Abstract
Background
As neoadjuvant chemotherapy (NAC) for breast cancer has become more widely used, so has nipple-sparing mastectomy. A common criterion for eligibility is a 1 cm tumor-to-nipple distance (TND), but its suitability after NAC is unclear. In this study, we examined factors predictive of negative nipple pathologic status (NS−) in women undergoing total mastectomy after NAC.
Methods
Women with invasive breast cancer treated with NAC and total mastectomy from August 2014 to April 2018 at our institution were retrospectively identified. Following review of pre- and post-NAC magnetic resonance imaging (MRI) and mammograms, the association of clinicopathologic and imaging variables with NS− was examined and the accuracy of 1 cm TND on imaging for predicting NS− was determined.
Results
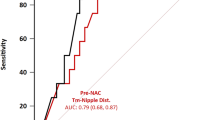
Among 175 women undergoing 179 mastectomies, 74% of tumors were cT1-T2 and 67% were cN+ on pre-NAC staging; 10% (18/179) had invasive or in situ carcinoma in the nipple on final pathology. On multivariable analysis, after adjusting for age, grade, and tumor stage, three factors, namely number of positive nodes, pre-NAC nipple-areolar complex retraction, and decreasing TND, were significant predictors of nipple involvement (p < 0.05). The likelihood of NS− was higher with increasing TND on pre- and post-NAC imaging (p < 0.05). TND ≥ 1 cm predicted NS− in 97% and 95% of breasts on pre- and post-NAC imaging, respectively.
Conclusions
Increasing TND was associated with a higher likelihood of NS−. A TND ≥ 1 cm on pre- or post-NAC imaging is highly predictive of NS− and could be used to determine eligibility for nipple-sparing mastectomy after NAC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
In women with invasive breast cancer, neoadjuvant chemotherapy (NAC) using modern chemotherapy and targeted therapy regimens is associated with improved pathologic complete response rates and is increasingly used to treat operable breast cancer.1,2,3 As more women undergo NAC, interest in the use of nipple-sparing mastectomy in this population has also increased.4,5,6,7,8,9,10 Among several recent institutional reports on outcomes following nipple-sparing mastectomy, 6–27% of these surgeries performed for therapeutic purposes were in patients who had received NAC.7,8,9,11 Studies examining the proportion of patients undergoing nipple-sparing mastectomy after NAC show a steady increase in its use over the past 10 years.4,9
In the primary surgery setting, a 1 cm tumor-to-nipple distance (TND) on imaging is often used to determine nipple-sparing mastectomy eligibility,12,13,14,15,16,17 and, combined with intraoperative retroareolar biopsy, has been reported to be the best criteria for achieving a negative nipple pathologic status (NS−).12 However, the accuracy of these criteria for predicting NS− pathology after NAC is unclear. In this study, we examine the accuracy of the 1 cm TND cut-off to predict NS− based on findings on pre- and post-NAC magnetic resonance imaging (MRI) and mammogram in a cohort of women undergoing total mastectomy after NAC.
Methods
After approval by the Institutional Review Board (IRB), we retrospectively collected clinicopathologic data from electronic medical records of women with invasive breast cancer treated with NAC and total mastectomy (non-nipple-sparing) at Memorial Sloan Kettering Cancer Center between August 2014 and April 2018. Total mastectomy cases were selected for study to allow complete pathologic examination of the nipple. Women with clinical T4 tumors, clinical nipple involvement, or pathologic nipple discharge were excluded. We also excluded women who did not have both a pre- and post-NAC MRI available for review, whose pre- and post-NAC MRIs were < 12 weeks apart, and those with technically inadequate MR image quality.
Pre- and post-NAC mammograms and MRIs were reviewed by three radiologists specializing in breast imaging, who were blinded to the final nipple pathology results. Performance of post-NAC mammogram was based on the presence of pre-NAC calcifications and surgeon discretion.
Pre- and post-NAC imaging review documented suspected tumor size, nipple-areolar complex thickening, nipple-areolar complex retraction or invasion, presence of multifocal or multicentric disease, and distances of biopsy clip, mass and non-mass enhancement, and calcifications from the nipple. Findings of nipple retraction or invasion, mass and non-mass enhancement, or suspicious calcifications were classified as suspected disease and were included in TND measurement. Mastectomy specimens were stratified according to TND into the following categories: < 1 cm, 1–2 cm, or ≥ 2 cm.
Routine processing of all mastectomy specimens began with inking and sectioning at approximately 0.5 cm intervals. If the nipple appeared grossly normal and uninvolved by tumor, the nipple-areolar complex was amputated in a plane parallel to the skin surface and sectioned perpendicularly. The entire nipple was submitted for examination. A second deeper section was taken in the plane parallel to the skin surface to demonstrate a cross-section of lactiferous ducts approaching the nipple. If the tumor grossly extended to or was within 1 cm of the nipple, perpendicular sections showing the interface between tumor and nipple were submitted for examination. For this study, only ductal carcinoma in situ (DCIS) or invasive carcinoma reported in the nipple sections were considered positive pathology findings.
The association between clinicopathologic and imaging variables and nipple involvement was examined using the t-test or Wilcoxon rank-sum test for continuous variables, and the Chi-square or Fisher’s exact tests for categorical variables. The accuracy of 1 cm TND for estimating the probability of negative nipple pathology was determined using the epidemiological parameters sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV). Sensitivity was defined as the ability of imaging studies to detect nipple involvement in women who had positive nipple pathology, specificity was defined as the ability to exclude nipple involvement in individuals who had NS−, PPV was defined as the ability of a positive imaging finding to correctly predict nipple involvement, and NPV was defined as the ability of a negative finding to correctly exclude nipple involvement. All statistical tests were performed using R 3.5.3 (R Core Development Team, Vienna, Austria) and SAS 9.4 (SAS Institute, Cary, NC, USA).
Results
We identified 298 mastectomies in 292 women who had undergone NAC followed by total mastectomy during the study period. Of these, 209 women had both pre- and post-NAC MRIs available for review. After excluding T4 tumors and those with clinical nipple involvement or pathologic nipple discharge, 175 eligible women who had undergone 179 mastectomies were analyzed. Median age was 48 years (interquartile range [IQR] 41–57) (Table 1). Based on pre-NAC staging, 74% of tumors were cT1–T2, 67% were clinically node-positive, and 77% were of ductal histology. With respect to molecular subtype, 42% of tumors were hormone receptor (HR)-positive and human epidermal growth factor receptor 2 (HER2)-negative, 24% were triple-negative, and 34% were HER2-positive.
On final pathologic examination, 10% (18/179) of mastectomy specimens had invasive carcinoma or DCIS detected in the nipple. On univariate analysis, multiple pathologic variables were significantly associated with nipple involvement, including lower grade, HR+/HER2− subtype, pT3, pN+, and greater numbers of positive nodes (Table 1). On univariate analysis of imaging findings, nipple involvement was associated with nipple-areolar complex thickening on pre-NAC mammogram, greater tumor extent, nipple retraction, and nipple-areolar complex thickening on pre- and post-NAC MRI, and multifocality/multicentricity on post-NAC MRI (p < 0.05).
When breasts were classified based on TND on imaging, we found that the likelihood of having an NS− pathology was higher with increasing TND on both pre- and post-NAC imaging (Fig. 1). In breasts with a pre-NAC TND < 1 cm, 83% had NS−, compared with 96% of breasts with a TND of 1–2 cm and 97% with a TND of > 2 cm (p = 0.006). We found a similar pattern of nipple involvement on examination of post-NAC TND: 73% of breasts with a TND < 1 cm had NS−, compared with 95% with a TND of 1–2 cm and 96% of those with a TND > 2 cm (p < 0.001). On multivariable analysis, after adjusting for age, histologic grade, and tumor stage, significant (p < 0.05) predictors of nipple involvement remained the number of positive nodes, nipple-areolar complex retraction on pre-NAC MRI, and decreasing TND (Table 2).
We further examined measures of accuracy for TND in predicting nipple pathologic status (Table 3). Using a cut-off of ≥ 1 cm, TND had a sensitivity and specificity of 84% and 56% in the pre-NAC settings, respectively. When applying this cut-off in the post-NAC setting, sensitivity decreased to 67% and specificity increased to 79%. A ≥ 1 cm TND on pre-NAC imaging had an NPV of 97%. In other words, if we applied imaging eligibility criteria for nipple-sparing mastectomy of ≥ 1 cm TND, 90 of 93 breasts in this cohort would go on to have a negative nipple pathology. On post-NAC imaging, the NPV of ≥ 1 cm TND cut-off decreased very slightly to 95%. We further examined those breasts that had a complete imaging response post-NAC, defined as no residual tumor enhancement seen on MRI or suspicious calcification/masses on mammogram. In the 13 breasts with TND < 1 cm on pre-NAC imaging and a complete response on post-NAC imaging, all were NS− (p = 0.4).
Discussion
In this study, we used findings on both pre- and post-neoadjuvant mammogram and MRI to categorize women based on TND < 1 cm, 1–2 cm, and > 2 cm from the nipple. We found that increasing TND was associated with a higher likelihood of having a negative nipple status on final pathology, and use of a cut-off TND of ≥ 1 cm on pre- or post-NAC mammogram and MRI could rule out nipple involvement in 97% and 95% of breasts, respectively.
To our knowledge this is the first study to examine this question in the neoadjuvant setting. Prior studies have evaluated the optimal TND cut-off on MRI to determine nipple-sparing mastectomy eligibility in the upfront surgery setting. In one such study by Koh et al., among 249 patients who had preoperative MRI, 24 (9.6%) had nipple involvement on final pathology.17 Nipple enhancement and TND ≤ 1 cm showed the best performance (AUC 0.88) in predicting nipple-areolar complex involvement, with a sensitivity of 92%, specificity of 84%, PPV of 38%, and NPV of 99%. However, that study also included patients having breast conservation who may have had undetected nipple pathology, likely explaining the higher reported PPV in that study compared with our study (27% on post-NAC MRI). In a retrospective study of 195 patients, Mariscotti et al. also found a TND distance of 1 cm to be most appropriate for selecting patients likely to have negative nipple pathology.12 A TND of 1 cm had a sensitivity of 82%, specificity of 72%, PPV of 84%, and NPV of 69%. Increasing this threshold to 1.5 cm decreased the sensitivity to 69% and increased specificity to 77%, while decreasing the threshold to 0.5 cm increased the sensitivity to 92% and decreased specificity to 57%. Using a TND cut-off of ≥ 1 cm to select patients for nipple-sparing mastectomy would have correctly identified 81% of patients without nipple involvement. These results are consistent with other studies16,18 that sought to identify a threshold lower than the previously accepted cut-off TND of 2 cm, which excluded many patients without nipple involvement from having nipple-sparing mastectomy. Our study found that this cut-off had a sensitivity and specificity of 84% and 56% for pre-NAC imaging, with an NPV of 97%, compared with 67%, 79%, and 95%, respectively, on post-NAC imaging.
The relatively high NPV seen in our study compared with the studies by both Mariscotti et al.12 and Ponzone et al.18 is perhaps explained by the high sensitivity of MRI and imaging criteria set for suspected disease, where we considered as suspicious any focus of non-mass enhancement on pre- and post-NAC images. We also included distance of mammographic calcifications to the nipple when accounting for the proximity to the nipple of suspicious imaging findings. Although the NPV for both pre- and post-NAC imaging are comparable, the most stringent method of selecting patients without nipple involvement would be ≥ 1 cm TND cut-off on pre-NAC imaging. However, using the pre-NAC cut-offs would also lead to exclusion of a larger proportion of patients with negative nipple status from undergoing nipple-sparing mastectomy.
We found a low PPV for both pre- and post-NAC MRI in determining nipple involvement, i.e. 17% and 26%, respectively. In patients having upfront surgery, there is also variation in the PPV of MRI, ranging from 36 to 84%,12,17 indicating that a large proportion of patients deemed ineligible for nipple-sparing mastectomy on this basis will have NS−. In our cohort, based on the pre-NAC imaging cut-off of ≥ 1 cm TND, 86 breasts were ineligible, and only 15 of these had positive nipple pathology. Similarly, using post-NAC imaging, 45 patients would have been ineligible, of whom 12 had positive pathology. This is an area that requires further study, as offering nipple-sparing mastectomy to patients with a TND < 1 cm relies heavily on a negative retroareolar biopsy result. A negative retroareolar biopsy is considered mandatory, in addition to imaging selection criteria, for nipple-sparing mastectomy. In a study including 232 prospectively collected therapeutic mastectomy specimens with grossly uninvolved nipples, Brachtel et al. found a sensitivity of 80% and NPV of 96% for the retroareolar biopsy with respect to ruling out nipple involvement.19 These studies have not yet been replicated in the neoadjuvant setting, therefore, although using a ≥ 1 cm TND cut-off on post-NAC imaging overestimates the extent of disease, at present it may be the most oncologically sound method of selecting women without suspected disease, for sparing the nipple-areolar complex.
On multivariable analysis, increasing the number of positive lymph nodes and increasing TND were significantly associated with nipple involvement. This is consistent with prior studies in patients having upfront surgery16,20 and suggests that increasing tumor burden as manifested by residual nodal disease after NAC is correlated with nipple involvement. Thus, careful consideration should be given to attempting nipple-sparing mastectomy in patients with clinical evidence of persistent nodal disease after NAC. An interesting finding in this study was that 13 patients who had suspicious disease within 1 cm of the nipple on pre-NAC imaging showed complete resolution on mammogram and MRI after NAC, and none of these patients had nipple involvement on final pathology. Although this is a small number of patients, it suggests that in patients with an excellent response to chemotherapy it may be possible to further reduce the ≥ 1 cm TND cut-off, although this requires further study in a larger population.
Limitations of our study include its retrospective design. In addition, although MRIs were reviewed by radiologists specializing in breast imaging, determination of what constitutes a suspicious finding can be subjective and therefore may differ based on radiologist. A strength of this study is the inclusion of mammographic findings in determination of TND because, particularly in the post-neoadjuvant setting, evidence of residual disease such as calcifications may not be detected on MRI. Furthermore, although TND is an important factor in determining whether the nipple-areolar complex can be successfully spared, it is important to choose appropriate candidates for nipple-sparing mastectomy, such that the entire breast parenchyma and disease can be adequately removed from all quadrants. Most recurrences occur not at the nipple-areolar complex but along the superior and lateral borders of the mastectomy flaps.21 Therefore, even if the patient meets the criteria for nipple-sparing mastectomy based on radiologic TND cut-offs, eligibility remains a clinical decision that should take into account factors such as disease volume and location, breast size, and incision size and location.
Conclusion
We found that increasing TND on pre- or post-NAC imaging was associated with a higher likelihood of having an NS−. A TND of ≥ 1 cm on pre- or post-NAC imaging had a high NPV and could be used to determine eligibility for nipple-sparing mastectomy after NAC. Further study of imaging accuracy in women with TND < 1 cm pre-NAC who achieve complete imaging response post-NAC is needed.
References
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72.
Gianni L, Pienkowski T, Im YH, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800.
Singh JC, Mamtani A, Barrio A, et al. Pathologic complete response with neoadjuvant doxorubicin and cyclophosphamide followed by paclitaxel with trastuzumab and pertuzumab in patients with HER2-positive early stage breast cancer: a single center experience. Oncologist. 2017;22(2):139–43.
Young WA, Degnim AC, Hoskin TL, et al. Outcomes of > 1300 nipple-sparing mastectomies with immediate reconstruction: the impact of expanding indications on complications. Ann Surg Oncol. 2019;26(10):3115–23.
Bartholomew AJ, Dervishaj OA, Sosin M, et al. Neoadjuvant chemotherapy and nipple-sparing mastectomy: timing and postoperative complications. Ann Surg Oncol. 2019;26(9):2768–72.
Jadeja P, Ha R, Rohde C, et al. Expanding the criteria for nipple-sparing mastectomy in patients with poor prognostic features. Clin Breast Cancer. 2018;18(3):229–33.
Santoro S, Loreti A, Cavaliere F, et al. Neoadjuvant chemotherapy is not a contraindication for nipple sparing mastectomy. Breast. 2015;24(5):661–6.
Coopey SB, Tang R, Lei L, et al. Increasing eligibility for nipple-sparing mastectomy. Ann Surg Oncol. 2013;20(10):3218–22.
Valero MG, Muhsen S, Moo TA, et al. Increase in utilization of nipple-sparing mastectomy for breast cancer: indications, complications, and oncologic outcomes. Ann Surg Oncol. 2020;27(2):344–51.
Wong SM, Chun YS, Sagara Y, Golshan M, Erdmann-Sager J. National patterns of breast reconstruction and nipple-sparing mastectomy for breast cancer, 2005–2015. Ann Surg Oncol. 2019;26(10):3194–203.
Galimberti V, Morigi C, Bagnardi V, et al. Oncological outcomes of nipple-sparing mastectomy: a single-center experience of 1989 patients. Ann Surg Oncol. 2018;25(13):3849–57.
Mariscotti G, Durando M, Houssami N, et al. Preoperative MRI evaluation of lesion-nipple distance in breast cancer patients: thresholds for predicting occult nipple-areola complex involvement. Clin Radiol. 2018;73(8):735–43.
Schecter AK, Freeman MB, Giri D, Sabo E, Weinzweig J. Applicability of the nipple-areola complex-sparing mastectomy: a prediction model using mammography to estimate risk of nipple-areola complex involvement in breast cancer patients. Ann Plast Surg. 2006;56(5):498–504 (discussion 504).
Ryu JM, Nam SJ, Kim SW, et al. Feasibility of nipple-sparing mastectomy with immediate breast reconstruction in breast cancer patients with tumor-nipple distance less than 2.0 cm. World J Surg. 2016;40(8):2028–35.
Alsharif E, Ryu JM, Choi HJ, et al. Oncologic outcomes of nipple-sparing mastectomy with immediate breast reconstruction in patients with tumor-nipple distance less than 2.0 cm. J Breast Cancer. 2019;22(4):613–23.
D’Alonzo M, Martincich L, Biglia N, et al. Clinical and radiological predictors of nipple-areola complex involvement in breast cancer patients. Eur J Cancer. 2012;48(15):2311–8.
Koh J, Park AY, Ko KH, Jung HK. MRI diagnostic features for predicting nipple-areolar-complex involvement in breast cancer. Eur J Radiol. 2020;122:108754.
Ponzone R, Maggiorotto F, Carabalona S, et al. MRI and intraoperative pathology to predict nipple-areola complex (NAC) involvement in patients undergoing NAC-sparing mastectomy. Eur J Cancer. 2015;51(14):1882–9.
Brachtel EF, Rusby JE, Michaelson JS, et al. Occult nipple involvement in breast cancer: clinicopathologic findings in 316 consecutive mastectomy specimens. J Clin Oncol. 2009;27(30):4948–54.
Laronga C, Kemp B, Johnston D, Robb GL, Singletary SE. The incidence of occult nipple-areola complex involvement in breast cancer patients receiving a skin-sparing mastectomy. Ann Surg Oncol. 1999;6(6):609–13.
Moo TA, Pinchinat T, Mays S, et al. Oncologic outcomes after nipple-sparing mastectomy. Ann Surg Oncol. 2016;23(10):3221–5.
Acknowledgment
None of the authors have financial incentives associated with publishing this article.
Funding
This study was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748 to Memorial Sloan Kettering Cancer Center.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to the material submitted for publication, and all have read and approved the manuscript.
Corresponding author
Ethics declarations
Disclosures
Elizabeth Morris reports grants from the National Institutes of Health/National Cancer Institute, during the conduct of the study; and grants from Grail, Inc. outside the submitted work. Monica Morrow has received honoraria from Genomic Health. Tracy-Ann Moo, Carolina Rossi Saccarelli, Elizabeth J. Sutton, Varadan Sevilimedu, Kate R. Pawloski, Timothy M. D’Alfonso, Mary C. Hughes, Jill S. Gluskin, Almir Bitencourt, Audree Tadros, Mary L. Gemignani, and Virgilio Sacchini have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moo, TA., Saccarelli, C.R., Sutton, E.J. et al. Tumor-Nipple Distance of ≥ 1 cm Predicts Negative Nipple Pathology After Neoadjuvant Chemotherapy. Ann Surg Oncol 28, 6024–6029 (2021). https://doi.org/10.1245/s10434-021-09902-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-09902-2