Abstract
Infection with the parasite Toxoplasma gondii causes serious public health problems and is of great economic importance worldwide. No vaccine is currently available, so the design of efficient vaccine strategies is still a topical question. In this study, we evaluated the immunoprophylactic potential of a T. gondii virulence factor, the rhoptry kinase ROP18, in a mouse model of chronic toxoplasmosis: first using a recombinant protein produced in Schneider insect cells adjuvanted with poly I:C emulsified in Montanide SV71 by a parenteral route or adjuvanted with cholera toxin by the nasal route and second using a DNA plasmid encoding ROP18 adjuvanted with GM-CSF ± IL-12 DNA. If both intranasal and subcutaneous recombinant ROP18 immunizations induced predominantly anti-ROP18 IgG1 antibodies and generated a mixed systemic Th1-/Th2-type cellular immune response characterized by the production of IFN-γ, IL-2, Il-10 and IL-5, only intranasal vaccination induced a mucosal (IgA) humoral response in intestinal washes associated with a significant brain cyst reduction (50 %) after oral challenge with T. gondii cysts. DNA immunization induced antibodies and redirected the cellular immune response toward a Th1-type response (production of IFN-γ and IL-2) but did not confer protection. These results suggest that ROP18 could be a component of a subunit vaccine against toxoplasmosis and that strategies designed to enhance mucosal protective immune responses could lead to more encouraging results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasma is an obligate intracellular protozoan parasite which is the cause of toxoplasmosis in almost all warm-blooded animals. Nearly, one-third of the world’s population is infected with T. gondii, which can cause Toxoplasmosis in the developing fetus and life-threatening disease in people whose immune systems are compromised through diseases like AIDS or chemotherapy [1–3]. Toxoplasma gondii is also recognized as a major cause of abortion in farm animals such as sheep and goats [2]. Acquired resistance to T. gondii infection is mediated by a mucosal and systemic Th1 cellular immunity, which depends mainly on the ability of T cells to produce IFN-γ [4, 5]. When humans or animals become infected, there is no drug treatment available that will eliminate the parasite; hence, an effective vaccine against T. gondii would be extremely valuable for controlling toxoplasmosis [6–8]. Thus, in the last years, the immunogenic efficacy of many T. gondii antigens has been extensively investigated as potential vaccine candidates; among them is ROP18, a polymorphic rhoptry protein. ROP18, a member of the ROP2 family, is localized to the rhoptry bulb and is an active kinase [9]. Upon invasion, ROP18, ROP17, ROP5 and GRA7 are secreted into the host cell and subsequently traffic back to the PVM, where they cooperate to inhibit the localization and the function of immunity-related GTPases [10, 11]. The efficiency of vaccination depends on several factors including the adjuvanticity of the formulation component(s), the route of administration and the strain of mouse used. Because the main natural site of infection for T. gondii is the mucosal surface of the intestine, the protective immunity obtained after natural infection with T. gondii points to the importance of developing a vaccine that stimulates both mucosal and systemic defenses. In previous studies, we have shown that intranasal vaccination with SAG1 plus CT induced humoral and cellular immune responses at local and systemic sites with a Th1 cytokine pattern and protects CBA/J mice (H-2k) against chronic toxoplasmosis following oral challenge with T. gondii cysts [12, 13]. Previous studies used parenteral immunizations with recombinant ROP18 protein, and only one investigated protective efficacy in H-2k major histocompatibility haplotype (C3H mice) [14]. We were therefore interested in evaluating the potential of T. gondii ROP18 using an intranasal vaccination strategy compared with a subcutaneous vaccination strategy similar to that of Grzybowski et al. [14] except that ROP18 and poly I:C were emulsified with Montanide ISA 71 an adjuvant known to enhance cellular immune responses [15].
We also compared the immune responses and the protective efficacy elicited in mice immunized with a bicistronic plasmid encoding ROP18 and GM-CSF with or without a plasmid encoding IL-12. Intramuscular DNA vaccination has been shown to induce Th1-type CD4+ T cells and cytotoxic CD8+ T cells, while maintaining the capability of stimulating antibody responses [16], immune responses which are potentially protective against T. gondii. We previously showed that co-administration of a plasmid encoding GM-CSF with a plasmid encoding a T. gondii antigen or administration of a bicistronic plasmid (pIRESopt) encoding both the antigen and GM-CSF enhanced the humoral and cellular immune responses and improved the protective efficacy in mice against toxoplasmosis [17, 18]. Furthermore, IL-12 has been used to modulate the humoral and T cell responses toward a Th1 profile and to enhance the generation and activity of CTLs in many studies including vaccine strategies against T. gondii [19–22]. Based on this background information, DNA encoding GM-CSF and IL-12 were used in this study.
Materials and methods
Mice
Female CBA/J mice (H-2k), 6–8 weeks old, were obtained from Janvier (Le Genest St. Isle, France) and maintained under pathogen-free conditions in our animal house for use throughout these experiments. Experiments were performed in accordance with the guidelines for the animal experimentation, and the protocol was approved by the local ethics committee (CEEA VdL).
Parasites
Cysts of the 76 K strain of T. gondii (type II) were obtained from the brains of orally infected CBA/J mice and maintained by monthly passage. They were used for all experimental infections.
Recombinant T. gondii ROP18 protein (ROP18S2)
The gene fragment encoding the mutated ROP18 (type I, ROP18mut, point mutation introduced in the catalytic domain) without its signal sequence and propeptide (AA 83-539) (1368 bp) was amplified by PCR from the plasmid pTracerROP18mutΔpro (D394 A) which was kindly provided by Maryse Lebrun [9] using the primers 5BglROP18S2 (5′-GGCGGCAGATCTGAAAGGGCTCAAC, forward) and 3XbaROP18S2 (5′-GCCCTCTAGATTCTG, reverse). The resulted PCR fragment was cloned into the expression vector pMT/BiP/V5-HisA (Invitrogen) at BglII/XbaI restriction sites to obtain pMT-ROP18mutsec. The recombinant vector was sequenced and Drosophila Schneider 2 cells (S2 cells) were transfected, using the drosophila expression system (DES) purchased from Invitrogen, as previously described [23]. ROP18S2 was purified under native conditions from the supernatant of stably transfected cells, using the HisTrap HP affinity column (GE Healthcare), as specified by the manufacturer. The purified protein was analyzed by SDS-PAGE with coomassie blue staining. Antigenic validation was performed by Western blot against anti-ROP18 rat polyclonal antibodies (kindly provided by Maryse Lebrun), sera from T. gondii (76 K) infected mice and sera from uninfected mice as negative control. Electrophoresis and immunoblotting were performed as previously described [24].
DNA vaccine (pROP18)
The eukaryotic expression vector pIRESopt-GMCSF [17] was used to co-express a secreted T. gondii ROP18 and mouse GM-CSF from the same plasmid. To obtain the secreted form of ROP18, the sequence encoding ROP18 (without its signal sequence and propeptide) was amplified by PCR from the plasmid pTracerROP18mutΔpro by using the primers 5XbaROP18sec (5′GCGGCTCTAGAGAAAGGGCTCAAC, forward) and 3NheROP18 (5′GAAGGGCTAGCTTTATTCTGTGTGGAG, reverse). The signal sequence of MIC3 (ssMIC3) was amplified from the plasmid pcDNA3-EGF [17] by using the primers, 5NheSigMIC3 (5′-GACCCAAGCTAGCCTTGTCC, forward) and 3XbaSigMIC3 (5′-CAGCTTCTAGAAGCCTCCGC, reverse). Then the two XbaI-digested PCR fragments were ligated together, and the ligation product was PCR-amplified with primers, 5NheSigMIC3 and 3NheROP18. The resulted PCR fragment was cloned at NheI site of the plasmid pIRESopt-GMCSF to obtain pIRESopt-GMCSF-ROP18secmut (pROP18). IL-12-containing plasmid (pIL12) kindly provided by Dr A.L. Rakhmile-vich (University of Wisconsin Comprehensive Cancer Center, Madison, WI, USA), pIRESopt-GMCSF (pIRES) and pROP18 were purified from transformed Escherichia coli DH5α by endotoxin-free DNA purification kit (Qiagen GmBH, Hilden, Germany) as specified by the Manufacturer.
To test its expression, the recombinant eukaryotic expression plasmid pROP18 was transiently transfected into 293 T cells (Invitrogen) using a polycationic liposome reagent (LipofectAMINE™, Invitrogen) as instructed by the manufacturer. After 48 h of culture, cells were lysed and antigenic validation was performed by Western blot against anti-ROP18 rat antibodies.
Mice immunizations and challenge
For protein immunizations, mice were immunized either with ROP18S2 (15 µg) emulsified in Montanide™ ISA 71 (SEPPIC, France) combined with 10 µg poly I:C (Invivogen, size >1.5 kb) by subcutaneous injection (sc-M-P-ROP18 group), or with ROP18S2 (15 µg) plus 1 µg cholera toxin (CT, Sigma) intranasally (in-CT-ROP18 group), three times at intervals of 2 weeks. Control groups were either untreated (untreated group) or inoculated with adjuvants alone (in-CT-control and sc-M-P-control groups).
For DNA immunization, mice received three intramuscular injections at 2-week intervals of pROP18 (100 µg) without or with adjuvant, IL-12-containing plasmid (pIL12) at 100 µg/mouse (pROP18 group and pROP18-pIL12 group, respectively), as previously described [24]. Control mice were injected either with empty plasmid pIRESopt-GMCSF without or with pIL12 (pIRES-control group and pIRES-pIL12-control group, respectively), or kept untreated (control group).
Two weeks after the last immunization, mice were infected orally with 60 cysts of the 76 K strain. One month after challenge, mice were killed and their brains removed. Each brain was homogenized in 5 ml of RPMI medium. The mean number of cysts per brain was determined microscopically by counting eight samples (10 μl each) of each homogenate.
Analysis of antibody humoral responses
The levels of antigen-specific IgG antibodies in serum samples were determined 2 weeks after the third immunization as previously described [24]. Briefly, recombinant ROP18S2 at 4 µg/ml was used to coat microtiter plates (Maxisorp; Nunc). Twofold serial dilutions of serum samples from immunized mice diluted in PBS were added to the wells. Bound antibodies were detected with a goat anti-mouse IgG-alkaline phosphatase conjugate (Sigma) diluted 1:5000 in PBS-4 % BSA. The anti-ROP18 IgG subclass was determined by ELISA as described above except that sera were added to the plates at a single dilution and alkaline phosphatase-conjugated anti-mouse IgG1 and IgG2a (BD Pharmingen) were used and were diluted 1:1000 in PBS-4 % BSA. Intestinal IgA antibody responses to ROP18S2 were measured in intestinal washes by ELISA using a goat anti-mouse IgA-alkaline phosphatase conjugate (Sigma) diluted 1:1000 in PBS-4 % BSA. Intestinal washes were performed with a syringe by passing 5 ml of PBS–1 mM phenylmethylsulfonyl fluoride through the gut of mice killed 1 week after the third immunization. After clarification by centrifugation and concentration to 500 µl using poly(ethyleneglycol) (Sigma), positive rates were determined by the cutoff value calculated as the mean of optic density at 405 nm of six intestinal washes from untreated mice ±3 standard deviations.
Cytokine assays
Spleens and mesenteric lymph nodes were aseptically removed from mice 1 week after the third immunization. Single-cell suspensions were prepared as previously described [24]. They were seeded in duplicate in flat-bottomed, 96-well microtiter plates (Costar) at 5 × 105 cells per well in 200 µl of culture medium either alone or stimulated by adding 5 µg of rROP18S2 protein or 10 µg of concanavalin A (Con A) per ml. The plates were incubated for 3 days under 5 % CO2 at 37 °C. Cell-free supernatants were harvested and assayed for interleukin-2 (IL-2), IL-10 and IL-5, and gamma interferon (IFN-γ) activity at 72 h. The concentrations of IL-2, IL-10 and IL-5 and of IFN-γ were determined with an ELISA kit (eBioscience ELISA Ready-SET-Go), as specified by the manufacturer.
Analysis of the CD8+ T cells recruited in vivo following challenge with L929-ROP18 cells
T. gondii-specific CD8+ T cell responses elicited by protein/DNA vaccinations versus untreated mice were analyzed using a peritoneal antigenic challenge model, essentially as described by Jongert et al. [25], with some modifications. To generate syngeneic target cells expressing ROP18, the sequence encoding ROP18 was cleaved by NotI/XbaI from pTracerROP18mutΔpro and cloned into the NotI/XbaI sites of pcDNA3. L929 cells (fibroblastic cell line from C3H/An mice, H-2k) were stably transfected with the resulted plasmid pcDNA3-ROP18. One month after oral challenge with T. gondii, mice were injected intraperitoneally (i.p.) with 105 L929-ROP18 cells and peritoneal exudates cells (PECs) were harvested 16 h later by peritoneal drainage performed with 10 ml PBS. After centrifugation at 500g for 10 min, PECs were prepared for direct ex vivo staining and cytofluorometric analysis. Standard procedures were used to stain 2–5 × 105 PECs in 5 % FCS as previously described [26]. Antibodies for the detection of CD8 (eBioH35-17.2) were purchased from eBioscience. Cell acquisition was undertaken with a BD FACSCalibur cytometer and analyzed using CellQuest software (BD Bioscience).
Statistical analysis
All statistical tests were performed using GraphPad Prism software. Details of the tests applied are in the figure legends.
Results
The recombinant secreted T. gondii ROP18mut (ROP18S2) produced in Schneider 2 cells
The recombinant T. gondii ROP18mut (ROP18S2) was produced using eukaryotic S2 cells to avoid bacterial endotoxin contamination and was purified under native conditions by nickel affinity. The purified ROP18S2 showed electrophoretic behavior compatible with the expected molecular weight (56 kDa) under non-reducing conditions (Fig. 1a). Immunoblot analysis showed that ROP18S2 is recognized not only by rat anti-ROP18 antibodies, but also by sera from mice infected with T. gondii 76 K cysts, while sera from uninfected mice did not recognize ROP18S2, which confirms the antigenicity of ROP18S2 (Fig. 1b).
Recombinant T. gondii ROP18 proteins. The purified ROP18S2 expressed in drosophila cells was resolved on 10 % SDS-PAGE in non-reducing conditions and stained with coomassie blue (a) or revealed by immunoblot (b) using a TgROP18-specific rat serum (1), sera from uninfected mice (2) or sera from T. gondii (76 K strain) infected mice (3). MW: molecular weight markers. Sizes of molecular weight markers are shown on the left in kDa. c Transient expression of ROP18 by pROP18 in 293T cells. Lysates of 293T cells transfected with pIRES (1) or pROP18 (2) were analyzed by immunoblot in non-reducing conditions using a TgROP18-specific rat serum. MW: molecular weight markers. Sizes of molecular weight markers are shown on the left in kDa
The T. gondii ROP18mut protein encoded by the DNA vaccine
A bicistronic plasmid construct, expressing GM-CSF and a secreted form of T. gondii ROP18mut (pIRESopt-GMCSF-ROP18secmut, pROP18), was constructed. The production of ROP18 from pROP18 was investigated by transfecting 293FT cells. Rat anti-ROP18 antibodies recognized a major band of about 55 kDa which corresponds to the expected molecular weight on Western blot of 293T cell lysates transfected with pROP18 (Fig. 1c), while any band was detected in non-transfected cell lysates.
Immune responses and protection in mice immunized with ROP18S2 by a mucosal or a parenteral route
To determine whether mucosal immunization could induce protection against chronic T. gondii infection, CBA/J mice were immunized intranasally with ROP18S2 combined with CT (in-CT-ROP18 group). The non-parenteral immunization was compared to a parenteral subcutaneous immunization using ROP18S2 and poly I:C emulsified with Montanide, an adjuvant known to enhance cellular immune responses (sc-M-P-ROP18 group).
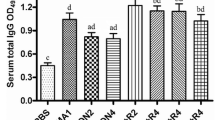
IgG antibody titers in sera were determined by ELISA with ROP18S2 2 weeks after the last immunization. IgG antibody titers were significantly higher in the sc-M-P-ROP18 immunization group than in the in-CT-ROP18 immunization group (Fig. 2a). To find out whether a Th1 and/or a Th2 humoral response was induced by immunization, the IgG subclasses were analyzed (Fig. 2b). Predominant productions of IgG1 and low productions of IgG2a were detected in sera of both immunized groups, suggesting a predominant humoral Th2-type response. Intranasal immunization induced a weak anti-ROP18S2 intestinal IgA response. Anti-ROP18S2 IgA antibodies were detected by ELISA in two of six immunized mice (Fig. 2c). Immunoblot analysis of intestinal washes confirmed ELISA results (data not shown).
IgG and IgA responses to ROP18S2 immunizations in mice. a Determination of specific anti-ROP18 antibody titers 2 weeks after the last immunization. Sera were tested by ELISA using ROP18S2. Sera from 8 mice in each group immunized with ROP18S2 (15 µg) emulsified in Montanide™ ISA 71 combined with 10 µg poly I:C by subcutaneous injection (sc-M-P-ROP18 group), with ROP18S2 (15 µg) plus 1 µg CT intranasally (in-CT-ROP18 group) or inoculated with adjuvants alone (in-CT-control and sc-M-P-control groups) were analyzed individually. The antigen-specific antibody titer is given as the reciprocal of the highest dilution producing an optical density (OD) that was 2.5-fold greater than that of the serum of non-immunized mice. Results are expressed as the mean log2 titers ± SEM. Unpaired t test. ***p < 0.001. b Determination of the IgG subclass profiles by ELISA using ROP18S2. Results are expressed as the mean of the optical density (OD) ± SEM. Sera from 6 mice in each group collected after the third immunization were tested at a single dilution. c IgA response in intestinal washes. Undiluted, concentrated intestinal washes collected after the third immunization were analyzed by ELISA using ROP18S2. The graph shows optical density (OD) for each mouse (n = 6), horizontal bars indicated the mean values, and the dotted line depicts the cutoff value calculated as the mean of OD at 405 nm of six intestinal washes from untreated mice ±3 standard deviations
The potencies of the intranasal and s.c. immunizations to induce T cell immune responses were investigated by measuring the specific cytokine responses in spleen cells and mesenteric lymph nodes 1 week after the last immunization. Stimulation of splenocytes with ROP18S2 induced significant IFN-γ (Fig. 3a) and IL-2 (Fig. 3b) responses in immunized mice compared to the control groups. The specific secretion of IFN-γ and IL-2 of spleen cells from mice of the in-CT-ROP18 immunization group was not statistically different from that from the sc-M-P-ROP18 immunization group. Re-stimulated splenocytes from both immunized groups produced significant IL-5 levels compared to their respective controls (Fig. 3c). Splenocytes from sc-M-P-ROP18 immunization group responded to ROP18S2 stimulation by higher production of IL-5; however, the difference between the two immunized groups did not reach statistical significance. IL-10 (Fig. 3d), a regulatory cytokine that is also found to counterbalance Th2 immune responses, was produced in both immunized groups at similar levels; however, the IL-10 productions did not reach statistical significance compared to their respective controls. No secretion of cytokines was detected in the supernatants of mesenteric lymph node cells of the in-CT-ROP18 immunization group. These results suggest that in our experimental conditions, both immunization routes induced a mixed Th1/Th2 systemic cellular immune response, but Th1 was predominant, and in parallel, intranasal immunization induced a weak humoral response in intestinal site.
Cellular immune response after immunization with ROP18S2. Mice were immunized on days 0, 14 and 28 with ROP18S2 (15 µg) emulsified in Montanide™ ISA 71 combined with 10 µg poly I:C by subcutaneous injection (sc-M-P-ROP18 group), ROP18S2 (15 µg) plus 1 µg CT intranasally (in-CT-ROP18 group) or inoculated with adjuvants alone (in-CT-control and sc-M-P-control groups). Splenocytes from vaccinated mice (n = 6/group) were recovered 1 week after the third immunization and cultured with 4 µg/ml ROP18S2. Cell-free supernatants were harvested and assayed for IFN-γ (a), IL-2 (b), IL-5 (c) and IL-10 (d) activities. Each symbol represents a single mouse, and the horizontal line is the median. The limit of the detection of each assay as determined by the manufacturer was 15 pg/ml (IFN-γ), 2 pg/ml (IL-2), 4 pg/ml (IL-5) and 32 pg/ml (IL-10). ND, no cytokine detected. Kruskal–Wallis nonparametric test, Dunn’s multiple comparisons test. **p < 0.01; *p < 0.05
In order to evaluate the protective effect of these vaccines against chronic toxoplasmosis, all groups of mice were orally challenged with 60 cysts of the 76 K strain 2 weeks after the last immunization. Furthermore, to investigate whether or not CD8+ T cell responses were elicited by ROP18S2 immunizations, 1 day before killing for brain cyst counting, mice were i.p. challenged with L929-ROP18 cells and PECs were harvested 16 h later for ex vivo staining of CD8+ T cells. In response to L929-ROP18 challenge, no significant recruitment of CD8+ T cells was observed in vaccinated mice compared with control groups (Fig. 4a).
Analyses 1 month post-infection (after challenge infection) in mice immunized with ROP18S2. Two weeks after the last immunization, all groups of mice were orally infected with 60 cysts of 76 K T. gondii strain. a One day before killing for brain cyst load analysis, mice (n = 5/group) were challenged i.p with 105 L929-ROP18 cells. Peritoneal exudates cells (PECs) were harvested 16 h later for direct ex vivo staining of CD8+ T cells. Percentage of CD8+ T cells in the peritoneum are represented as mean ± SEM. b Brain cysts load was evaluated 1 month after challenge (n = 8/group). The results are expressed as the mean number of cysts for each group of mice ± SEM and are representative of two independent experiments. ANOVA, Bonferroni’s multiple comparison test. **p < 0.001; *p < 0.05
Compared to their respective controls, if a significant reduction was found in brain cyst load of the in-CT-ROP18 immunization group (reduction in brain cyst load of 52 %, p value <0.05), the reduction in the brain cyst load of the sc-M-P-ROP18S2 immunization group did not reach statistical significance (Fig. 4b).
Immune responses and protection in mice immunized with pROP18
The initial rationale for developing DNA vaccines was to find a means of delivering antigen into the MHC-I processing pathway in order to induce cytotoxic T cells, while maintaining the capability of stimulating T cell helper and antibody responses [16]. To further potentiate a CD8+ T cell response, we co-administrated a plasmid encoding IL-12. Immune responses and protective efficacy were evaluated in mice immunized through the intramuscular route with a plasmid encoding both ROP18 and GM-CSF administrated without (pROP18 group) or with a plasmid expressing IL-12 (pROP18-pIL12 group).
ROP18S2-specific IgG antibodies were detected in all vaccinated mice. Similar IgG antibody titers were found in both immunized groups (Fig. 5a). Both groups produced IgG1 and IgG2a, and the levels of IgG2a exceeded those of IgG1 without significant differences between the two immunized groups (Fig. 5b). These results suggest a mixed Th1/Th2 humoral-type response with a slight bias toward a Th1 response. Stimulated splenocytes from both immunized groups produced significant IFN-γ (Fig. 5c) and IL-2 (Fig. 5d) levels compared to their respective controls, without significant difference between the two immunized groups. IL-5 and IL-10 were not detected. These results suggest that DNA immunization induced a Th1 cellular immune response. Co-administration of pIL12 did not enhance the cellular immune response and was not able to influence the humoral response both in terms of magnitude and IgG subclasses.
Humoral and cellular responses after immunization with pROP18. Mice were immunized on days 0, 14 and 28 with pROP18 (pROP18 group) or pROP18 plus pIL12 (pROP18-pIL12 group). Control groups were either untreated (untreated group) or received empty plasmids (pIRES-control and pIRES-pIL12 groups). a Determination of specific anti-ROP18 antibody titers 2 weeks after the last immunization. Sera were tested by ELISA using ROP18S2. Sera from 8 mice in each group were analyzed individually. The antigen-specific antibody titer is given as the reciprocal of the highest dilution producing an optical density (OD) that was 2.5-fold greater than that of the serum of non-immunized mice. Results are expressed as the mean log2 titers ± SEM. b Determination of the IgG subclass profiles by ELISA using ROP18S2. Results are expressed as the mean of the optical density (OD) ± SEM. Sera from 6 mice in each group collected after the third immunization were tested at a single dilution. c, d Splenocytes from vaccinated mice (n = 6/group) were recovered 1 week after the third immunization and cultured with 4 µg/ml ROP18S2. Cell-free supernatants were harvested and assayed for IFN-γ (c) and IL-2 (d) activities. Each symbol represents a single mouse and the horizontal line is the median. The limit of the detection of each assay as determined by the manufacturer was 15 pg/ml (IFN-γ), 2 pg/ml (IL-2). ND no cytokine detected. Kruskal–Wallis nonparametric test, Dunn’s multiple comparisons test.*p < 0.05
DNA immunization with pROP18 did not confer a significant reduction in the brain cyst load, and co-administration of pIL12 did not potentiate the protection (Fig. 6a). Following in vivo challenge with L929-ROP18 cells, no significant attraction of CD8+ T cells was observed in mice immunized with pROP18 plus pIL12 and infected with T. gondii compared with untreated and infected control group (Fig. 6b).
Analyses 1 month post-infection (after challenge infection) in mice immunized with pROP18. Two weeks after the last immunization, all groups of mice were orally infected with 60 cysts of 76 K T. gondii strain. a Brain cysts load was evaluated 1 month after challenge (n = 8/group). The results are expressed as the mean number of cysts for each group of mice ± SEM and are representative of two independent experiments. b One day before killing for brain cyst load analysis, mice (n = 5/group) were challenged i.p. with 105 L929-ROP18 cells. Peritoneal exudates cells (PECs) were harvested 16 h later for direct ex vivo staining of CD8+ T cells. Percentages of CD8+ T cells in the peritoneum are represented as mean ± SEM
Discussion
In this study, we investigated the immunoprophylactic potential of T. gondii ROP18 against chronic toxoplasmosis in CBA/J mice after oral challenge with T. gondii cysts. We first compared a mucosal vaccination to a subcutaneous vaccination route using recombinant protein of ROP18 (ROP18S2) produced through the drosophila insect cell system and purified under native conditions.
Significant (but partial) protection was observed in mice immunized by the nasal route with ROP18S2 plus cholera toxin (50 % brain cyst reduction) as compared to the control groups following oral challenge with T. gondii cysts. However, no significant brain cyst reduction was observed in mice immunized s.c. with ROP18S2 and poly I:C emulsified in Montanide. This result is similar to that obtained by Grzybowski et al. [14] with another strain of mice carrying the same H-2k haplotype, immunized s.c. with a recombinant ROP18 protein and poly I:C following i.p. challenge with T. gondii cysts. However, in the same study, Balb/c mice exhibited significant brain cyst reduction (52 %) as compared to the poly I:C control group. Significant brain cyst reduction was also observed in Kunming (H-2d) mice immunized intramuscularly with a recombinant canine adenovirus type 2 expressing ROP18 following oral challenge with T. gondii cysts (57 % brain cyst reduction) [27]. Furthermore, intramuscular immunization of Kunming mice (H-2d) with a plasmid encoding ROP18 conferred partial protection after lethal challenge [28].
The initial rationale for developing DNA vaccines or recombinant viral vectors was to find a means of delivering antigen into the MHC-I processing pathway in order to induce cytotoxic T cells, while maintaining the capability of stimulating T cell helper and antibody responses [16, 29]. This strategy could be particularly important in the case of intracellular pathogens such as T. gondii. Indeed, immunization of Kunming mice with either a recombinant canine adenovirus type 2 expressing ROP18 or a plasmid encoding ROP18 has been shown to activate T CD8+ T cell, and significant CTL activities have been reported [27, 28]. Bioinformatic analysis for H-2k, H-2d and H-2b MHC class I binding epitopes (8–11 mers) using the NetMHC3.0 algorithm [30, 31] showed that putative CTL epitopes are present in ROP18, and the identified epitopes had a uniform affinity range with a mixture of strong and weak binders. The stronger binders were found for H-2Kk MHC class I binding epitopes (Supplementary Figure S1). In our experimental conditions, DNA immunization of CBA/J mice with pROP18 induced specific humoral and cellular immune responses and co-administration of pIL12 did not enhance these responses. The induced Th1 biased immune responses did not confer significant protection against oral challenge with T. gondii cysts. To evaluate the CD8+ T cell response in ROP18S2 or pROP18 vaccinated mice, we used an in vivo challenge with syngeneic cells expressing ROP18, 1 month post-T. gondii infection. The percentages of recruited CD8+ T cells were only slightly higher in immunized mice compared to controls. Our results are quite similar to those obtained in C3H mice immunized s.c with ROP18 plus poly I:C [14]. In the same study, compared to C3H mice, the ratio CD4+/CD8+ in Balb/c mice was the lowest which, as suggested by the authors, may indicate a weak stimulation of the cytotoxic immune mechanisms in Balb/c mice [14]. In our experimental conditions, even in strategies which may favor MHC class I presentation, CBA/J did not generate detectable specific CD8+ T cell responses. Collectively these results suggest that Balb/c mice are probably more effective in inducing CD8+ T cell than CBA/J mice. Failure to generate a sufficient CD8 response may contribute to the lack of protection observed after parenteral immunizations in CBA/J mice. However, a mucosal administration route was able to confer partial protection in these mice.
Mice immunized by the nasal route with ROP18S2 plus CT exhibited lower levels of anti-ROP18S2 IgG antibodies than mice immunized s.c. with ROP18S2 and poly I:C emulsified in Montanide. Both mucosal and parenteral vaccinations generated a mixed Th1/Th2 response polarized toward the IgG1 antibody isotype. In addition to the production of IFN-γ, and IL-2, IL-10 and IL-5 were also produced by the spleen cells of the immunized mice stimulated with ROP18S2, suggesting that a mixed systemic Th1-/Th2-type cellular immune response occurred in both immunized groups. Beside the systemic immune response, a mucosal humoral immune response at the intestinal site was detected in some mice immunized by the nasal route with ROP18S2 plus CT; however, cellular immune responses were not detected in the mesenteric lymph nodes of any mouse. This mucosal humoral response was weak, since IgA could be detected only after concentration of the intestinal washes. These results suggest that mucosal immunity may augment vaccine efficacy. It therefore remains to identify at both local and systemic sites the mechanisms of vaccine efficacy and correlates of protection. It is possible that such mechanisms will be different for each administration route. Such observations have already been reported [32, 33]. One potential concern with CT intranasal immunization is a possible adverse side effect since CT could be transported to the central nervous system. We recently showed the potential of nanoparticles to improve the immunogenicity of nasal vaccine against T. gondii; therefore, this strategy could be used to improve the efficiency of ROP18 [34].
References
Robert-Gangneux F, Dardé ML (2012) Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 25(3):264–296
Innes EA (2010) Vaccination against Toxoplasma gondii: an increasing priority for collaborative research? Expert Rev Vaccines 9(10):1117–1119
Montoya JG, Remington JS (2008) Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis 47(4):554–566
Denkers EY, Gazzinelli RT (1998) Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev 11(4):569–588
Suzuki Y, Remington J (1990) The effect of anti-IFN-gamma antibody on the protective effect of Lyt-2+ immune T cells against toxoplasmosis in mice. J Immunol 144(5):1954–1960
Hiszczyńska-Sawicka E, Gatkowska JM, Grzybowski MM, Długońska H (2014) Veterinary vaccines against toxoplasmosis. Parasitology 141(11):1365–1378
Verma R, Khanna P (2013) Development of Toxoplasma gondii vaccine: a global challenge. Hum Vaccin Immunother 9(2):291–293
Jongert E, Roberts CW, Gargano N, Förster-Waldl E, Petersen E (2009) Vaccines against Toxoplasma gondii: challenges and opportunities. Mem Inst Oswaldo Cruz 104(2):252–266
El Hajj H, Lebrun M, Arold ST, Vial H, Labesse G, Dubremetz JF (2007) ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog 3(2):e14
Etheridge RD, Alaganan A, Tang K, Lou HJ, Turk BE, Sibley LD (2014) The Toxoplasma pseudokinase ROP5 forms complexes with ROP18 and ROP17 kinases that synergize to control acute virulence in mice. Cell Host Microbe 15(5):537–550
Alaganan A, Fentress SJ, Tang K, Wang Q, Sibley LD (2014) Toxoplasma GRA7 effector increases turnover of immunity-related GTPases and contributes to acute virulence in the mouse. Proc Natl Acad Sci USA 111(3):1126–1131
Bonenfant C, Dimier-Poisson I, Velge-Roussel F, Buzoni-Gatel D, Del Giudice G, Rappuoli R et al (2001) Intranasal immunization with SAG1 and nontoxic mutant heat-labile enterotoxins protects mice against Toxoplasma gondii. Infect Immun 69(3):1605–1612
Debard N, Buzoni-Gatel D, Bout D (1996) Intranasal immunization with SAG1 protein of Toxoplasma gondii in association with cholera toxin dramatically reduces development of cerebral cysts after oral infection. Infect Immun 64(6):2158–2166
Grzybowski MM, Dziadek B, Gatkowska JM, Dzitko K, Długońska H (2015) Towards vaccine against toxoplasmosis: evaluation of the immunogenic and protective activity of recombinant ROP5 and ROP18 Toxoplasma gondii proteins. Parasitol Res 114(12):4553–4563
Aucouturier J, Ascarateil S, Dupuis L (2006) The use of oil adjuvants in therapeutic vaccines. Vaccine 24(Suppl 2):S44–S45
Liu MA (2011) DNA vaccines: an historical perspective and view to the future. Immunol Rev 239(1):62–84
Ismael AB, Hedhli D, Cérède O, Lebrun M, Dimier-Poisson I, Mévélec MN (2009) Further analysis of protection induced by the MIC3 DNA vaccine against T. gondii. CD4 and CD8 T cells are the major effectors of the MIC3 DNA vaccine-induced protection, both Lectin-like and EGF-like domains of MIC3 conferred protection. Vaccine 27(22):2959–2966
Mévélec MN, Bout D, Desolme B, Marchand H, Magne R, Bruneel O et al (2005) Evaluation of protective effect of DNA vaccination with genes encoding antigens GRA4 and SAG1 associated with GM-CSF plasmid, against acute, chronical and congenital toxoplasmosis in mice. Vaccine 23(36):4489–4499
Xue M, He S, Zhang J, Cui Y, Yao Y, Wang H (2008) Comparison of cholera toxin A2/B and murine interleukin-12 as adjuvants of Toxoplasma multi-antigenic SAG1-ROP2 DNA vaccine. Exp Parasitol 119(3):352–357
Cui YL, He SY, Xue MF, Zhang J, Wang HX, Yao Y (2008) Protective effect of a multiantigenic DNA vaccine against Toxoplasma gondii with co-delivery of IL-12 in mice. Parasite Immunol 30(5):309–313
Zhang J, He S, Jiang H, Yang T, Cong H, Zhou H et al (2007) Evaluation of the immune response induced by multiantigenic DNA vaccine encoding SAG1 and ROP2 of Toxoplasma gondii and the adjuvant properties of murine interleukin-12 plasmid in BALB/c mice. Parasitol Res 101(2):331–338
Letscher-Bru V, Villard O, Risse B, Zauke M, Klein JP, Kien TT (1998) Protective effect of vaccination with a combination of recombinant surface antigen 1 and interleukin-12 against toxoplasmosis in mice. Infect Immun 66(9):4503–4506
Khaznadji E, Boulard C, Moiré N (2003) Expression of functional hypodermin A, a serine protease from Hypoderma lineatum (Diptera, Oestridae), in Schneider 2 cells. Exp Parasitol 104(1–2):33–39
Ismael AB, Sekkai D, Collin C, Bout D, Mévélec MN (2003) The MIC3 gene of Toxoplasma gondii is a novel potent vaccine candidate against toxoplasmosis. Infect Immun 71(11):6222–6228
Jongert E, Lemiere A, Van Ginderachter J, De Craeye S, Huygen K, D’Souza S (2010) Functional characterization of in vivo effector CD4(+) and CD8(+) T cell responses in acute Toxoplasmosis: an interplay of IFN-gamma and cytolytic T cells. Vaccine 28(13):2556–2564
Akbar H, Germon S, Berthon P, Dimier-Poisson I, Moiré N (2012) Depletion of CD25+ cells during acute toxoplasmosis does not significantly increase mortality in Swiss OF1 mice. Mem Inst Oswaldo Cruz 107(2):155–162
Li XZ, Wang XH, Xia LJ, Weng YB, Hernandez JA, Tu LQ et al (2015) Protective efficacy of recombinant canine adenovirus type-2 expressing TgROP18 (CAV-2-ROP18) against acute and chronic Toxoplasma gondii infection in mice. BMC Infect Dis 15:114
Yuan ZG, Zhang XX, Lin RQ, Petersen E, He S, Yu M, He XH, Zhou DH, He Y, Li HX, Liao M, Zhu XQ (2011) Protective effect against toxoplasmosis in mice induced by DNA immunization with gene encoding Toxoplasma gondii ROP18. Vaccine 29(38):6614–6619
Appaiahgari MB, Vrati S (2015) Adenoviruses as gene/vaccine delivery vectors: promises and pitfalls. Expert Opin Biol Ther 15(3):337–351
Buus S, Lauemoller SL, Worning P, Kesmir C, Frimurer T, Corbet S et al (2003) Sensitive quantitative predictions of peptide-MHC binding by a ‘Query by Committee’ artificial neural network approach. Tissue Antigens 62(5):378–384
Nielsen M, Lundegaard C, Worning P, Lauemoller SL, Lamberth K, Buus S et al (2003) Reliable prediction of T-cell epitopes using neural networks with novel sequence representations. Protein Sci 12(5):1007–1017
Orr MT, Beebe EA, Hudson TE, Argilla D, Huang PW, Reese VA et al (2015) Mucosal delivery switches the response to an adjuvanted tuberculosis vaccine from systemic TH1 to tissue-resident TH17 responses without impacting the protective efficacy. Vaccine 33(48):6570–6578
McKay PF, King DF, Mann JF, Barinaga G, Carter D, Shattock RJ (2016) TLR4 and TLR7/8 adjuvant combinations generate different vaccine antigen-specific immune outcomes in minipigs when administered via the ID or IN routes. PLoS ONE 11(2):e0148984
Dimier-Poisson I, Carpentier R, N’Guyen TT, Dahmani F, Ducournau C, Betbeder D (2015) Porous nanoparticles as delivery system of complex antigens for an effective vaccine against acute and chronic Toxoplasma gondii infection. Biomaterials 50:164–175
Acknowledgments
This work was supported by the Higher Education Commission (HEC), Islamabad, Pakistan, and University of Veterinary and Animal Sciences (UVAS), Lahore, Pakistan. We would like to thank T. Papin and S. Bigot for their excellent technical assistance. We thank Dr A.L. Rakhmile-vich, University of Wisconsin Comprehensive Cancer Center, Madison, WI, for providing the IL-12-containing plasmid (pIL12). We thank Dr. M. Lebrun, Université de Montpellier 2, CNRS, UMR 5539, France, for providing the anti-ROP18 rat polyclonal antibodies and the plasmid pTracerROP18mutΔpro (D394 A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest among the authors regarding the work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Prediction analysis of H-2Kk, H-2Ld, H-2Kd, H-2Dd, H-2Db, H-2Kb; MHC class I binding epitopes in ROP18 T. gondii antigen. Predictions of peptide binding were made for peptides ranging from 8 to 11 amino acids using the NetMHC3.0 server which predicts binding of peptides to different alleles using artificial neural networks (ANNs). The affinity with which different peptides bind MHC molecules are given as IC50 values (50 % inhibitory concentration), and threshold values of 50 and 500 nM define peptides with strong and weak binding affinity, respectively. The number of 8–11 mers peptides is represented by their binding affinities (IC50) in a scatter dot plot. The threshold for strong binders (<50 nM) is represented by a dotted line (PPTX 43 kb)
Rights and permissions
About this article
Cite this article
Rashid, I., Moiré, N., Héraut, B. et al. Enhancement of the protective efficacy of a ROP18 vaccine against chronic toxoplasmosis by nasal route. Med Microbiol Immunol 206, 53–62 (2017). https://doi.org/10.1007/s00430-016-0483-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00430-016-0483-9