Abstract
CD34+ fibrocytes are constitutive elements of the connective tissue where they play a role in matrix synthesis and tumor-associated stromal remodeling. Secreted protein, acidic, and rich in cysteine (SPARC) is a pivotal mediator of stromal remodeling precipitated by invasive carcinomas. The present study was undertaken to investigate CD34+ fibrocytes in the stroma of the tumor-free urinary bladder, chronic cystitis, and urothelial carcinomas together with stromal expression of α-smooth muscle actin (α-SMA), CD117, and SPARC. In tumor-free urinary bladder and chronic cystitis, CD34+ fibrocytes were found in the deep lamina propria and tunica muscularis, whereas the superficial lamina propria disclosed a CD34-negative and α-SMA-positive fibrocyte-like cell. Invasive urothelial carcinomas revealed a complete loss of CD34+ fibrocytes and concomitant appearance of α-SMA-reactive myofibroblasts which showed strong expression of SPARC. CD117 expression of tumor-free and tumor-associated stroma revealed no differences. We in this study for the first time describe CD34+ fibrocytes in the urinary bladder and an up-to-now unknown population of α-SMA-positive fibrocytes exclusively occurring in the superficial lamina propria. Stromal remodeling associated with invasive carcinomas in the urinary bladder is characterized by a loss of CD34+ fibrocytes paralleled by a gain of α-SMA-positive myofibroblasts and increased expression of SPARC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
CD34+ fibrocytes have been described in the stroma of the upper aerodigestive tract [6], the lung [22] and heart [7], the gastrointestinal tract [4, 20, 21], the cornea [14], the breast [3, 8, 11, 24], and the female genital tract [5], where they constitute the most prevalent cell population. In most of these locations, stromal remodeling associated with invasive carcinomas is characterized by a virtually complete loss of CD34+ fibrocytes, paralleled by a gain of α-smooth muscle actin (α-SMA)-positive myofibroblasts which are not observed in the normal tumor-free stroma [3–6, 8, 11, 20, 24]. Moreover, the carcinoma-associated stromal phenotype change is accompanied by strong expression of secreted protein, acidic, and rich in cysteine (SPARC) [8, 17, 23, 26] and to a lesser extent of CD117 [6]. SPARC and TGFβ act together in the regulation of CD34 and α-SMA expression [1, 2, 14, 25], and SPARC mediates stromal de-adhesion which might constitute a pivotal step for tumor invasiveness. CD34+ fibrocytes, originally described by Bucala et al. [10] in 1994, are derived from circulating CD45+ myeloid cells. As shown by several subsequent investigations, CD34+ fibrocytes are involved in wound healing and matrix synthesis, and they appear to act as potent antigen presenting cells [1, 10, 12, 13, 16]. It has therefore been speculated that a loss of CD34+ fibrocytes might be essential for a malignant tumor to escape host immunosurveillance. Although, meanwhile, many studies as to this cell population have been published, the precise anatomical distribution, histogenesis, function, and fate in the tumor stroma of CD34+ fibrocytes remain in part enigmatic. Up to now, reports concerning the occurrence of CD34+ fibrocytes in the urinary bladder have due to our knowledge not been published. We therefore undertook the present study to investigate whether CD34+ fibrocytes occur in the stroma of the tumor-free urinary bladder, chronic cystitis, and urothelial carcinomas and to what extent the stromal expression of α-SMA, CD117, and SPARC is altered in carcinoma-associated stromal remodeling.
Materials and methods
The present study comprises a total of 58 cystoscopic biopsies of the urinary bladder obtained from 58 patients. In 18 patients (Table 1), biopsies showed chronic cystitis. All patients with chronic cystitis had a pertinent history and clinical presentation. The presumed cause of chronic cystitis was prostatic hyperplasia in men and long-standing ascending infection in women. Biopsies were taken to rule out malignancy and showed a moderate to severe lymphocytic infiltrate of the lamina propria. Squamous metaplasia as well as eosinophilia was each reported in four cases.
Patients with histologically proven urothelial carcinomas in previous or subsequent biopsies were excluded from the chronic cystitis group. Urothelial carcinomas were present in 40 patients. The carcinomas were graded in two tiers according to the WHO (2004); tumor staging was performed according to the UICC TNM classification (2002). Patients’ age, gender, and underlying diagnosis are summarized together with the major tumor characteristics in Table 1. Thirty-two of the 40 cases with urothelial carcinomas were initial diagnoses, and the remaining cases were recurrent tumors. In all cases, tumor-free biopsy particles or tumor-free biopsies located remotely from the tumor were available for comparison. However, the tumor-free biopsy particles varied greatly in size because some of them only covered superficial parts of the lamina propria, whereas others included the lamina propria in full thickness and adjacent tunica muscularis. Tissues were fixed in a 10% formalin solution, embedded in paraffin, cut, and stained with H&E and PAS for routine purposes.
Immunohistochemistry
CD34, α-SMA, SPARC, and CD117 were detected immunohistochemically by means of the standard avidin biotin complex (ABC)-peroxidase method (ABC Elite Kit; Vector, Burlingame, CA, USA) using 3,3′-diaminobenzidine (DAB) as chromogen. The primary antibodies and type of tissue pretreatment are listed in Table 2. Microwave pretreatment was performed by heating the deparaffinized and rehydrated sections, immersed in 10 mM sodium citrate buffer (pH 6.0), in a microwave oven at 600 W for three or four times for 5 min (see Table 2).
Double-staining immunohistochemistry
Double-label immunohistochemistry was performed for CD34, shown in brown color, and for α-SMA, shown in green color. In detail, as the first step, sections were stained for CD34 using a microwave oven heating for antigen retrieval and the ChemMate Detection Kit Peroxidase/DAB (DakoCytomation) which included biotinylated secondary antibodies, streptavidin peroxidase, and DAB as chromogen, producing a brown staining reaction. Sections were then again subjected to microwave oven heating (three times for 5 min). After cooling to room temperature, for the second step, sections were reacted with the monoclonal antibody against α-SMA. Bound antibodies were detected using the same ChemMate Detection Kit as described above, but the staining reaction was based on HistoGreen (Linaris, Wertheim-Bettingen, Germany), producing a green staining reaction. Sections were counterstained using nuclear fast red. Incubations were performed on an automated immunohistochemistry apparatus (Autostainer plus; DakoCytomation). As controls, the primary antibody of the first step (anti-CD34) was substituted by an irrelevant monoclonal antibody while all other steps remained unchanged, or the primary antibody of the second step (anti-α-SMA) was substituted by an irrelevant monoclonal antibody leaving the other steps unchanged. These controls yielded the expected results.
Semiquantitative assessment of immunohistochemistry
Immunoreactivity of CD34, CD117, α-SMA, and SPARC was assessed semiquantitatively in the tumor-free tissue and tumor-associated stroma as previously described [8]. In brief, the percentage of stromal cells expressing the respective antigen was categorized as “0”, “+”, “++”, or “+++” when up to 5%, more than 5% and up to 25%, more than 25% and up to 50%, or more than 50% of stromal cells, respectively, disclosed immunoreactivity. Percentages were assessed by two independent observers, assuming that a microscopic high-power field (objective 40×, microscopic magnification: 400×) harbors 100 stromal cells (range: 75–150). Double-stained (CD34 and α-SMA) slides were assessed qualitatively.
Statistical analyses
Comparison of frequencies was performed using the chi-square test assuming p < 0.01 to be statistically significant.
Results
Tumor-free urinary bladder
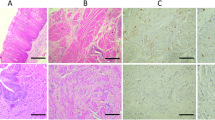
The loose connective tissue of the lamina propria harbored small stromal cells with inconspicuous cytoplasm and slender dendrite-like projections. In the superficial lamina propria, these cells were arranged in parallel to the basal lamina of the covering urothelium and immunohistochemically disclosed α-SMA reactivity (40 of 40) (Fig. 1a), whereas CD34 was not detected (Fig. 1c). Stromal cells within the deeper lamina propria were predominantly located around larger veins and arteries; those located remotely from vessels were haphazardly arranged and disclosed in partly multipolar cytoplasmic projections. In contrast to the superficial lamina propria, stromal cells of the deeper lamina propria were negative for α-SMA (Fig. 1b) and positive for CD34 constituting characteristic CD34+ fibrocytes (Fig. 1d). Accordingly, in the tumor-free lamina propria, CD34+ fibrocytes were found in 23 of 40 cases (Table 3). This value precisely reflects the number of biopsies covering the full thickness of the lamina propria, whereas small superficial biopsies of the lamina propria contained no CD34+ fibrocytes. Fascicles of smooth muscle in the tunica muscularis were surrounded by densely packed CD34+ fibrocytes.
Stromal cells of the superficial lamina propria exhibit a fibrocyte-like appearance and strong α-SMA positivity (a), whereas those of the deep lamina propria and tunica muscularis are negative for α-SMA (b). In contrast, the stroma of the superficial lamina propria discloses no reactivity for CD34 (c); in the deep lamina propria and tunica muscularis, CD34+ fibrocytes surround the vessels and fascicles of the smooth muscle (d). Double labeling discloses a mutually exclusive reactivity of α-SMA (green) (e superficial lamina propria) and CD34 (brown) in the stromal cells (f deep lamina propria)
No dual expression of CD34 and α-SMA was found by means of double labeling indicating two distinct populations of α-SMA-positive fibrocytes in the superficial lamina propria (Fig. 1e) and CD34+ fibrocytes in the deep lamina propria and tunica muscularis (Fig. 1f).
CD117 expression was found in a small population of stromal cells within the tumor-free stroma, whereas SPARC positivity appeared in up to 50% of the stromal cells. Strong CD117 staining was also found in mast cells which were scattered throughout the tumor-free lamina propria and tunica muscularis. The smooth muscle within the lamina propria and tunica muscularis was negative for both CD117 and SPARC.
Chronic cystitis
In chronic cystitis cases, the distribution of CD34+ fibrocytes and α-SMA-positive stromal cells was similar compared to that observed in the tumor-free bladder. Double labeling disclosed two distinct populations of CD34+ fibrocytes in the deep and α-SMA-positive stromal cells in the superficial lamina propria. There was also no significant difference as to the number of CD117- and SPARC-reactive stromal cells (Table 3). Compared to the tumor-free samples, the number of mast cells as detected by CD117 appeared to be increased.
Noninvasive papillary urothelial tumors (pTa)
With respect to the expression of CD117 and SPARC, the stroma of noninvasive papillary urothelial tumors disclosed no qualitative or quantitative differences compared to the tumor-free urinary bladder, whereas CD34 expression was completely absent from the stroma of these lesions (Table 3). Moreover, α-SMA-reactive stromal cells were more numerous in the stroma adjacent to and within noninvasive carcinomas, where they disclosed stronger α-SMA reactivity and a myofibroblastic phenotype.
Invasive urothelial carcinomas
As in noninvasive tumors, CD34+ fibrocytes were also completely absent from invasive carcinomas (Fig. 2a, Table 3). The stroma of invasive papillary carcinomas consisted of plump elongated myofibroblasts arranged in parallel or in a herringbone-like pattern. These cells were positive for α-SMA, featuring a characteristic tram-tracking of thick intracytoplasmic α-SMA-positive fibers (Fig. 2b). The stromal cells also mostly disclosed strong SPARC positivity (Fig. 2c), whereas CD117 was only observed in a small subpopulation of tumors displaying no significant difference in comparison to the tumor-free stroma (Table 3). Double labeling showed no dual positivity for α-SMA and CD34 in the carcinoma-associated myofibroblasts (Fig. 2d). Areas of antecedent biopsies were found in four cases and disclosed α-SMA-positive and CD34-negative myofibroblasts which showed weak SPARC positivity. CD117 was not observed in this cell population.
The stroma of invasive carcinomas shows a complete loss of CD34+ fibrocytes (a note preserved CD34 reactivity of endothelial cells) accompanied by a gain of α-SMA-positive myofibroblasts (b). Tumor-associated myofibroblasts show a strong reactivity for SPARC (c). No coexpression of α-SMA (green) and CD34 (brown) was observed in the myofibroblasts by means of double labeling (d)
Discussion
The present study is the first to report on CD34+ fibrocytes within the urinary bladder, where they occur in the deep lamina propria and the tunica muscularis. CD34+ fibrocytes, originally described by Bucala et al. in 1994 [10], have meanwhile been shown to be an important and constitutive element of the connective tissue of most organs which have been investigated until now [3–6, 8, 11, 20–22, 24]. The major functions of CD34+ fibrocytes are matrix synthesis and antigen presentation, suggesting a putative role in host response to invasive carcinomas [1, 10, 12, 13].
In addition, the present study reveals a novel cell type located in the superficial lamina propria which is negative for CD34, shows strong α-SMA reactivity, and morphologically resembles fibrocytes. Owing to their morphologic aspect and at least partial reactivity for SPARC and CD117, these cells are distinct from smooth muscle cells and myofibroblasts. Due to their α-SMA expression, it is likely that the superficial stromal cells play a role in providing and maintaining mechanical properties of the lamina propria, whereas CD34+ fibrocytes in the deeper lamina propria are mainly involved in matrix synthesis.
In the cervix uteri and the breast, in situ carcinomas are characterized by a loss of CD34+ fibrocytes in the adjacent stroma [3, 5]. This phenomenon was not observed in the urinary bladder because the superficial lamina propria is devoid of CD34+ fibrocytes. Therefore, the diagnostic value of CD34+ fibrocytes in the diagnosis of carcinomas of the urinary bladder is comparably low. Nevertheless, the presence of CD34+ fibrocytes separates tumor-free urinary bladder and chronic cystitis on one side from invasive and noninvasive carcinomas. In invasive urothelial carcinomas, the stromal composition is similar to that observed in various other carcinomas of whatever histologic type or anatomical location recapitulating the stereotypic transition from the CD34+ α-SMA− fibrocytes in the tumor-free stroma toward the tumor-associated CD34− α-SMA+ myofibroblast [3–6, 8]. This finding is in keeping with Shimasaki et al. [28] who reported myofibroblasts in the stroma of urothelial carcinomas of the urinary bladder.
In squamous cell carcinomas, stromal remodeling is at least in part due to recruitment of additional growth-regulating pathways mediated by CD117 [6]. This is not the case in urothelial carcinomas. However, except for CD117 expression, stromal remodeling in the urinary tract closely resembles that observed in various other organs. Thus, our study confirms the role of SPARC which has been claimed to play a role in stromal invasion by carcinomas. SPARC is a matricellular protein, serving several functions, the most important of which in the present context is induction of stromal de-adhesion [9, 15, 19]. It is interesting to note that in the present study, SPARC was also observed in the tumor-free lamina propria, reflecting the morphologic aspect of a loose “de-adhesive” stroma. SPARC expression is upregulated by TGFβ and vice versa [2, 25, 27], precipitating many features of carcinoma-associated stromal remodeling as TGFβ has been shown to downregulate CD34 and upregulate α-SMA [1, 14]. Accordingly, immunohistochemical double labeling showed the expression of CD34 and α-SMA to be mutually exclusive in the tumor-free as well as in the tumor-associated stroma. Downregulation of CD34 might further increase stromal de-adhesion induced by SPARC because CD34 signaling has been reported to create and maintain cell–cell contacts and homotypic adherence of mesenchymal cells [18]. Because CD34+ fibrocytes play a pivotal role in matrix synthesis and repair as they are a major source of collagen types I and III [1, 10, 13], a loss of this cell population and the adhesion of stromal cells may constitute an important prerequisite for tumor invasion, tumor cell migration, and subsequent distant tumor spread. This assumption is underlined by the fact that a CD34+ fibrocyte loss develops stereotypically in virtually all invasive carcinomas investigated up to now [3–6, 8, 11, 20–22, 24]. When considering the immunohistochemical analysis of the CD34+ stromal cell population as a diagnostic tool, it should be kept in mind that stromal response to unspecific damage such as previous biopsies is similar to that observed in invasive carcinomas [11]. Therefore, a loss of CD34+ fibrocytes does not prove malignancy although in contrast, the presence of this cell population excludes malignancy with a high degree of probability.
References
Abe R, Donnelly SC, Peng T, Bucala R, Metz CN (2001) Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 166:7556–7562
Abe K, Hibino T, Mishima H, Shimomura Y (2004) The cytokine regulation of SPARC production by rabbit corneal epithelial cells and fibroblasts in vitro. Cornea 23:172–179
Barth PJ, Ebrahimsade S, Ramaswamy A, Moll R (2002) CD34+ fibrocytes in invasive ductal carcinoma, ductal carcinoma in situ, and benign breast lesions. Virchows Arch 440:298–303
Barth PJ, Ebrahimsade S, Hellinger A, Moll R, Ramaswamy A (2002) CD34+ fibrocytes in neoplastic and inflammatory pancreatic lesions. Virchows Arch 440:128–133
Barth PJ, Ramaswamy A, Moll R (2002) CD34+ fibrocytes in normal cervical stroma, cervical intraepithelial neoplasia III, and invasive squamous cell carcinoma of the cervix uteri. Virchows Arch 441:564–568
Barth PJ, Schenck zu Schweinsberg T, Ramaswamy A, Moll R (2004) CD34+ fibrocytes, α-smooth muscle antigen-positive myofibroblasts, and CD117 expression in the stroma of invasive squamous cell carcinomas of the oral cavity, pharynx, and larynx. Virchows Arch 444:231–234
Barth PJ, Koster H, Moosdorf R (2005) CD34+ fibrocytes in normal mitral valves and myxomatous mitral valve degeneration. Pathol Res Pract 201:301–304
Barth PJ, Moll R, Ramaswamy A (2005) Stromal remodeling and SPARC (secreted protein acid rich in cysteine) expression in invasive ductal carcinomas of the breast. Virchows Arch 446:532–536
Bradshaw AD, Sage EH (2001) SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 107:1049–1054
Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A (1994) Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1:71–81
Chauhan H, Abraham A, Phillips JR, Pringle JH, Walker RA, Jones JL (2003) There is more than one kind of myofibroblast: analysis of CD34 expression in benign, in situ, and invasive breast lesions. J Clin Pathol 56:271–276
Chesney J, Bacher M, Bender A, Bucala R (1997) The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA 94:6307–6312
Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R (1998) Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol 160:419–425
Espana EM, Kawakita T, Liu CY, Tseng SC (2004) CD-34 expression by cultured human keratocytes is downregulated during myofibroblast differentiation induced by TGF-beta1. Invest Ophthalmol Vis Sci 45:2985–2991
Framson PE, Sage EH (2004) SPARC and tumor growth: where the seed meets the soil? J Cell Biochem 92:679–690
Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN (2001) Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J 15:2215–2224
Iacobuzio-Donahue CA, Argani P, Hempen PM, Jones J, Kern SE (2002) The desmoplastic response to infiltrating breast carcinoma: gene expression at the site of primary invasion and implications for comparisons between tumor types. Cancer Res 62:5351–5357
Majdic O, Stockl J, Pickl WF, Bohuslav J, Strobl H, Scheinecker C, Stockinger H, Knapp W (1994) Signaling and induction of enhanced cytoadhesiveness via the hematopoietic progenitor cell surface molecule CD34. Blood 83:1226–1234
Murphy-Ullrich JE (2001) The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest 107:785–790
Nakayama H, Enzan H, Miyazaki E, Kuroda N, Naruse K, Hiroi M (2000) Differential expression of CD34 in normal colorectal tissue, peritumoral inflammatory tissue, and tumour stroma. J Clin Pathol 53:626–629
Nakayama H, Enzan H, Miyazaki E, Kuroda N, Naruse K, Kiyoku H, Toi M, Hiroi M (2001) CD34 positive stromal cells in gastric adenocarcinomas. J Clin Pathol 54:846–848
Nakayama H, Enzan H, Yamamoto M, Miyazaki E, Hidaka C, Okumichi T, Okumichi A, Kajihara H (2003) CD34-positive stromal cells in primary lung carcinomas. Oncol Rep 10:1313–1316
Porter PL, Sage EH, Lane TF, Funk SE, Gown AM (1995) Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem 43:791–800
Ramaswamy A, Moll R, Barth PJ (2003) CD34+ fibrocytes in tubular carcinomas and radial scars of the breast. Virchows Arch 443:536–540
Reed MJ, Vernon RB, Abrass IB, Sage EH (1994) TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J Cell Physiol 158:169–179
Ryu B, Jones J, Hollingsworth MA, Hruban RH, Kern SE (2001) Invasion-specific genes in malignancy: serial analysis of gene expression comparisons of primary and passaged cancers. Cancer Res 61:1833–1838
Schiemann BJ, Neil JR, Schiemann WP (2003) SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-β-signaling system. Mol Biol Cell 14:3977–3988
Shimasaki N, Kuroda N, Miyazaki E, Hayashi Y, Toi M, Hiroi M, Enzan H, Shuin T (2006) The distribution pattern of myofibroblasts in the stroma of human bladder carcinoma depends on their invasiveness. Histol Histopathol 21:349–353
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nimphius, W., Moll, R., Olbert, P. et al. CD34+ fibrocytes in chronic cystitis and noninvasive and invasive urothelial carcinomas of the urinary bladder. Virchows Arch 450, 179–185 (2007). https://doi.org/10.1007/s00428-006-0347-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-006-0347-6