Abstract
The heavy incidence and severe or lethal damages of toxoplasmosis clearly indicate the need for the development of a more effective vaccine. In the present study, we constructed a multiantigenic DNA vaccine, eukaryotic plasmid pcDNA3.1-SAG1-ROP2, expressing surface protein SAG1 and rhoptry protein ROP2 of Toxoplasma gondii, and examined the expression ability of the DNA vaccine in HeLa cells by Western blot. Afterwards, we investigated the efficacy of pcDNA3.1-SAG1-ROP2 with or without co-administration of a plasmid encoding murine interleukin-12 (pIL-12) as a genetic adjuvant to protect Bagg albino/c mice against toxoplasmosis. After T. gondii RH strain challenge, mice immunized with pcDNA3.1-SAG1-ROP2 displayed significant high survival rates. Moreover, the protection was markedly enhanced by pIL-12 co-administration. The results of lymphocyte proliferation assay, cytokine, and antibody determinations show that mice immunized with pcDNA3.1-SAG1-ROP2 elicited stronger humoral and Th1-type cellular immune responses than those immunized with single-gene plasmids, empty plasmid, or phosphate-buffered saline. Furthermore, co-immunization with IL-12 genes resulted in a dramatic enhancement of these responses. Our study indicates that the introduction of multiantigenic DNA vaccine is more powerful and efficient than single-gene vaccine, and the co-delivery of pIL-12 further enhanced the potency of multiantigenic DNA vaccine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Toxoplasma gondii (T. gondii) is an obligate intracellular parasite capable of infecting virtually any warm-blood animal (Black and Boothroyd 2000). In humans, T. gondii infection is widespread and can lead to severe opportunistic disease (toxoplasmosis) in congenitally infected babies and immuno-compromised individuals (Dubey 1998). In veterinary medicine, T. gondii infection has economic importance due to abortion and neonatal loss in livestock, mainly sheep and goats (Buxton 1998; Dubey 1990), or as source of transmission to humans (Dubey and Thulliez 1993). Current primary control measures depend on chemotherapy. However, the chemotherapeutic agents used presently are inadequate, expensive, and often toxic. To date, there is no commercial vaccine for use in humans. One developed for veterinary use has low efficiency (Buxton 1998). Thus, the development of an effective vaccine against T. gondii would be of great value to both human and veterinary medicine.
In recent years, some T. gondii proteins were investigated as candidates for vaccine (Saavedra et al. 1996; Mevelec et al. 2005; Vercammen et al. 2000). Among these vaccine candidates, the main surface antigen 1 (SAG1) is the best characterized. SAG1, highly conserved in T. gondii strains (Windeck and Gross 1996), was shown to induce both humoral and cellular immune responses (Lunden 1995; Nielsen et al. 1999). The T. gondii life cycle has three infectious stages: tachyzoite, bradyzoite, and sporozoite (Dubey 1998). SAG1 is a tachyzoite-specific antigen. Unfortunately, vaccination with stage-specific antigens leads to stage-limited protection (Alexander et al. 1996), and subunit vaccines are weakly immunogenic. To circumvent these limitations, we choose another vaccine candidate, rhoptry protein 2 (ROP2). ROP2 is expressed at all invasive stages of T. gondii (Beckers et al. 1994) and is critical for parasite invasion, replication, and parasite–host cell interaction (Nakaar et al. 2003). If the epitopes of the two molecules are simultaneously presented to the immune system, the immunopotentiating properties of the two antigens can be utilized, and successful protection against T. gondii may be achieved.
However, the immunogenicity of DNA vaccines remain to be enhanced because the immune responses induced by DNA vaccines are often weak (Donnelly et al. 2005). By the use of molecular adjuvants, the immune responses may be enhanced and modulated. We chose a plasmid encoding murine interleukin-12 (pIL-12) as a genetic adjuvant to enhance the immunogenicity and protective efficacy of anti-toxoplasmosis DNA vaccine, seeing that IL-12 is a critical cytokine for the control of toxoplasmosis during acute and chronic stages of infection (Hunter et al. 1995; Yap et al. 2000). IL-12, which is secreted by the macrophages and the dendritic cells during antigen stimulation, plays a key role in the activation of NK cells and the development of Th1 effector cells and can effectually stimulate the production of gamma interferon (IFN-γ), which is essential for resistance to this parasite (Denkers and Gazzinelli 1998; Denkers et al. 2003).
In this study, we constructed a multiantigenic DNA vaccine expressing SAG1 and ROP2 antigens, and examined the immunogenicity and protective efficacy of the DNA vaccine with or without pIL-12 as a genetic adjuvant in Bagg albino/c (BALB/c) mice.
Materials and methods
Plasmid construction
To construct the SAG1-ROP2 fusion expression plasmid, the coding sequence of the SAG1 gene (786 bp, without the stop codon, encoding amino acid residues 74–335) and ROP2 gene (1,023 bp, encoding amino acid residues 188–528) were amplified by polymerase chain reaction (PCR) from genomic DNA of T. gondii (RH strain), with two pairs of oligonucleotide primers (SAG1, forward primer: 5′-CGGAATTCATGACGGAGAACCACTTCACTC-3′ and reverse primer: 5′-ATGTCGACGACACAAGCTGCGATAG-3′, introduced EcoRI and SalI recognition site, respectively, underlined; ROP2, forward primer: 5′-ATGTCGACAACCCTATGTATTTCCGCG-3′ and reverse primer: 5′-GGAAGCTTTCACCGATCCTCTTTCGAG-3′, introduced SalI and HindIII recognition site, respectively, underlined). The two kinds of PCR products were respectively digested with the above corresponding restriction enzymes and purified from agarose gel. Two independent SAG1 and ROP2 gene fragments were ligated into pMD18-T simple vector (TaKaRa, China) and then introduced into the eukaryotic expression plasmid pcDNA3.1(−) vector, generating the pcDNA3.1-SAG1-ROP2 plasmid (pSAG1-ROP2). The recombinant plasmid clones were screened by diagnostic restriction digestion and PCR and confirmed by sequencing (Bioasia, China). All plasmids were propagated in Escherichia coli DH5α.
Plasmid extraction and purification
The single-gene expression plasmids pcDNA3.1-SAG1 (pSAG1) and pcDNA3.1-ROP2 (pROP2), respectively encoding the SAG1 (amino acid residues 74–335) and ROP2 (amino acid residues 188–528) antigens, were constructed by our laboratory (Yang et al. 2005). Murine IL-12 expression plasmid containing the p35 and p40 sequences and designated as pUMVC3-mIL-12 (pIL-12) was kindly provided by Dr. Alexander Rakhmilevich (University of Wisconsin-Madison, USA). Plasmids were maintained and propagated in E. coli DH5α. Endotoxin free plasmid DNA was isolated using plasmid purification kit (Qiagen, Germany). After purification, plasmid concentration was determined by spectrophotometry at λ = 260 and 280 nm. The 260:280 UV absorption ratio was between 1.8 to 2.0.
Expression of compound gene in vitro
The recombinant eukaryotic expression plasmid pcDNA3.1-SAG1-ROP2 was transiently transfected into HeLa cells to test its expression. Lipofectamine™ 2000 reagent (Invitrogen, USA) was mixed with 1.0 μg plasmid DNA at a concentration of 10 μg/ml in Dulbecco’s modified Eagle’s medium (DMEM), without fetal calf serum (FCS) and antibiotics, and was incubated at room temperature for 20 min. The lipofectin DNA mixture was then overlayed on 75% confluent HeLa cells. The cells were incubated with the transfection mix for 6 h at 37°C, 5% CO2. At the end of incubation, fresh medium was supplemented (DMEM 10% FCS, 2 mmol/l glutamine and antibiotics), and plates were returned for further incubation. After 48 h, cells were lysed and the proteins were collected. Synthesis of SAG1-ROP2 protein in a eukaryotic system was tested by Western blot (Fachado et al. 2003).
Mice
Female BALB/c mice were purchased from Shandong University Laboratory Animal Center. All mice were maintained under specific-pathogen-free conditions and were at 5 to 6 weeks of age when immunizations were initiated.
Parasite
T. gondii tachyzoites (RH strain) were kindly provided by the Department of Parasitology in Yat-Sen University of Medical Science (Guangzhou, China) and maintained by serial intraperitoneal passage in BALB/c mice. The tachyzoites were collected from the peritoneal fluids, washed by centrifugation, and then suspended in sterile phosphate-buffered saline (PBS). For enzyme-linked immunoadsorbent assay (ELISA), soluble tachyzoite antigens (STAg) was obtained by sonication and stored at −70°C.
DNA immunization and challenge
Six groups of mice (14 per group) were injected intramuscularly with 100 μg of plasmid DNA suspended in 100 μl sterile PBS, 50 μl in each thigh skeletal muscle, whereas control mice received PBS alone. Group I was injected with PBS as control, group II with empty pcDNA3.1 vector also as control, group III with pSAG1, group IV with pROP2, group V with pSAG1-ROP2, and group VI with pSAG1-ROP2 plus pIL-12 (100 μg each). Mice were immunized using the same protocol on days 0, 14, and 28 and were bled by orbital plexus puncture on days 13, 27, 41, and 55. Four weeks after the final inoculation (on day 56), spleens from seven immunized mice per group were collected under aseptic conditions, and another seven immunized mice per group were intraperitoneally challenged with 1 × 104 tachyzoites of virulent RH T. gondii.
IgG and subclass determination
Antigen-specific antibodies were measured by ELISA. In brief, 96-well Costar plates were coated with 1 μg of STAg in 100 μl of carbonate buffer, pH 9.2 and incubated overnight at 4°C. Mice sera diluted in PBS were applied to the wells, then the bound antibodies were detected by horseradish peroxidase-conjugated goat anti-mouse IgG, IgG1, or IgG2a (Southern Biotechnology Associates, USA), diluted in 1:4,000. Immune complexes were revealed by incubating with orthophenylene diamine (Sigma) and 0.15% H2O2 for 30 min. The reaction was stopped by the addition of 1 M H2SO4, and the absorbance was measured at 490 nm using ELISA reader (Bio-Tek EL × 800, USA). All samples were run in triplicate.
Lymphocyte proliferation assay
Briefly, splenocyte suspensions were prepared from each group of mice by pushing the spleens through a wire mesh. After the red blood cells were removed using RBC lysis solution (Sigma), splenocytes were resuspended in DMEM medium supplemented with 10% FCS. Cells were then plated in 96-well Costar plates at a density of 5 × 105 cells per well and cultured with STAg (10 μg/ml) or concanavalin A (Con A; 5 μg/ml; Sigma; positive control) or medium alone (negative control) at 37°C with 5% CO2. After 72 h, each well was pulsed with 1 μCi of [3H] thymidine (China Institute of Atomic, Beijing) for 18 h and then harvested onto fiberglass filters with an automated cell harvester (Perkin Elmer Life and Analytical Sciences, USA) Incorporated radioactivity was measured using a liquid scintillation counter (Perkin Elmer). All assays were performed in triplicate.
Cytokine assays
For the detection of cytokines, splenocytes from immunized mice were cultured with different stimuli as described for the lymphocyte proliferation assay. Cell-free supernatants were harvested and assayed for IL-4 and IL-12 activities at 24 h and for IFN-γ activity at 96 h. The concentrations of IFN-γ and IL-4 were determined by ELISA kit (R&D Systems, Minneapolis, MN, USA), and IL-12 by ELISA kit (Bender MdeSystems, Austria), according to manufacturer’s instructions. All assays were performed in triplicate. The lower limits of detection of IFN-γ, IL-4, and IL-12 were 4, 7, and 3.96 pg/ml, respectively.
Statistical analysis
Survival times for vaccinated and control mice were compared using the Kaplan–Meier method. The difference in the level of proliferation assays, cytokine, and antibody production was determined by one-way ANOVA. Statistical analysis was performed using the Statistical Package for the Social Sciences for Windows version 10.0. Differences were considered significant when P < 0.05.
Results
The sequencing results of recombinant pSAG1-ROP2 were consistent with GenBank DNA sequences of SAG1 gene and ROP2 gene (GenBank accession numbers S76248 and Z36906 for SAG1 and ROP2, respectively; data not shown). As is seen from Fig. 1a, the digested fragment size was the same as with fragment size of PCR products (in lane 2 and lane 1, respectively). The Western blot analysis of SAG1-ROP2 protein after transfection is shown in Fig. 1b. The extract of cells transfected with pSAG1-ROP2 showed the presence of SAG1-ROP2 protein (about 67 kDa) when incubated with anti-T. gondii polyclonal antibody (lane 1) or anti-SAG1 Mab (lane 3), whereas the control empty plasmid-transfected cells did not show any band (lanes 2 and 4) upon incubation with the same antibodies.
a Identification of the recombinant expression plasmid. pcDNA3.1-SAG1-ROP2 with restriction enzyme digestion and PCR amplification. pcDNA3.1-SAG1-ROP2 plasmid without digestion (lane 1), pcDNA3.1-SAG1-ROP2 digested with EcoRI (lane 2), pcDNA3.1-SAG1-ROP2 digested with EcoRI and HindIII (lane 3), PCR product of pcDNA3.1-SAG1-ROP2 (lane 4), DNA marker (lane M). b Western blot analysis of extract of HeLa cells transfected with pcDNA3.1-SAG1-ROP2 or with empty pcDNA3.1 as a control. pSAG1-ROP2 transfected cells showed the presence of SAG1-ROP2 protein (about 67 kDa) when incubated with anti-T. gondii polyclonal antibody (lane 1) or anti-SAG1 Mab (lane 3); pcDNA3.1 transfected cells did not show any band upon incubation with anti-T. gondii polyclonal antibody (lane 2) or anti-SAG1 Mab (lane 4)
To test whether this vaccination protocol could induce protection against T. gondii, immunized mice were challenged with tachyzoites of the virulent RH strain. Survival percentage of the different groups of mice is shown in Fig. 2. Substantially higher survival rates were obtained in pSAG1-ROP2 vaccinated mice compared with PBS, empty plasmid, or single-gene plasmids (pSAG1 or pROP2) vaccinated mice. Moreover, the protection induced by pSAG1-ROP2 was markedly enhanced by pIL-12 co-administration. Finally, no significant difference was observed with the groups of mice immunized with single-gene plasmids in association with control groups. Mice immunized with PBS or pcDNA3.1 died within 4 to 8 days.
To determine the levels of anti-T. gondii antibodies, all sera were tested by ELISA. As shown in Fig. 3, significant high levels of IgG antibodies were detected in the sera of mice immunized with pSAG1-ROP2 alone or combined with pIL-12 as a genetic adjuvant (P < 0.05 versus other groups), especially after the third immunization (the levels of antibodies increased with successive immunizations). The levels of IgG antibodies were greater in the sera of mice co-immunized with pIL-12 than in the sera of mice immunized with pSAG1-ROP2 alone (P < 0.01). In contrast, there were no significant differences in the levels of IgG antibodies on days 13 and 27 among the single-gene immunized mice and control mice (P > 0.05) nor was there any significant difference between mice immunized with pSAG1 or pROP2 in each time (P > 0.05).
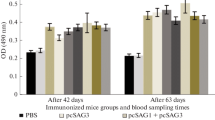
The levels of anti-T. gondii IgG subclasses are shown in Fig. 4. Predominances of the levels of IgG2a over IgG1 were observed in sera of the single- and multiple-gene immunized mice. Furthermore, IgG2a values in the pSAG1-ROP2 plus pIL-12 immunized group were significantly higher than the pSAG1-ROP2 immunized group (P < 0.001). In contrast, anti-T. gondii IgG1 antibodies were similar between the two groups (P = 0.722). These data suggest that pIL-12 augmented the predominance of IgG2a over IgG1 induced by pSAG1-ROP2 alone.
Determination of the specific anti-T. gondii IgG1 and IgG2a subclass antibodies in the sera of mice. Sera were collected 4 weeks after the last immunization. Results are expressed as means of the OD490±SD and representative of three experiments. *P < 0.05 and **P < 0.001, as compared to other groups
Four weeks after the final immunization (on day 56), after stimulation with STAg, splenocyte proliferation and cytokine production were studied in vitro. As shown in Fig. 5, splenocytes from mice injected with pSAG1-ROP2 resulted in a significant increase in proliferation when compared with single-gene immunized mice and control mice (P < 0.05). Furthermore, co-administration of pSAG1-ROP2 and pIL-12 augmented splenocyte proliferation by 3.5-fold when compared to proliferation by spleen cells from groups immunized with pSAG1-ROP2 alone (P < 0.001). In addition, splenocytes from all experimental and control groups proliferated to comparable levels in response to the mitogen Con A (data not shown).
In vitro proliferation of splenocytes from BALB/c mice after stimulation with T. gondii antigens. Splenocytes from mice were harvested 4 weeks after the last immunization. Results are expressed as means of the CPM±SD and are representative of three experiments. *P < 0.05 and **P < 0.001, as compared to other groups
The levels of IFN-γ, IL-4, and IL-12 produced in splenocytes from immunized mice stimulated with STAg are shown in Table 1. Significant high levels of IFN-γ and IL-12 were observed in spleen cell cultures from mice immunized with pSAG1-ROP2 compared with mice immunized with PBS, empty plasmid, or single-gene plasmids, and the levels were markedly enhanced by pIL-12 co-administration. On the other hand, low levels of IL-4 was evident in supernatants from spleen cells of pSAG1-ROP2 plus pIL-12 vaccinated mice compared with other five groups, and no statistically significant differences could be found between the five groups.
Discussion
DNA vaccination represents a promising strategy to protect animals and humans against pathogenic microorganisms, particularly intracellular parasites (Bunell and Morgan 1998), as DNA immunization enables the production of the native form of a given antigen, priming both cellular and humoral specific immune responses (Robinson 1997). Besides, DNA vaccines are attractive because of ease of production and low cost (Bunell and Morgan 1998) and the potential for long-lasting immunity (Gurunathan et al. 2000). In this study, we constructed a multiple antigen-encoding plasmid pSAG1-ROP2, and then investigated the ability of pSAG1-ROP2 to induce protective immunity in a DNA vaccine strategy with or without co-administration of pIL-12 as a genetic adjuvant.
After transient transfection of pSAG1-ROP2 into the HeLa cells, specific expression of the SAG1-ROP2 protein was detected by Western blot. Further, we investigated the potential of DNA vaccine in BALB/c mice. The results show that pSAG1-ROP2 immunization was able to strongly enhance specific IgG and subclass antibody production, IFN-γ and IL-12 production, and splenocyte proliferation compared to mice immunized with single-gene plasmids (pSAG1 or pROP2). Enhanced immune responses driven by pSAG1-ROP2 appear to give protection from RH strain of T. gondii challenge. When challenged with lethal doses (1 × 104) of T. gondii, mice immunized with single-gene died within 11 days after challenge. However, pSAG1-ROP2 vaccination resulted in longer survival of mice but not complete protection. The challenging dose of T. gondii is likely to be an important factor in the protection. Besides, no previous vaccine has been shown to completely protect against intraperitoneal challenge with the RH strain of T. gondii (Angus et al. 2000).
Immunization of BALB/c mice with a multiantigenic DNA vaccine pSAG1-ROP2 resulted in an improvement of the protective immunity against the T. gondii RH strain challenge as measured by the survival rate and the humoral and cellular immune response, in comparison with mice immunized with single-gene plasmid expressing SAG1 alone or ROP2 alone. These results suggested that multiple gene plasmid is more effective in the face of T. gondii challenge than single-gene plasmid. Our findings are consistent with earlier observations, which show that co-injection of low dose of two genes encoding SAG1 and ROP2 afforded protection, whereas single plasmids have no protective effect (Fachado et al. 2003). More recent study demonstrated that immunization with a mixture of plasmids expressing SAG1 and GRA4 resulted in an improvement of protection in comparison with plasmid expressing GRA4 alone or SAG1 alone (Mevelec et al. 2005). All these results imply that complete immunity against toxoplasmosis cannot be elicited by a single T. gondii protein. In contrast, one difference between our study and the above two is that we constructed SAG1-ROP2 fusion DNA so that the functional heterodimer could be expressed as a single open reading frame by a single messenger RNA. The SAG1-ROP2-fused heterodimeric DNA has the advantage of being delivered as a single vector that expresses a single heterodimeric protein encoded by a single mRNA transcript.
The magnitude and nature of these immune responses to DNA vaccines can be further manipulated by co-delivery of cytokine genes. Several studies show that immune response can be enhanced and modulated by co-injection of vectors containing IL-12 genes (Kim et al. 1997; Chow et al. 1998; Sin et al. 1999; Schadeck et al. 2006).
IL-12 administered in the form of DNA as an adjuvant can retain its high immunomodulatory properties and provide a significant advantage over protein IL-12 and the significant toxic effects associated with it (Marshall 1995; Schadeck et al. 2006). Interestingly, the administration of IL-12 encoding DNA has also been shown to be effective as a therapeutic vaccine (Lowrie et al. 1999). Therefore, we evaluated the ability of a plasmid expressing the murine IL-12 gene to enhance the level of protection in mice when co-injected with pSAG1-ROP2.
When compared with pSAG1-ROP2 alone group, introduction of pIL-12 with pSAG1-ROP2 further augmented Th1 type of immune response, with significant production of IgG2a antibodies, IFN-γ, and IL-12 but low levels of IL-4. The fact that IL-12 promotes the development of a type 1 cell-mediated immune response in part by suppressing IL-4 production by activated T cells (Manetti et al. 1993) may explain the low levels of IL-4. Moreover, mice immunized simultaneously with pSAG1-ROP2 and pIL-12 elicited higher survival rates against lethal T. gondii challenge than those immunized with pSAG1-ROP2 alone. The results clearly demonstrate that pIL-12 can act as a potent DNA vaccine adjuvant and significantly augment Th1-type cellular immune responses in BALB/c mice.
Immunity induced by T. gondii is complex, having both antibody and cellular components. However, a modulated Th1-type response plays a major role in controlling both acute and chronic T. gondii infections (Petersen et al. 1998). Fortunately, plasmid DNA itself has an adjuvant effect by polarizing the immune response toward a Type 1 phenotype (Klinman et al. 1997; Krieg et al. 1998), and in this study, the efficacy is further enhanced when combined with pIL-12.
In conclusion, these results show that the introduction of two functional genes encoding SAG1 and ROP2 via bicistronic vector is more powerful and efficient than single gene vaccines, and the use of murine IL-12-encoding plasmid as adjuvant in immunization protocol successfully enhances the level of protection induced by the multiple antigen-encoding plasmid alone. Therefore, this immunization regime may represent an effective vaccine strategy against T. gondii infection and provide a basis for further studies toward the use of multiantigenic DNA vaccines combined with cytokine plasmid in protection studies against T. gondii and other intracellular parasite infections.
References
Alexander J, Jebbari H, Bluethmann H, Satoskar A, Roberts CW (1996) Immunological control of Toxoplasma gondii and appropriate vaccine design. Curr Top Microbiol Immunol 219:183–195
Angus CW, Klivington-Evans D, Dubey JP, Kovacs JA (2000) Immunization with a DNA plasmid encoding the SAG1 (p30) protein of Toxoplasma gondii is immunogenic and protective in rodents. J Infect Dis 181:317–324
Beckers CJ, Dubremetz JF, Mercereau-Puijalon O, Joiner KA (1994) The Toxoplasma gondii rhoptry protein ROP2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J Cell Biol 127:947–961
Black MW, Boothroyd JC (2000) Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev 64:607–623
Bunell BA, Morgan RA (1998) Gene therapy for infectious diseases. Clin Microbiol Rev 11:42–56
Buxton D (1998) Protozoan infections (Toxoplasma gondii, Neospora caninum and Sarcocystis spp.) in sheep and goats: recent advances. Vet Res 29:289–310
Chow YH, Chiang BL, Lee YL, Chi WK, Lin WC, Chen YT, Tao MH (1998) Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol 160:1320–1329
Denkers EY, Gazzinelli RT (1998) Regulation and function of T-cell-mediated immunity during Toxoplasma gondii infection. Clin Microbiol Rev 11:569–588
Denkers EY, Del Rio L, Bennouna S (2003) Neutrophil production of IL-12 and other cytokines during microbial infection. Chem Immunol Allergy 83:95–114
Donnelly JJ, Wahren B, Liu MA (2005) DNA vaccines: progress and challenges. J Immunol 175:633–639
Dubey JP (1990) Status of toxoplasmosis in sheep and goats in the United States. J Am Vet Med Assoc 196:259–262
Dubey JP (1998) Advances in the life cycle of Toxoplasma gondii. Int J Parasitol 28:1019–1024
Dubey JP, Thulliez P (1993) Persistence of tissue cysts in edible tissues of cattle fed Toxoplasma gondii oocysts. Am J Vet Res 54:270–273
Fachado A, Rodriguez A, Angel SO, Pinto DC, Vila I, Acosta A, Amendoeira RR, Lannes-Vieira J (2003) Protective effect of a naked DNA vaccine cocktail against lethal toxoplasmosis in mice. Vaccine 21:1327–1335
Gurunathan S, Wu CY, Freidag BL, Seder RA (2000) DNA vaccines: a key for inducing long-term cellular immunity. Curr Opin Immunol 12:442–447
Hunter CA, Candolfi E, Subauste C, Van Cleave V, Remington JS (1995) Studies on the role of interleukin-12 in acute murine toxoplasmosis. Immunology 84:16–20
Kim JJ, Ayyavoo V, Bagarazzi ML, Chattergoon MA, Dang K,Wang B, Boyer JD, Weiner DB (1997) In vivo engineering of a cellular immune response by co-administration of IL-12 expression vector with a DNA immunogen. J Immunol 158:816–826
Klinman DM, Yamshchikov G, Ishigatsubo Y (1997) Contribution of CpG motifs to the immunogenicity of DNA vaccines. J Immunol 158:3635–3639
Krieg AM, Yi AK, Schorr J, Davis HL (1998) The role of CpG dinucleotides in DNA vaccines. Trends Microbiol 6:23–27
Lowrie DB, Tascon RE, Bonato VL, Lima VM, Faccioli LH, Stavropoulos E, Colston MJ, Hewinson RG, Moelling K, Silva CL (1999) Therapy of tuberculosis in mice by DNA vaccination. Nature 400:269–271
Lunden A (1995) Immune response in sheep after immunization with Toxoplasma gondii antigens incorporated into iscoms. Vet Parasitol 56:23–35
Manetti R, Parronchi P, Giudizi MG, Piccinni MP, Maggi E, Trinchieri G, Romagnani S (1993) Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med 177:1199–1204
Marshall E (1995) Cancer trial of IL-12 halted. Science 268:1555
Mevelec MN, Bout D, Desolme B, Marchand H, Magne R, Bruneel O, Buzoni-Gatel D (2005) Evaluation of protective effect of DNA vaccination with genes encoding antigens GRA4 and SAG1 associated with GM-CSF plasmid, against acute, chronical and congenital toxoplasmosis in mice. Vaccine 23:4489–4499
Nakaar V, Ngo HM, Aaronson EP, Coppens I, Stedman TT, Joiner KA (2003) Pleiotropic effect due to targeted depletion of secretory rhoptry protein ROP2 in Toxoplasma gondii. J Cell Sci 116:2311–2320
Nielsen HV, Lauemoller SL, Christiansen L, Buus S, Fomsgaard A, Petersen E (1999) Complete protection against lethal Toxoplasma gondii infection in mice immunized with a plasmid encoding the SAG1 gene. Infect Immun 67:6356–6363
Petersen E, Nielsen HV, Christiansen L, Spenter J (1998) Immunization with E. coli produced recombinant SAG1 with alum as adjuvant protect mice against lethal infection with Toxoplasma gondii. Vaccine 16:1283–1289
Robinson HL (1997) Nucleic acid vaccines: an overview. Vaccine 15:785–787
Saavedra R, Becerril MA, Dubeaux C, Lippens R, De Vos MJ, Herion P, Bollen A (1996) Epitopes recognized by human T lymphocytes in the ROP2 protein antigen of Toxoplasma gondii. Infect Immun 64:3858–3862
Schadeck EB, Sidhu M, Egana MA, Chong SY, Piacente P, Masood A, Garcia-Hand D, Cappello S, Roopchand V, Megati S, Quiroz J, Boyer JD, Felber BK, Pavlakis GN, Weiner DB, Eldridge JH, Israel ZR (2006) A dose sparing effect by plasmid encoded IL-12 adjuvant on a SIVgag-plasmid DNA vaccine in rhesus macaques. Vaccine 24:4677–4687
Sin JI, Kim JJ, Arnold RL, Shroff KE, McCallus D, Pachuk C, McElhiney SP, Wolf MW, Pompa-de Bruin SJ, Higgins TJ, Ciccarelli RB, Weiner DB (1999) IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J Immunol 162:2912–2921
Vercammen M, Scorza T, Huygen K, De Braekeleer J, Diet R, Jacobs D, Saman E, Verschueren H (2000) DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2 induces partially protective immunity against lethal challenge in mice. Infect Immun 68:38–45
Windeck T, Gross U (1996) Toxoplasma gondii strain-specific transcript levels of SAG1 and their association with virulence. Parasitol Res 82:715–719
Yang TT, He SY, Jiang H, Gu QM, Cong H, Zhou HY, Zhang JQ, Li Y, Zhao QL (2005) Construction of monovalent and compound nucleic acid vaccines against Toxoplasma gondii with gene encoding p30. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 23:14–17
Yap G, Pesin M, Sher A (2000) Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol 165:628–631
Acknowledgment
This study was sponsored by the National Natural Science Foundation of China (Grant 30371257) and by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (Grant 2003[406]). We declare that the experiments we performed comply with the current laws of China.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jie Zhang and Shenyi He contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, J., He, S., Jiang, H. et al. Evaluation of the immune response induced by multiantigenic DNA vaccine encoding SAG1 and ROP2 of Toxoplasma gondii and the adjuvant properties of murine interleukin-12 plasmid in BALB/c mice. Parasitol Res 101, 331–338 (2007). https://doi.org/10.1007/s00436-007-0465-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-007-0465-3