Abstract
Merkel cell polyomavirus (MCV) is frequently detectable in Merkel cell carcinoma (MCC) tumors, but the significance of MCV infection is not yet totally understood. Thus far, no key regulatory miRNA has been identified for MCC tumorigenesis. However, distinct miRNA expression profiles have been suggested for MCV-positive and MCV-negative tumors. We used microarray hybridization to identify miRNA expression differences in MCC tumor samples according to MCV status and further validated these results by quantitative reverse transcription polymerase chain reaction (qRT-PCR). When compared with MCV-negative tumors, we detected overexpression of miR-34a, miR-30a, miR-142-3p, and miR-1539 in those MCV positives. In addition, slight underexpression was detectable in MCV-positive tumors of miR-181d. We confirmed the distinct expression of miRNAs in MCV-positive and MCV-negative tumors and confirmed statistically significant underexpression of miR-34a in MCV-negative tumors by both array analysis and qRT-PCR. Neither tumor location nor development of metastases affected miRNA expression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Merkel cell carcinoma (MCC) is a rare and aggressive neuroendocrine carcinoma of the skin. In 2008, Merkel cell polyomavirus (MCV) was discovered clonally integrated into the tumor DNA both of primary tumors and of metastases, lending support for its fundamental causative role in the majority of MCCs [1–3]. Later studies showed that after integrating with the host genome, MCV loses its ability to replicate [4], suggesting further that MCV infection is already contributing in the early steps of MCC tumorigenesis. Other factors, such UV exposure, advancing age, Caucasian race, and an immunocompromised state, lead to a predisposition for MCC [5–8].
Earlier work by our group and others has revealed the existence of two distinct subgroups, based on the amount of MCV DNA, on differing oncogenic pathways and on clinical presentation [1, 5, 9–11]. MCV infection associates with better prognosis, tumor location on the extremities, and greater genomic stability [2, 12, 13]. A key step in its viral oncogenesis is binding and inactivation of RB by the large T-antigen (LTA) of MCV. Loss of retinoblastoma protein (RB) function seems, however, to be important also in MCV-negative tumors (as they are characterized by loss of RB expression) [9, 13, 14].
MicroRNAs (miRNAs) are non-coding single-stranded RNAs that negatively regulate gene expression at transcriptional or posttranscriptional level by binding to messenger RNA (mRNA) [15]. A single miRNA can target multiple gene networks involved in the oncogenesis via mRNA regulation [16]. Regarding MCC, to the best of our knowledge, only one study has addressed the miRNA profiles in MCV-negative and MCV-positive MCC tumors [17].
We hypothesized that dysregulation of specific miRNAs plays a part in the distinctive molecular features of such tumors. The aim of this present study was therefore to study the expression of miRNAs in MCC tumors by miRNA microarray and qRT-PCR analyses. Further aims were to compare the miRNA profiles in MCV-negative and MCV-positive MCC tumors, to find distinctions in miRNA expression by viral status that might offer therapeutic targets, and to explain the driving forces behind MCV-negative MCC.
Patients and methods
An institutional ethics committee approved the study protocol, and permission to analyze the tissue samples came from the National Agency for Medicolegal Affairs, Finland.
Subjects
Data on 181 patients diagnosed with MCC in Finland during 1979–2004 came from the Finnish Cancer Registry. Clinical details were extracted from clinical files and hospital records. Formalin-fixed, paraffin-embedded tissue blocks were retrieved from the pathology archives. The MCC diagnoses were confirmed from the tissue samples. We required for histological diagnosis of MCC that tissue morphology was compatible with MCC in microscopy and that the cancer cells stained positively for CK-20 and negatively for TTF-1. MCV detection from the paraffinized tumor blocks was as described in detail elsewhere [2]. Whenever MCV DNA copy number per reference gene was greater than 0.1, the sample was considered positive.
Array analysis
RNA preparation and miRNA microarrays
From our pool of 181 MCC patients, we chose 15 with MCV-positive and 15 with MCV-negative primary MCC tumors. Their selection was based on the amount of paraffinized tumor sample available and their known MCV status. Total RNA was extracted from the tumor samples and from a paraffinized control sample of normal skin. Qiagen’s miRNeasy mini Kit (Qiagen, Valencia, CA, USA) allowed extraction of total RNA from the paraffin blocks, including miRNA, according to manufacturer’s instructions. The NanoDrop-1000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA) served for quantification of RNA. Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) served in checking of the quality of both RNA and miRNA. We excluded two MCV-negative samples from our study due to insufficient RNA quantity, so microarray hybridization involved 15 MCV-positive and 13 MCV-negative samples.
Labeling and hybridization of RNA samples were with the Agilent’s miRNA Complete Labeling and Hybridization kit protocol version 2.4 (Agilent Technologies, Santa Clara, CA, USA), as described earlier [18]. Briefly, the samples were hybridized on Agilent’s miRNA Microarray System V16 (1205 human and 144 human viral miRNAs, Sanger miRBase 16.0; Agilent Technologies, Santa Clara, CA, USA). The microarrays were then washed with the manufacturer’s washing buffers. Arrays were scanned with Agilent’s Feature Extraction (v.11.0.1.1.), and default parameters were used to extract the data.
miRNA microarray data analysis
GeneSpring GX Analysis Software v11.0.2 (Agilent) was used for the statistical analysis of the microarray data. The data was preprocessed by log 2 transformation, and normalization between all arrays was by the use of the 75th percentile method. We excluded miRNAs not detected in any samples or controls from further analysis. miRNAs not expressed in at least 100 % of one group of samples were excluded. Significance of differential expression between every two groups of samples was estimated by t test for those miRNAs with at least a 2.0-fold reduced or increased mean expression level between the two groups. Expression of mirRNAs was considered to differ significantly when the adjusted p value (q value) was <0.2 (Benjamini correction for multiple testing).
We used the following databases to search for predicted mRNA targets of the differentially expressed miRNAs: TargetScan (http://www.targetscan.org), miRanda (http://www.microrna.org/microrna/home.do), mirTarget2 (http://mirdb.org/miRDB), Tarbase (http://diana.cslab.ece.ntua.gr/tarbase), miRBase target prediction database (http://www.mirbase.org/), and PICTAR (pictar.mdc-berlin.de). Only those mRNA targets that were predicted by at least four of the six programs were listed in order to minimize the possibility of false positives. Predicted mRNA targets of the differentially expressed miRNAs were screened by Chipster v1.4.7 (http://chipster.csc.fi/) for significant enrichment of over-represented pathways in the ConsensusPathDB (CPDB) by the hypergeometric test (p < 0.05).
qRT-PCR validation
qRT-PCR
Sufficient RNA for the qRT-PCR validation was extracted from 14 MCV-positive tumors and 12 MCV-negative tumors. Clinical data for the tumors and respective patients is summarized in Table 1. Reverse transcription of the RNA was by use of the miScript II RT Kit (50) (QIAGEN) according to the manufacturer’s instructions. Quantitative RT-PCR was executed with miScript SYBR Green PCR Kit (QIAGEN) and LightCycler 480 (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturers’ protocols. The primers for the amplification of miRNAs (miR-34a, miR-30a, miR-181d, miR-142-3p, and miR-1539) and U6 were purchased from QIAGEN. U6 expression served for normalization in statistical analysis. PCR for each RNA sample was performed in duplicate, with a negative control (no template of cDNA) included in every run. Melting curve analysis was also performed to check for nonspecific amplification.
Statistical analysis of the qRT-PCR data
We used the ΔΔCt method for relative quantification of miRNA expression by the equation: ΔΔCt = (CtmiRNA − CtU6)control skin − (CtmiRNA − CtU6)tumor sample. The relative quantity (RQ) for each miRNA, compared with the quantity of U6, was calculated by the equation: 2−ΔΔCt. Mean and median RQs for MCV-positive and MCV-negative tumors were calculated for miRNAs. Expression data of miRNAs are represented also as fold change (fold change = log 2 RQ). Student’s t test served to evaluate statistically significant differences in miRNA expression between the two groups: the MCV-negative tumors and MCV-positive tumors. Since the distribution of the RQ values was skewed, miRNA expression according to MCV status was confirmed with the Mann-Whitney U test using the median RQs. In addition to comparison according to MCV status, tumors were divided also into subgroups based on the progression to metastatic disease and the location on sun-exposed versus non-exposed areas. Sun-exposed areas were the head and neck and the arms. The Mann-Whitney U test served to compare miRNA expression in these groups (location and metastasis). p values less than 0.05 were considered statistically significant.
Results
miRNA expression profiles of MCV-positive and MCV-negative groups by microarray
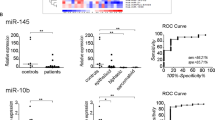
We conducted a microarray analysis to define possible global differences in miRNA expression between the MCV-positive and MCV-negative MCC subgroups. The microarray study comprised of 15 MCV-negative and 13 MCV-positive MCC tumors. Clustering analysis revealed no distinct clusters for MCV-positive and MCV-negative samples. Five miRNAs were expressed differently between these groups: miR-34a (FC = 4.1, q = 0.0097), miR-30a (FC = 2.3, q = 0.08), miR-1539 (FC = 4.6, q = 0.13), and miR-142-3p (FC = 3.8, q = 0.17) were significantly underexpressed, and miR-181d (FC = 3.5, q = 0.17) was overexpressed in MCV-negative tumors as compared to MCV positives (Table 2).
miRNA targets and biological pathways
We identified 184 predicted targets for the differently expressed miRNAs in those MCV positives versus MCV negatives (Supplementary Table 1). Further analysis of predicted target mRNAs of the differentially expressed miRNAs showed three pathways significantly affected in MCV-positive samples (Supplementary Table 2).
Validation of miRNA expression by qRT-PCR
The five miRNAs that were differently expressed in the array analysis were included in the qRT-PCR. Results of qRT-PCR showed underexpression of miR-34a, miR-30a, miR-142-3p, and miR-1539 in MCV-negative samples. Results were in consensus with the array results (Figs. 1 and 2). However, only miR-34a showed statistically significant underexpression in MCV-negative samples (Table 3). The relative expression level of miR-34a in individual MCC tumors is depicted in Fig. 3. The result of miR-181d was discrepant (Table 3). Detailed information on the findings is provided in Supplementary Table 3. No correlation emerged between the differently expressed miRNAs and either location of the tumors or their progression to metastasis.
Discussion
In this study, comparing miRNA profiles of MCV-positive and MCV-negative primary MCC tumor samples by microarray analysis, miR-34a, miR-1539, miR-30a, and miR-142-3p were underexpressed, and miR-181d was overexpressed in MCV-negative tumors. Underexpression of miR-34a was also validated as statistically significant by qRT-PCR. To the best of our knowledge, this is the largest study addressing miRNA expression in MCC.
The definition of MCV-negative versus MCV-positive MCC tumors is not as yet absolute. Traces of MCV genomic material are detectable in non-cancerous and cancerous tissues, representing a passenger virus without a causal role [19–21]. Rodig and colleagues recently suggested that with detection methods sufficiently sensitive, MCV DNA occurs in all MCC tumors [22]. Here, the threshold of viral copy numbers determining MCV positivity was set at 0.1, which has correlated well with LTA expression with the mouse monoclonal antibody CM2B4. The protocol is generally accepted and widely used [2, 10, 12, 23]. Detection of LTA expression with CM2B4 in studies had led to the consensus that MCV is in fact a carcinogenic virus [1, 4]. No in vitro studies exist that show the causality for very low copy number MCV. Albeit the exact threshold copy number that defines MCV status is still uncertain, the biological, molecular, and clinical differences in the outcome point to two distinct MCC subgroups [2, 9, 11–14, 17, 24].
miR-34a is a known tumor suppressor mediated via p53-dependent apoptosis promotion and cell cycle regulation [25, 26]. Its commonly recognized target genes are listed in Supplementary Table 1. miR-34a forms a positive feedback loop with p53. The activation of p53 by miR-34a leads to increased miR-34a production [25, 26] and to further suppression of miR-34a target genes, one of which is the SIRT1 gene. SIRT1, an enzyme of the sirtuin protein family, deacetylates p53, thus abolishing the tumor-suppressive effects of p53. The tumor-suppressor properties of miR-34a are therefore partly explained by blocking SIRT1 [26, 27]. Immunohistochemical detection of p53 is dependent upon TP53 gene mutations that lead to inhibition of protein degradation. In MCC, only MCV LTA-negative MCC tumors have TP53 mutations, suggesting p53 regulation is involved in the oncogenesis of MCV-negative tumors [14]. Prominent miR-34a reduction has been reported in human papillomavirus-induced cervical cancer. The viral oncoprotein E6 reduces miR-34a expression partly by destabilizing p53 [28]. Considering the signal loop of mir-34a and p53, the TP53 mutations and the underexpression of miR-34a could contribute to oncogenesis of MCV-negative tumors. Whether viral oncoproteins are behind the upregulation of miR-34a in MCC—and the possible mechanisms underlying such an effect—remain to be deciphered.
Another transcription factor regulating miR-34a is c-Myc, which is frequently expressed in MCC tumors [11] Unlike p53, c-Myc suppresses miR-34a expression [29]. miR-34a enhances tumor cell survival by reducing p53 levels in c-Myc-overexpressed Burkitt’s lymphoma cell lines during chemotherapy [30]. This suggests that miR-34a could have implications in other tumors with c-Myc deregulation and that miR-34a could be considered as a therapeutic target in such malignancies [30]. Although c-Myc expression does not correlate with MCV status [11], miR-34a may interact with c-Myc in MCC. Further, the overexpression of miR-34a in MCV-positive tumors could decrease the p53 expression.
We have reported earlier that expression of the anti-apoptotic protein bcl-2 indicates better prognosis in MCC patients [31]. miR-34a is a negative regulator of bcl-2 [32]; overexpression of miR-34a inhibits bcl-2, for example, in hepatocellular carcinoma [33]. If miR-34a inhibits bcl-2 also in MCC, this would lend support to a tumor-suppressive role for miR-34a in MCC. However, the role of bcl-2 as a positive prognostic factor in MCC hints that other mechanisms than bcl-2 inhibition might be more important in MCC oncogenesis.
In this study, we found no correlation between miRNA expression and disease stage or survival. Interestingly, the mir34a RQ value was 5- to 19-fold greater in the sample from the only patient who progressed to metastatic disease than in the six tumors taken from those patients who survived for more than 5 years. Larger tumor series are required to determine whether the differently expressed miRNAs in fact have prognostic value.
miR-34a overexpression in MCV-positive MCC was first reported by Xie et al. [17] Distinct expression of miR-34a and miR-30a according to MCV status was detected both in our study and by Xie and colleagues. They, however also confirmed significant overexpression of miR-375 and miR-769-5p and underexpression of miR-203 in MCV-positive tumors [17], whereas the mean miR-142-3p relative quantity was over 2.5-fold that our (own) MCV-positive tumors. As the differently expressed miRNAs show some partial differences between these studies, it is reasonable to propose possible fluctuations in MCC tumors’ miRNA profiles. However, since both of these independent studies found expression of miR-34a to differ according to tumor viral status, it is possible that there exist few key miRNAs whose dysregulation is relevant in MCC oncogenesis. For example, the overexpression of miR-203 detected in MCV-negative tumors by Xie and colleagues suppressed survivin expression and led to cell cycle arrest in MCV-negative tumor cells, suggesting its role in MCC as a tumor suppressor [17].
We acknowledge the limitations of this study, mainly the small sample sizes. To overcome this limitation, however, we used various methodologies to validate our results, together with thorough statistical analysis.
In conclusion, we found underexpression of miR-34a, miR-30a, miR-1539, and miR-142-3p in MCV-negative MCCs compared to expression in MCV positives as well as overexpression of miR-181d in MCV-negative MCCs by array analysis. This underexpression of miR-34a, ascertained to be statistically significant also by qRT-PCR, may play a role in the oncogenesis of MCV-negative tumors. None of the differently expressed miRNAs were of any prognostic value, yet more research is needed to study the correlation between miRNA expression and disease stage or survival. These results suggest that the miRNA profiles of MCC deviate according to MCV status, and this offers further evidence that the tumorigenesis of MCV-positive tumors diverges from that of MCV-negative tumors.
References
Feng H, Shuda M, Chang Y, Moore PS (2008) Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319:1096–1100
Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H (2009) Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J Natl Cancer Inst 101:938–945
Kassem A, Technau K, Kurz AK, Pantulu D, Loning M, Kayser G, Stickeler E, Weyers W, Diaz C, Werner M, Nashan D, Zur Hausen A (2009) Merkel cell polyomavirus sequences are frequently detected in nonmelanoma skin cancer of immunosuppressed patients. Int J Cancer 125:356–361
Shuda M, Feng H, Kwun HJ, Rosen ST, Gjoerup O, Moore PS, Chang Y (2008) T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc Natl Acad Sci U S A 105:16272–16277
Kukko H, Bohling T, Koljonen V, Tukiainen E, Haglund C, Pokhrel A, Sankila R, Pukkala E (2012) Merkel cell carcinoma—a population-based epidemiological study in Finland with a clinical series of 181 cases. Eur J Cancer 48:737–742
Koljonen V, Kukko H, Pukkala E, Sankila R, Bohling T, Tukiainen E, Sihto H, Joensuu H (2009) Chronic lymphocytic leukaemia patients have a high risk of Merkel-cell polyomavirus DNA-positive Merkel-cell carcinoma. Br J Cancer 101:1444–1447
Koljonen V, Kukko H, Tukiainen E, Bohling T, Sankila R, Pukkala E, Sihto H, Joensuu H, Kyllonen L, Makisalo H (2009) Incidence of Merkel cell carcinoma in renal transplant recipients. Nephrol Dial Transplant 24:3231–3235
Cardoso JC, Teixeira V, Tchernev G, Wollina U (2013) Merkel cell carcinoma: a review and update on aetiopathogenesis, diagnosis and treatment approaches. Wien Med Wochenschr. doi:10.1007/s10354-013-0218-0
Harms PW, Patel RM, Verhaegen ME, Giordano TJ, Nash KT, Johnson CN, Daignault S, Thomas DG, Gudjonsson JE, Elder JT, Dlugosz AA, Johnson TM, Fullen DR, Bichakjian CK (2013) Distinct gene expression profiles of viral- and nonviral-associated Merkel cell carcinoma revealed by transcriptome analysis. J Invest Dermatol 133:936–945
Higaki-Mori H, Kuwamoto S, Iwasaki T, Kato M, Murakami I, Nagata K, Sano H, Horie Y, Yoshida Y, Yamamoto O, Adachi K, Nanba E, Hayashi K (2012) Association of Merkel cell polyomavirus infection with clinicopathological differences in Merkel cell carcinoma. Hum Pathol 43:2282–2291
Kouzmina M, Hayry V, Leikola J, Haglund C, Bohling T, Koljonen V, Hagstrom J (2012) BMI1 expression identifies subtypes of Merkel cell carcinoma. Virchows Arch 461:647–653. doi:10.1007/s00428-012-1327-7; 10.1007/s00428-012-1327-7
Bhatia K, Goedert JJ, Modali R, Preiss L, Ayers LW (2010) Merkel cell carcinoma subgroups by Merkel cell polyomavirus DNA relative abundance and oncogene expression. Int J Cancer 126:2240–2246
Bhatia K, Goedert JJ, Modali R, Preiss L, Ayers LW (2010) Immunological detection of viral large T antigen identifies a subset of Merkel cell carcinoma tumors with higher viral abundance and better clinical outcome. Int J Cancer 127:1493–1496
Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H (2011) Merkel cell polyomavirus infection, large T antigen, retinoblastoma protein and outcome in Merkel cell carcinoma. Clin Cancer Res 17:4806–4813
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Calin GA, Croce CM (2006) MicroRNA signatures in human cancers. Nat Rev Cancer 6:857–866
Xie H, Lee L, Caramuta S, Hoog A, Browaldh N, Bjornhagen V, Larsson C, Lui WO (2013) microRNA expression patterns related to Merkel cell polyomavirus infection in human Merkel cell carcinoma. J Invest Dermatol. doi:10.1038/jid.2013.355; 10.1038/jid.2013.355
Mosakhani N, Sarhadi VK, Borze I, Karjalainen-Lindsberg ML, Sundstrom J, Ristamaki R, Osterlund P, Knuutila S (2012) MicroRNA profiling differentiates colorectal cancer according to KRAS status. Genes Chromosome Cancer 51:1–9
Shuda M, Arora R, Kwun HJ, Feng H, Sarid R, Fernandez-Figueras MT, Tolstov Y, Gjoerup O, Mansukhani MM, Swerdlow SH, Chaudhary PM, Kirkwood JM, Nalesnik MA, Kant JA, Weiss LM, Moore PS, Chang Y (2009) Human Merkel cell polyomavirus infection I. MCV T antigen expression in Merkel cell carcinoma, lymphoid tissues and lymphoid tumors. Int J Cancer 125:1243–1249
Loyo M, Guerrero-Preston R, Brait M, Hoque MO, Chuang A, Kim MS, Sharma R, Liegeois NJ, Koch WM, Califano JA, Westra WH, Sidransky D (2010) Quantitative detection of Merkel cell virus in human tissues and possible mode of transmission. Int J Cancer 126:2991–2996
Foulongne V, Kluger N, Dereure O, Mercier G, Moles JP, Guillot B, Segondy M (2010) Merkel cell polyomavirus in cutaneous swabs. Emerg Infect Dis 16:685–687
Rodig SJ, Cheng J, Wardzala J, DoRosario A, Scanlon JJ, Laga AC, Martinez-Fernandez A, Barletta JA, Bellizzi AM, Sadasivam S, Holloway DT, Cooper DJ, Kupper TS, Wang LC, DeCaprio JA (2012) Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J Clin Invest 122:4645–4653. doi:10.1172/JCI64116; 10.1172/JCI64116
Waltari M, Sihto H, Kukko H, Koljonen V, Sankila R, Bohling T, Joensuu H (2011) Association of Merkel cell polyomavirus infection with tumor p53, KIT, stem cell factor, PDGFR-alpha and survival in Merkel cell carcinoma. Int J Cancer 129:619–628
Paulson KG, Lemos BD, Feng B, Jaimes N, Penas PF, Bi X, Maher E, Cohen L, Leonard JH, Granter SR, Chin L, Nghiem P (2009) Array-CGH reveals recurrent genomic changes in Merkel cell carcinoma including amplification of L-Myc. J Invest Dermatol 129:1547–1555
He L, He X, Lowe SW, Hannon GJ (2007) microRNAs join the p53 network—another piece in the tumour-suppression puzzle. Nat Rev Cancer 7:819–822
Yamakuchi M, Ferlito M, Lowenstein CJ (2008) miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci USA 105:13421–13426. doi:10.1073/pnas.0801613105; 10.1073/pnas.0801613105
Yamakuchi M, Lowenstein CJ (2009) MiR-34, SIRT1 and p53: the feedback loop. Cell Cycle 8:712–715
Wang X, Wang HK, McCoy JP, Banerjee NS, Rader JS, Broker TR, Meyers C, Chow LT, Zheng ZM (2009) Oncogenic HPV infection interrupts the expression of tumor-suppressive miR-34a through viral oncoprotein E6. RNA 15:637–647. doi:10.1261/rna.1442309; 10.1261/rna.1442309
Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, Dang CV, Thomas-Tikhonenko A, Mendell JT (2008) Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet 40:43–50
Sotillo E, Laver T, Mellert H, Schelter JM, Cleary MA, McMahon S, Thomas-Tikhonenko A (2011) Myc overexpression brings out unexpected antiapoptotic effects of miR-34a. Oncogene 30:2587–2594. doi:10.1038/onc.2010.634; 10.1038/onc.2010.634
Sahi H, Koljonen V, Kavola H, Haglund C, Tukiainen E, Sihto H, Bohling T (2012) Bcl-2 expression indicates better prognosis of Merkel cell carcinoma regardless of the presence of Merkel cell polyomavirus. Virchows Arch 461:553–559. doi:10.1007/s00428-012-1310-3; 10.1007/s00428-012-1310-3
Raver-Shapira N, Marciano E, Meiri E, Spector Y, Rosenfeld N, Moskovits N, Bentwich Z, Oren M (2007) Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell 26:731–743
Yang F, Li QJ, Gong ZB, Zhou L, You N, Wang S, Li XL, Li JJ, An JZ, Wang DS, He Y, Dou KF (2013) MicroRNA-34a targets Bcl-2 and sensitizes human hepatocellular carcinoma cells to sorafenib treatment. Technol Cancer Res Treat. doi:10.7785/tcrt.2012.500364
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Tuukka Veija and Helka Sahi contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Table 1
(XLSX 19 kb)
Supplementary Table 2
(XLSX 10 kb)
Supplementary Table 3
(XLSX 14 kb)
Rights and permissions
About this article
Cite this article
Veija, T., Sahi, H., Koljonen, V. et al. miRNA-34a underexpressed in Merkel cell polyomavirus-negative Merkel cell carcinoma. Virchows Arch 466, 289–295 (2015). https://doi.org/10.1007/s00428-014-1700-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-014-1700-9