irreversible monoamine oxidase (MAO)-A inhibitor, on morphine-induced hyperlocomotion and antinociception. A single administration of morphine (30 mg/kg, i.p.) to male ICR mice induced a hyperlocomotion. ANOVA analysis revealed the statistical significance of the morphine effect on horizontal locomotion and of the clorgyline pretreatment × morphine interaction effect, but not of the effect of clorgyline pretreatment. The initial (5 min after challenge) phase of morphine actions vs. saline challenge appeared as if morphine had a strong inhibitory effect on locomotor activity in combination with different doses of clorgyline. The mice administered with morphine in combination of clorgyline (1 and 10 mg/kg) did not show any stereotypic behaviors. Clorgyline at a dose of 0.1 mg/kg but not other doses tested significantly potentiated morphine-induced antinociception evaluated by tail flick but not hot plate test. During the measurements of locomotor activity and antinociception, clorgyline at doses of 1 and 10 mg/kg significantly inhibited monoamine metabolism through MAO. These results suggest that clorgyline showed an inhibitory effect on morphine-induced hyperlocomotion, but not antinociception, through MAO inhibition. There is not a possibility that clorgyline pretreatment enhanced morphine action on motor activity, resulting in the abnormal behavior from hyperlocomotion to stereotypic movements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morphine is commonly used to treat pain. The principal protein responsible for the antinociceptive effect of morphine is μ-opioid receptor, since mice lacking the μ-opioid receptor show a loss of morphine-induced analgesia [1] and increased sensitivity to painful stimuli [2, 3].
In rats, morphine hyperpolarizes γ-aminobutylic acid (GABA)-containing interneurons via the activation of μ-opioid receptor, reducing spontaneous GABA-mediated synaptic input to dopamine neurons in the ventral tegmental area (VTA) [4]. As a result, morphine enhances dopamine release in the nucleus accumbens (NAc) via the activation of dopamine neurons in the VTA, leading to hypermotility [5]. Cocaine reinforcement decreased in mice lacking the μ-opioid receptor in parallel with increased GABAergic input to VTA dopaminergic neurons [6], although there is a contradictory report [7]. Also, the rewarding property of mother-related stimuli, infant attachment behavior, decreased in mouse puppies lacking the μ-opioid receptor [8]. In line with these observations, the opioid system which is responsible for morphine action (i.e. antinociception and hypermotility) is likely to modulate the mesocorticolimbic dopaminergic pathway, which is attributed to both addictive drugs and natural reward systems [9–14].
The psychostimulant methamphetamine (METH)-induced hyperlocomotion and behavioral sensitization were inhibited by clorgyline, a monoamine oxidase (MAO)-A inhibitor [15, 16]. The change in monoamine turnover in specific brain regions was associated with this inhibition [15, 16]. These observations predicted that clorgyline treatment could inhibit morphine-induced hyperlocomotion and antinociception via alterations in monoamine turnover. To address the prediction, we examined the effect of clorgyline treatment on morphine-induced hyperlocomotion and antinociceptive effect in mice.
Experimental procedure
Reagents
Morphine hydrochloride was purchased from Takeda Chemical Industries (Osaka, Japan). Dexmedetomidine hydrochloride was kindly provided by Prof. K. Noguchi of Hyogo College of Medicine (Nishinomiya, Japan). N-Methyl-N-propargyl-3-(2,4-dichlorophenoxy)propylamine hydrochloride (clorgyline) and all standard reagents for HPLC were from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used were of the highest commercially available purity. The doses of drugs refer to the weight of salt. All drugs were dissolved in sterile saline. Clorgyline was administered subcutaneously (s.c.) in a volume of 0.05 ml/10 g of body weight. Morphine and dexmedetomidine were administered intraperitoneally (i.p.) in a volume of 0.1 ml/10 g of body weight. An identical volume of saline was used for the control.
Subjects
Male ICR mice (7 weeks old at purchase; Japan SLC, Shizuoka, Japan) were housed in groups of 6–8 in a temperature- (22 ± 2°C) and humidity- (50 ± 10%) controlled environment under a 12-h light/dark cycle (lights on at 0700 h) with food and water available ad libitum except during the locomotor activity measurements using Supermex apparatus and nociception test (see below). Animal handling and care were conducted according to the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy Press 1996; NIH publication number 85–23, revised 1996) and all experiments were approved by the Institutional Animal Research Committee. After at least 3 weeks’ habituation in this facility, mice were used in the experiments described below.
Behavioral analysis
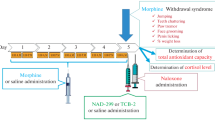
Mice were weighed (34–47 g) and injected s.c. with 0.05 ml/10 g volume of sterile saline or clorgyline (0, 0.1, 1 or 10 mg/kg) 2 h before i.p. morphine treatment with 0.1 ml/10 g volume (Fig. 1A). The mice were returned to their home cages for 1 h, then placed into a transparent acrylic box (37 × 24 × 27 cm) with an infrared sensor that detects thermal radiation from animals (for horizontal locomotion; Supermex; Muromachi Kikai Co., Ltd., Tokyo, Japan) in a quiet, ventilated chamber (53 × 45 × 45 cm) for next 1 h. The mice were injected i.p. with saline or morphine (30 mg/kg) and immediately returned to the same test box and subjected to a measurement of horizontal locomotor activity for 2 h 30 min at 5-min intervals [15, 16]. During the measurements, mice were given tap water ad libitum. After locomotion measurements, mice were sacrificed by cervical dislocation and decapitation. The brains were immediately removed, and the regions of striata and accumbens were isolated, weighed, and frozen in liquid nitrogen until assay by HPLC (brain dissection 4 h 30 m after clorgyline treatment).
Schematic representation of the experimental protocols for measurements of locomotor activity (A) and nociception assays (B). Horizontal locomotion was measured during the periods indicated by hatched bars. Arrows show the time when mice were subjected to injections with the drug solutions indicated or antinociception assays. ±DEX, with or without 30 μg/kg dexmedetomidine
Nociception test
The mice injected with saline (0.05 ml/10 g, s.c.) or clorgyline (0.1, 1 or 10 mg/kg, s.c.) were returned to their home cages for 1 h 40 min (Fig. 1B). Antinociceptive responses to morphine alone or with dexmedetomidine, an α2-adrenoceptor agonist, were examined using a hot plate and tail flick tests [17] with slight modifications described below.
Hot plate assay
Antinociceptive effects were assessed in mice injected at 20-min intervals with saline (0.1 ml/10 g, i.p.) and then with ascending morphine doses producing total drug doses of 1, 3, 10 and 30 mg/kg, i.p. with or without 30 μg/kg dexmedetomidine. Each mouse was placed on a hot plate (55 ± 1°C, Model KN-205D, Natsume Seisakusho, Co., Ltd., Tokyo, Japan) after 20 min of saline injection for the baseline measurement just prior to drug administration. Through the test, a cut-off time of 30 s was set to minimize tissue damage, and mice were immediately removed from the hot plate when they displayed nociceptive responses, which were jumping or paw-licking. Baseline latencies were between 4 s and 25 s among all the mice examined.
Tail flick assay
Following the hot plate test, the tail flick test was conducted. Each mouse wrapped in cotton cloth was softly restrained upright by hand. About 3–4 cm length of the tail tip was placed in a hot water bath at 53 ± 1°C until flicked out. Through the test, a cut-off time of 15 s was set to minimize tissue damage, and mice were immediately removed from the hot water bath and returned to the home cage. Baseline latencies were between 1 s and 7 s.
For each test time in both assays, the percentage maximum possible effect (%MPE) was calculated as (test latency−baseline latency)/(cut off time−baseline latency) × 100% [17].
HPLC analysis
Brain dissections 2 h and 3 h 20 min after clorgyline treatment were conducted as shown in Fig. 1C. Each frozen brain sample was homogenized with a Teflon/glass homogenizer in 10 volumes (w/v) of ice-cold 0.1 N perchloric acid with 30 μM Na2EDTA containing 3,4-dihydroxybenzylamine hydrobromide and isoproterenol as internal standards for the catechols and indoles, respectively. The homogenates were centrifuged at 10,000g for 10 min at 4°C and the supernatants were filtered through a 0.20-μm membrane filter (Millipore Co., Bedford, MA, USA). The filtrates (10 μl) were injected directly into an HPLC system (system controller, model SCL-10A; auto-injector, model SIL-10A; pump, model LC-10AD; Shimadzu Co., Kyoto, Japan) equipped with a reversed-phase ODS-column (MCM column 150; 4.6 × 150 mm; MC Medical, Inc., Osaka, Japan) and an electrochemical detector (Coulochem Model 5100A, ESA, Inc., Chelmsford, MA, USA). The column temperature was maintained at 24°C, and the detector potentials were set at +0.40 V, +0.15 V and −0.35 V on the conditioning cell, Detectors 1 and 2, respectively. The mobile phase was a 1000:35.2:85.8 (v/v) mixture of a buffer (50 mM Na2HPO4, 50 mM citric acid, 4.4 mM 1-heptanesulfonic acid and 0.1 mM Na2EDTA, pH 3.0), acetonitrile and methanol, and the flow rate was set at 0.9 ml/min [18]. The monoamines measured in this study were dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOAPC), 3-methoxytyramine (3-MT), homovanillic acid (HVA), norepinephrine (NE), 3-methoxy-4-hydroxyphenylglycol (MHPG), 5-hydroxytryptamine (serotonin, 5-HT), and 5-hydroxyindoleacetic acid (5-HIAA).
Statistical analysis
Values are shown as the means with bars representing the standard errors of the means (S.E.M.). Statistical analysis was performed using one-way or two-way analysis of variance (ANOVA) with or without repeated measures, followed by Tukey–Kramer’s comparisons or Student’s t-test packaged in the computer program StatView 5.0. for Apple Macintosh. Results were considered significant when P-values were <0.05.
Results
Effects of pretreatment with clorgyline on spontaneous locomotion and morphine-induced hyperlocomotion in mice
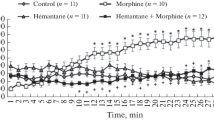
Pretreatment of the mice with different doses of clorgyline had no effect on spontaneous locomotor activities evaluated in terms of horizontal locomotion in the period between 2 h and 4 h 30 min after clorgyline injection (Fig. 2A, Saline challenge) (clorgyline pretreatment effect, F(3,1200) = 0.381, P = 0.7669, repeated-measure two-way ANOVA). In the habituation period between 1 h and 2 h after clorgyline injection, no effect of clorgyline pretreatment on exploring activities was observed (data not shown).
Effect of clorgyline pretreatment on horizontal hyperlocomotion in response to morphine challenge. Horizontal locomotor activity counts within each 5-min interval after saline (A) and morphine challenge (B, total, 2 h 30 min) are shown. Mice were administered with saline or clorgyline (0.1, 1, and 10 mg/kg, s.c.). Two-hours after pretreatment, animals were challenged with morphine (30 mg/kg, i.p.) or saline. The values are shown as the means ± S.E.M. of 11 mice. CLO, clorgyline. *P < 0.05, compared with saline (one-way ANOVA followed by Tukey–Kramer’s comparisons)
ANOVA analysis revealed statistical significance of the morphine effect (Fig. 2B, one-way repeated-measure ANOVA, Morphine challenge, F(29,1200) = 10.632, P < 0.001) and of the clorgyline pretreatment × morphine interaction effect (inhibition, two-way repeated-measure ANOVA, F(87,1200) = 1.375, P < 0.05), but not of the effect of clorgyline pretreatment (one-way repeated-measure ANOVA, clorgyline dose, F(3,1200) = 1.059, P = 0.3773).
To reveal statistically the apparent inhibitory action of morphine on locomotor activity during the initial phase after the drug challenge (0–5 min after morphine challenge vs. saline, Fig. 2), a mixed factorial ANOVA was performed. There were significant differences between the locomotion prior to the drug challenge (−5 to 0 min after morphine/saline injection, see Fig. 1A) and that after the drug challenge (0–5 min after morphine/saline injection) (F(1,40) = 8.979, P < 0.01) and between the challenge types (morphine vs. saline) (F(1,40) = 4.386, P < 0.05). The interaction effect (challenge types × locomotion prior to and after the drug challenge) was also revealed to be significant (F(1,40) = 14.678, P < 0.01).
The mice administered with morphine in combination of clorgyline (1 and 10 mg/kg) did not show any stereotypic behaviors (data not shown) as assessed by the method reported previously [19].
Effects of pretreatment with clorgyline on morphine-induced antinociception in mice
Cumulative morphine administration to mice caused dose-dependent antinociceptive effects in the hot plate and tail flick tests (Fig. 3A and B, F(3,284) = 29.042, P < 0.001 and F(3,284) = 148.883, P < 0.001, respectively, repeated-measure two-way ANOVA). In the hot plate test, co-administration of dexmedetomidine (30 μg/kg, i.p.) with morphine or pretreatment with clorgyline had no effect on the antinociceptive effect induced by morphine (Fig. 3A, F(12,284) = 1.253, P = 0.2488). In the tail flick test, co-administration of dexmedetomidine with morphine and pretreatment with clorgyline (0.1 mg/kg) significantly potentiated the antinociceptive effect induced by morphine (at doses of 1 and 10, and 10 mg/kg, respectively) (Fig. 3B, F(12,284) = 1.997, P < 0.05). Apparent hyperalgesic effect of lower doses of morphine was observed in the hot plate but not in the tail flick test.
Effect of clorgyline pretreatment on morphine-induced antinociceptive effect in hot plate (A) and tail flick tests (B). Mice were injected i.p. with morphine at the indicated concentrations in a cumulative manner. The values are shown as the means ± S.E.M. of 14–20 mice. CLO, clorgyline; DEX, 30 μg/kg dexmedetomidine. *P < 0.05, compared with control animals (saline challenge) (Student’s t-test)
Tissue levels of monoamines and their metabolites
The results obtained from HPLC-based measurements of the contents of brain monoamines and their metabolites after the clorgyline pretreatment and morphine challenge are presented in Table 1. ANOVA analysis revealed statistical significance of the clorgyline pretreatment effect, morphine effect (shown in Table 1), and of a clorgyline pretreatment × morphine interaction effect in all monoamines and metabolites except DOPAC (F(3,40) = 3.514, P < 0.05, F(3,40) = 7.329, P < 0.001, F(3,40) = 5.593, P < 0.01, F(3,40) = 4.809, P < 0.01, F(3,40) = 3.592, P < 0.05, F(3,40) = 3.549, P < 0.05, and F(3,40) = 7.367, P < 0.001 for dopamine, 3-MT, HVA, norepinephrine, MHPG, 5-HT, and 5-HIAA, respectively). In DOPAC content, no significant clorgyline pretreatment x morphine interaction effect was observed (and F(3,40) = 1.652, P = 0.1926). It should be noted that the data on 3-MT level might not be valid, since post-mortem changes in 3-MT levels were not prevented even by focused microwaves.
Apparent monoamine turnover
As shown in Fig. 4A–C, apparent monoamine turnovers in saline-challenged mice pretreated with clorgyline (protocol shown in Fig. 1A) and mice pretreated with clorgyline (protocol shown in Fig. 1C) were evaluated by calculating the ratio of the tissue contents of monoamines and their metabolites. ANOVA analysis revealed statistical significance of the clorgyline effect (dose) (F(3,44) = 105.492, P < 0.001, F(3,44) = 74.945, P < 0.001, F(3,44) = 193.127, P < 0.001, F(3,44) = 19.647, P < 0.001, and F(3,44) = 69.726, P < 0.001 for the ratio of DOPAC to dopamine, 3-MT to dopamine, HVA to dopamine, MHPG to norepinephrine, and 5-HIAA to 5-HT, respectively), but not of the effect of clorgyline treatment period (time) (F(2,44) = 0.572, P = 0.5683, F(2,44) = 0.007, P = 0.9927, F(2,44) = 0.686, P = 0.5091, F(2,44) = 1.796, P = 0.178, and F(2,44) = 3.201, P = 0.62 for the ratio of DOPAC to dopamine, 3-MT to dopamine, HVA to dopamine, MHPG to norepinephrine, and 5-HIAA to 5-HT, respectively) or of the dose × time interaction effect (F(6,44) = 0.822, P = 0.5587, F(6,44) = 1.856, P = 0.1102, F(6,44) = 2.109, P = 0.0712, F(6,44) = 1.928, P = 0.0975, and F(6,44) = 0.74, P = 0.62 for the ratio of DOPAC to dopamine, 3-MT to dopamine, HVA to dopamine, MHPG to norepinephrine, and 5-HIAA to 5-HT, respectively).
Apparent monoamine turnover in the striatum + accumbens of the mice at 2 h (A), 3 h 20 min (B), and 4 h 30 min (C) after clorgyline pretreatment. Each column represents the mean ± S.E.M. (n = 4–6). CLO, clorgyline. *P < 0.05, significantly different change in apparent monoamine turnover between control (saline) and clorgyline-pretreated mice (two-way ANOVA interaction effect)
Discussion
A single administration of morphine induced hyperlocomotion in mice with a maximal level attained at 75 min after drug administration, then hypermotility gradually decreased to the basal level at 150 min after drug administration (Fig. 2B). The time course was similar to that reported by Mori et al. [20] in which mice were subjected to a single morphine administration (20 mg/kg). Pretreatment with increasing doses of clorgyline, an irreversible MAO-A inhibitor [21], 2 h prior to morphine administration inhibited morphine-induced hyperlocomotion in a dose-dependent manner, reaching significant inhibition with a dose of 10 mg/kg (Fig. 2B). Statistical evaluation revealed a significant difference in morphine effect (i.e. hypermotility) × clorgyline pretreatment interaction effect. However, there was no statistical significance in the clorgyline pretreatment effect itself because of individual variations in the locomotion data (Fig. 2A). Based on the statistical analysis, it is suggested that clorgyline pretreatment delayed the onset of morphine-induced hyperlocomotion, although clorgyline pretreatment appeared to reduce the peak activity of morphine-induced hyperlocomotion. This hypothesis is supported by evidence that the initial (5 min after challenge) phase of morphine actions versus saline challenge appeared as if morphine had a strong inhibitory effect on locomotor activity in combination with various doses of clorgyline (Fig. 2; see Results section for statistical analysis). No stereotypic behavior was observed in mice treated with morphine in combination with clorgyline, as assessed by the method reported previously [19]. Therefore, there is not a possibility that clorgyline pretreatment enhanced morphine action on motor activity, resulting in the abnormal behavior from hyperlocomotion to stereotypy.
In this study, the ratio of the major metabolite to the corresponding monoamine is used as an index of monoamine turnover. For 5-HT metabolism, the ratio of 5-HIAA/5-HT is a good index of apparent serotonin turnover [22]. HPLC analysis revealed that clorgyline pretreatment showed typical pharmacological properties as an irreversible MAO-A inhibitor throughout the period of measurement of morphine-induced hyperlocomotion (Fig. 4A–C). Significant alteration in monoamine turnover was observed at doses of 1 and 10 mg/kg of clorgyline. In parallel with this, significant inhibition by clorgyline (1 and 10 mg/kg) of morphine-induced hyperlocomotion was observed (Fig. 2B), suggesting that the inhibitory effect of clorgyline on morphine administration-induced hyperlocomotion was attributed primarily to MAO-A inhibition by clorgyline. The clorgyline effect on monoamine turnover appeared to peak 2 h after pretreatment, since the maximal inhibition of 5-HT metabolism by clorgyline (1 and 10 mg/kg, s.c.) in the rat brain was reported to be observed 2 h after pretreatment [23].
Inhibition by dopamine D3 receptor agonist of the levels of dopamine metabolites (DOPAC and HVA), but not dopamine, in NAc was accompanied by the inhibition of morphine-induced hyperlocomotion in mice, suggesting that the inhibitory effect of D3 receptor agonist on morphine-induced hypermotility might be attributed to decrease in the apparent dopamine turnover (ratio of DOPAC to dopamine) [24]. Similarly, in this study, clorgyline (10 mg/kg) pretreatment inhibited dopamine turnover (ratio of DOPAC to dopamine) by 14.9 and 12.4% in mice after saline and morphine challenges, respectively (Fig. 4C). However, in contrast to the result of Suzuki et al. [24] that no change in the level of dopamine was observed after D3 receptor agonist, pretreatment with clorgyline significantly decreased the striatal level of dopamine after morphine challenge, compared with the control (saline challenge) (P < 0.05, Table 1). Assuming that the synthesis of dopamine decreases in the striatum after clorgyline pretreatment, animal motility might be reduced, accompanied by a decreased level of striatal dopamine. There is another possibility that the increased release of striatal dopamine from presynaptic storage into the synaptic cleft might explain the decreased level of dopamine, and it was seen that pretreatment of rats with clorgyline (4 mg/kg) increased in extracellular dopamine in the brain [25]. Further study is needed to clarify why the striatal dopamine was decreased after clorgyline pretreatment.
Clorgyline, in spite of a well-known selective MAO-A inhibitor, can irreversibly inhibit MAO-B as well as MAO-A at higher doses, as reported previously [23]. The MAO-B inhibitory action of clorgyline depends on doses and treatment period. In our previous study, l-deprenyl, a MAO-B inhibitor, did not block METH (1 mg/kg)-induced hyperlocomotion in mice [15]. This observation led us to a speculation that the unexpected inhibitory action of clorgyline on MAO-B could not contribute prominently to the inhibitory effect of clorgyline on morphine-induced hyperlocomotion.
Morphine caused dose-dependent antinociception in both hot plate and tail flick tests (Fig. 3A and B). Apparent hyperalgesic effect of morphine (1 and 3 mg/kg) was observed in the hot plate test (Fig. 3A). This might be attributed to the degree of habituation to the hot plate test apparatus. Therefore, we tried to habituate the mice to the apparatus 1 day before nociception tests, and got the same data as that shown in Fig. 3A (data not shown), excluding the possibility of habituation. No apparent hyperalgesic effect of morphine was observed when μ-opioid receptor gene knockout mice (and the corresponding wild type mice) were used [17]. The μ-receptor KO mice had a mixed C57BL/6/129Sv background [2, 17], and appeared to show lower spontaneous locomotion than that observed in ICR mice used in this study. It is considered that the apparent hyperalgesic effect of lower doses of morphine (Fig. 3A) might be due to strain differences, i.e. difference between the genetic background of ICR (closed colony) and C57/BL6 (inbred). If the apparent hyperalgesic effect of morphine is attributed to stress-induced analgesia when testing in the baselines, the effect ought to be observed more strongly in the tail flick test than in the hot plate test since the mice were forced to wrap in cotton cloth, but was not (Fig. 3B).
In the hot plate assay, doses of morphine tested did not induce ca. 100% of MPE (Fig. 3A). To address whether the mice could show 100% MPE in this assay, co-administration of morphine with dexmedetomidine, an α2-adrenoceptor agonist, was performed. Dexmedetomidine in combination with 30 mg/kg of morphine attained ca. 90% of antinociception, which was similar to the result reported by Tham et al. [26].
When the mice were pretreated with 0.1 mg/kg of clorgyline, the antinociceptive effect of morphine (10 mg/kg) was potentiated in the tail flick test (Fig. 3B). The effect appeared not to be associated with the clorgyline effect on MAO-A, since no significant alteration in the monoamine turnover was observed at 0.1 mg/kg of clorgyline (Fig. 4A–C). Therefore, a mechanism(s) other than MAO inhibition should be investigated to clarify the action of clorgyline as low as 0.1 mg/kg. There is evidence that clorgyline at a dose of 0.1 mg/kg, but not 1 nor 10 mg/kg, significantly delayed the onset of METH-induced stereotypy in mice, whereas higher doses of clorgyline (1–10 mg/kg) had no effect [19], suggesting that clorgyline interacted not only with MAO-A but also with σ-receptors and/or imidazoline I2-preferring receptors [27, 28] or unknown sites other than MAO depending on the dosage used.
As reported previously, natural form of morphine ((−)-morphine) is glucuronidated only at the 3-position and not at the 6-position in the rat liver by UDP-glucuronosyltransferase isoform, Ugt2b1 [29, 30]. A large fraction of morphine absorbed into the body is removed by this first-pass metabolism. Morphine 6β-glucuronide, but not morphine 3β-glucuronide is reported to stimulate locomotor activity in mice [31] and show analgesic activity in rats [32]. In contrast to metabolism of morphine, various cytochrome P450 enzymes, especially P450 2B1, are involved in metabolism of clorgyline. Clorgyline was found to inactivate P450 2B1 as well by forming a metabolic intermediate complex with the enzyme [33]. This process appears not to interact with liver glucuronidation. Therefore, the possibility that the interaction of clorgyline with central morphine effects is pharmacokinetic could be ruled out.
Overall, this study suggested that, in contrast to our prediction, clorgyline pretreatment showed an inhibitory effect on morphine-induced hyperlocomotion, but not antinociception, through MAO inhibition. Apparent strong inhibitory action of morphine on motor activity in mice pretreated with clorgyline in the initial phase is not negligible to better understand the change in the locomotion.
References
Matthes HWD, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dollé P, Tzavara E, Hanoune J, Roques BP, Kieffer BL (1996) Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the μ-opioid-receptor gene. Nature 383:819–823
Sora I, Takahashi N, Funada M, Ujike H, Revay RS, Donovan DM, Miner LL, Uhl GR (1997) Opiate receptor knockout mice define μ receptor roles in endogenous nociceptive responses and morphine-induced analgesia. Proc Natl Acad Sci USA 94:1544–1549
Uhl GR, Sora I, Wang Z (1999) The μ opiate receptor as a candidate gene for pain: polymorphisms, variations in expression, nociception, and opiate responses. Proc Natl Acad Sci USA 96:7752–7755
Johnson SW, North RA (1992) Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci 12:483–488
Di Chiara G, Imperato A (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278
Mathon DS, Lesscher HM, Gerrits MA, Kamal A, Pintar JE, Schuller AG, Spruijt BM, Burbach JP, Smidt MP, van Ree JM, Ramakers GM (2005) Increased GABAergic input to ventral tegmental area dopaminergic neurons associated with decreased cocaine reinforcement in μ-opioid receptor knockout mice. Neuroscience 130:359–367
Contarino A, Picetti R, Matthes HW, Koob GF, Kieffer BL, Gold LH (2002) Lack of reward and locomotor stimulation induced by heroin in μ-opioid receptor-deficient mice. Eur J Pharmacol 446:103–109
Moles A, Kieffer BL, D’Amato FR (2004) Deficit in attachment behavior in mice lacking the μ-opioid receptor gene. Science 304:1983–1986
De Vries TJ, Shippenberg TS (2002) Neural systems underlying opiate addiction. J Neurosci 22:3321–3325
Gardner EL (2004) Brain reward mechanisms. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG (eds) Substance abuse: a comprehensive textbook, 4th edn. Lippincott Williams & Wilkins, Philadelphia, pp 48–97
Kelley AE, Berridge KC (2002) The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci 22:3306–3311
Nestler EJ, Berhow MT, Brodkin ES (1996) Molecular mechanisms of drug addiction: adaptations in signal transduction pathways. Mol Psychiat 1:190–199
Przewlocki R (2004) Opioid abuse and brain gene expression. Eur J Pharmacol 500:331–349
Wise RA (2002) Brain reward circuitry: insights from unsensed incentives. Neuron 36:229–240
Kitanaka N, Kitanaka J, Takemura M (2005) Inhibition of methamphetamine-induced hyperlocomotion in mice by clorgyline, a monoamine oxidase-A inhibitor, through alteration of the 5-hydroxytryptamine turnover in the striatum. Neuroscience 130:295–308
Kitanaka N, Kitanaka J, Takemura M (2005) Repeated clorgyline treatment inhibits methamphetamine-induced behavioral sensitization in mice. Neurochem Res 30:445–451
Kitanaka N, Sora I, Kinsey S, Zeng Z, Uhl GR (1998) No heroin or morphine 6β-glucuronide analgesia in μ-opioid receptor knockout mice. Eur J Pharmacol 355:R1–R3
Kitanaka N, Kitanaka J, Takemura M (2003) Behavioral sensitization and alteration in monoamine metabolism in mice after single versus repeated methamphetamine administration. Eur J Pharmacol 474:63–70
Tatsuta T, Kitanaka N, Kitanaka J, Morita Y, Takemura M (2005) Effects of monoamine oxidase inhibitors on methamphetamine-induced stereotypy in mice and rats. Neurochem Res 30:1377–1385
Mori T, Ito S, Narita M, Suzuki T, Sawaguchi T (2004) Combined effects of psychostimulants and morphine on locomotor activity in mice. J Pharmacol Sci 96:450–458
Finberg JP, Youdim MB (1983) Selective MAO A and B inhibitors: their mechanism of action and pharmacology. Neuropharmacology 22:441–446
de Vries M, Odink J (1991) Simultaneous measurement of serotonin and 5-hydroxyindoleacetic acid in rat brain using a liquid chromatographic method with electrochemical detection. J Chromatogr 564:250–257
Felner AE, Waldmeier PC (1979) Cumulative effects of irreversible MAO inhibitors in vivo. Biochem Pharmacol 28:995–1002
Suzuki T, Maeda J, Funada M, Misawa M (1995) The D3-receptor agonist (±)-7-hydroxy-N,N-di-n-propyl-2-aminotetralin (7-OH-DPAT) attenuates morphine-induced hyperlocomotion in mice. Neurosci Lett 187:45–48
Segal DS, Kuczenski R, Okuda C (1992) Clorgyline-induced increases in presynaptic DA: changes in the behavioral and neurochemical effects of amphetamine using in vivo microdialysis. Pharmacol Biochem Behav 42:421–429
Tham SM, Angus JA, Tudor EM, Wright CE (2005) Synergic and additive interactions of the cannabinoid agonist CP55,940 with μ-opioid receptor and α2-adrenoceptor agonists in acute pain models in mice. Br J Pharmacol 144:875–884
Itzhak Y, Stein I, Zhang S-H, Kassim CO, Cristante D (1991) Binding of σ-ligands to C57BL/6 mouse brain membranes: effects of monoamine oxidase inhibitors and subcellular distribution studies suggest the existence of σ-receptor subtypes. J Pharmacol Exp Ther 257:141–148
Alemany R, Olmos G, García-Sevilla JA (1995) The effects of phenelzine and other monoamine oxidase inhibitor antidepressants on brain and liver I2 imidazoline-preferring receptors. Br J Pharmacol 114:837–845
Rane A, Gawronska-Sxklarz B, Svensson JO (1985) Natural (−)- and unnatural (+)-enantiomers of morphine: comparative metabolism and effect of morphine and phenobarbital treatment. J Pharmacol Exp Ther 234:761–765
King CD, Rios GR, Green MD, MacKenzie PI, Tephly TR (1997) Comparison of stably expressed UGT1.1 and UTG2B1 in the glucuronidation of opioid compounds. Drug Metab Dispos 25:251–255
Morland J, Jones BL, Palomares ML, Alkana RL (1994) Morphine-6-glucuronide: a potent stimulator of locomotor activity in mice. Life Sci 55:163–168
Lipkowski AW, Carr DB, Langlade A, Osgood PF, Szyfelbein SK (1994) Morphine-3-glucuronide: silent regulator of morphine actions. Life Sci 55:149–154
Sharma U, Roberts ES, Hollenberg PF (1996) Formation of a metabolic intermediate complex of cytochrome P4502B1 by clorgyline. Drug Metab Dispos 24:1247–1253
Acknowledgments
NK was supported by a Grant-in-Aid for Researchers, Hyogo College of Medicine.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kitanaka, N., Kitanaka, J. & Takemura, M. Modification of Morphine-induced Hyperlocomotion and Antinociception in Mice by Clorgyline, a Monoamine Oxidase-A Inhibitor. Neurochem Res 31, 829–837 (2006). https://doi.org/10.1007/s11064-006-9087-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-006-9087-x