Abstract
We investigated tumor-free mucosa and squamous cell carcinomas of the oral cavity, the pharynx, and larynx with respect to the presence of stromal CD34+ fibrocytes and α-smooth muscle antigen (SMA)-positive myofibroblasts. Additionally, stromal expression of CD117 was analyzed. A total of 39 squamous cell carcinomas were assessed immunohistochemically. In all cases investigated, CD34+ fibrocytes were found in the tumor-free stroma, whereas α-SMA-positive myofibroblasts were lacking. Areas of lymphocytic infiltration disclosed a focal reduction of CD34+ fibrocytes. CD117 expression was absent from the tumor-free stroma. Of 39 squamous cell carcinomas, 33 were free of stromal CD34+ fibrocytes, and, in 31 carcinomas, stromal α-SMA-positive myofibroblasts occurred at least focally. CD117-positive stromal spindle cells were found in 25 carcinomas. Compared with tumor-free mucosa, the number of tissue mast cells was significantly increased in carcinomas. We conclude that stromal remodeling induced by invasive carcinomas is characterized by a loss of CD34+ fibrocytes and subsequent gain of α-SMA-positive myofibroblasts. The diagnostic impact of this finding is, however, limited by the fact that chronic inflammation may also be accompanied by a focal loss of CD34+ fibrocytes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With respect to their cellular composition, tumor-laden and tumor-free stroma are clearly distinct. These differences are widely independent of the anatomical site and histological type of carcinoma and consist of a loss of CD34+ fibrocytes and a subsequent gain of α-smooth muscle antigen (SMA)-positive myofibroblasts [2, 3, 4, 6, 12, 17]. CD34+ fibrocytes appear to be constitutive elements of the connective tissue in a multitude of anatomical sites, such as the skin [12], the breast [3, 6], the gastrointestinal tract [2, 14, 15], and the cervix [4]. In all of the aforementioned locations, the stroma of invasive carcinomas discloses a reduction or complete loss of CD34+ fibrocytes paralleled by the occurrence of α-SMA-positive myofibroblasts [2, 3, 4, 6, 12, 17]. In addition to their function as matrix-producing [5, 8] and antigen-presenting cells [7], CD34+ fibrocytes secrete platelet-derived growth factor (PDGF) [10], the receptor of which, c-kit (CD117), a protein receptor with tyrosine kinase activity, is structurally identical to that of stem cell factor (SCF).
To our knowledge, investigations concerning the occurrence of CD34+ fibrocytes in the upper aero-digestive tract and their behavior in invasive carcinomas of this anatomical site, up to now, have not been published. α-SMA-positive myofibroblasts have recently been reported in squamous cell carcinomas of the larynx [20].
We undertook the present study to analyze the stroma of tumor-free mucosa and squamous cell carcinomas of the oral cavity, the pharynx, and larynx with respect to the presence or absence, respectively, of CD34+ fibrocytes and α-SMA-positive myofibroblasts. We additionally investigated whether stromal cells express the CD117 antigen.
Materials and methods
This study comprises a total of 39 squamous cell carcinomas of the oral cavity (n=16), the oropharynx (n=13), hypopharynx (n=6), and larynx (n=4) obtained from 34 patients. The patients, 28 of which were males, ranged in age between 44 years and 96 years (arithmetic mean: 61.1 years). The stroma within the border of the invasive carcinoma was defined to be tumor associated, whereas stroma beyond the border of the tumor was regarded as tumor free. In all cases, tumor-free tissue from resection margins was available for comparison. Tissues were fixed in a 10% formalin solution, embedded in paraffin, cut and stained with hematoxylin and eosin (H&E) and periodic acid-Schiff for routine purposes. The Giemsa stain was performed to distinguish tissue mast cells from CD117-positive stromal cells.
Immunohistochemistry
Immunohistochemistry was performed using standard avidin biotin complex-peroxidase method (ABC Elite Kit; Vector, Burlingame, CA) using 3,3′-diaminobenzidine as chromogen. CD34 antigen was detected using a monoclonal antibody (QBEND10, dilution 1:50; Dako, Hamburg, Germany) after microwave pretreatment. CD117 expression was detected accordingly, using a polyclonal rabbit antibody (c-kit, dilution 1:100; Dako, Hamburg, Germany). Microwave pretreatment was performed by heating the deparaffinized and rehydrated sections, immersed in 10 mM sodium citrate buffer (pH 6.0), in a microwave oven at 600 W for 3×5 min. α-SMA was detected using a monoclonal antibody (ASM-1, dilution 1:200; Progen, Heidelberg, Germany) after tissue pretreatment with 0.1% trypsin for 15 min at 37°C.
Quantification of mast cells
In each case, the number of mast cells per HPF (i.e. high power fields at 400× microscopic magnification) was assessed in tumor-free tissue and tumor-associated stroma as the arithmetic mean mast cells in five randomly selected microscopic fields. Comparison of values was performed by means of the student’s t-test. P<0.01 was considered to be statistically significant.
Results
Tumor-free mucosa
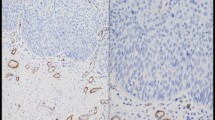
CD34+ fibrocytes characterized by an inconspicuous cytoplasm with slender elongated bipolar neurite-like projections and a small nucleus were found in the tumor-free stroma of all specimens investigated. The highest number of CD34+ fibrocytes was observed in the vicinity of vessels, around submucosal glands and adjacent to the covering epithelium (Fig. 1A). In areas with more pronounced lymphocytic infiltration, CD34+ fibrocytes were reduced in number or completely lacking. α-SMA myofibroblasts were absent from the tumor-free stroma, and CD117 expression was also not observed in the tumor-free stroma.
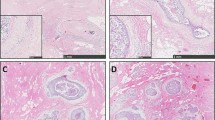
The stroma of the upper aero-digestive tract harbors diffusely scattered CD34+ fibrocytes, which are more densely packed in the subepithelial stroma (A). The stroma of invasive squamous cell carcinoma (to the upper right) is devoid of CD34+ fibrocytes (B); the infiltrating border of invasive carcinomas shows an abrupt loss of CD34+ fibrocytes. Tumor-associated spindled stromal cells are α-SMA positive (C), and 25 cases disclose CD117 positivity (D). The number of tissue mast cells in the normal mucosa (E, CD117 immunohistochemistry) is significantly lower than tumor-laden stroma (F, CD117 immunohistochemistry). In this case, stromal spindle cells were negative for CD117
Squamous cell carcinomas
The border of invasive squamous cell carcinomas was characterized by an abrupt loss of CD34+ fibrocytes, which was already visible at low microscopic magnification (Fig. 1B). In 33 cases, the stroma of invasive squamous cell carcinomas was completely devoid of CD34+ fibrocytes; 6 cases disclosed, at least, a focal loss of this cell type. Residual CD34+ fibrocytes showed no local predilection for the center or periphery of the lesion. The tumor-associated stroma was made up of diffusely scattered plump spindle-shaped α-SMA positive myofibroblasts with a characteristic tram-tracking of thick intracytoplasmatic α-SMA-reactive fibers in 12 cases. In 19 cases, these cells were focally distributed throughout the tumor stroma (Fig. 1C). The stroma of 8 carcinomas disclosed no α-SMA-positive myofibroblasts. Cytoplasmic CD117-immunoreactivity of stromal spindle cells was focal in 21 and diffuse in 4 cases; 14 cases showed no CD117 reactivity of stromal spindle cells in the tumor (Fig. 1D; Table 1).
The tumor stroma contained 23.9±9.5 (arithmetic mean ± standard deviation) mast cells per HPF, a value significantly higher than that observed in the tumor-free stroma (8.1±5.6; P<0.01) (Fig. 1E, F).
Discussion
In accordance with previous studies regarding various anatomical sites, the present investigation demonstrates a phenotypical change of the stroma associated with invasive squamous cell carcinomas of the upper aero-digestive tract, consisting of a loss of CD34+ fibrocytes paralleled by a gain of α-SMA-positive myofibroblasts. The mechanisms initiating this process are yet not well understood, but have been thought to be related to a soluble factor secreted by tumor cells [6, 18]. Experimental studies have shown that α-SMA is upregulated in CD34+ fibrocytes exposed to transforming growth factor (TGF)-β [1]. This might be the primary event of stromal remodeling, followed by a complete loss of CD34 expression, resulting in the aforementioned phenotypical alteration of tumor-associated stroma. In squamous cell carcinomas of the upper aero-digestive tract, TGF-β is upregulated [9, 13, 16], and we found tissue mast cells, another important source of TGF-β, to be significantly increased in number in the tumor stroma. The latter finding is not restricted to the upper aero-digestive tracts, since Humphreys and coworkers [11] described elevated numbers of mast cells in the stroma of cutaneous basal cell carcinomas and also suggested that mast cells might play a role in tissue remodeling associated with basal cell carcinoma. Regarding these data, it seems to be reasonable that the phenotypical change of CD34+ fibrocytes toward α-SMA-positive myofibroblasts is, at least in part, mediated by TGF-β.
In contrast to the tumor-free stroma, tumor-associated myofibroblasts were c-kit (CD117)-positive, indicating that SCF or PDGF or both might play a role in the proliferation of this cell type. Granulocyte-macrophage colony-stimulating factor (GM-CSF) is closely related to macrophage colony-stimulating factor (M-CSF), which is constitutively expressed in CD34+ fibrocytes [8, 10], downregulates CD117 expression in mast cells [19] and, as suggested by our data, in stromal myofibroblasts. In turn, upregulation of CD117 in stromal myofibroblasts in mast cells is likely to be a direct sequel of a reduction of CD34+ fibrocytes.
Phenotypical alterations of the stroma, chiefly the loss of CD34+ fibrocytes, have been considered valuable tools in distinguishing benign from malignant lesions [2, 3, 4, 17]. When applying this criterion, some precautions and recommendations should be regarded. The loss of CD34+ fibrocytes in the breast stroma is, in most cases, due to malignancy, but recent studies also described CD34-negative spindle cell proliferations in areas of previous biopsy [6]. A minority of radial scars of the breast also exhibit stromal areas negative for CD34 and positive for α-SMA [17]. In the present study, a loss of CD34+ fibrocytes was also of reduced value in detecting invasive carcinoma, since areas with lymphocytic infiltration also exhibited a loss of CD34+ fibrocytes. However, α-SMA myofibroblasts were not observed in these areas. Therefore, the diagnosis of malignancy should not solely be based on a loss of CD34+ fibrocytes.
The functional consequences of the loss of CD34+ fibrocytes, along with their value in diagnosing malignancy, remain a matter of speculation requiring further investigation. However, considering that CD34+ fibrocytes are antigen-presenting cells, their reduction or complete elimination enables an invasive tumor to escape immune-surveillance. This might constitute an important step in local tumor infiltration and distant tumor spread.
References
Abe R, Donnelly SC, Peng T, Bucala R, Metz CN (2001) Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 166:7556–7562
Barth PJ, Ebrahimsade S, Hellinger A, Moll R, Ramaswamy A (2002) CD34+ fibrocytes in neoplastic and inflammatory pancreatic lesions. Virchows Arch 440:128–133
Barth PJ, Ebrahimsade S, Ramaswamy A, Moll R (2002) CD34+ fibrocytes in invasive ductal carcinoma, ductal carcinoma in situ, and benign breast lesions. Virchows Arch 440:298–303
Barth PJ, Ramaswamy A, Moll R (2002) CD34+ fibrocytes in normal cervical stroma, cervical intraepithelial neoplasia III, and invasive squamous cell carcinoma of the cervix uteri. Virchows Arch 441:564–568
Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A (1994) Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1:71–81
Chauhan H, Abraham A, Phillips JR, Pringle JH, Walker RA, Jones JL (2003) There is more than one kind of myofibroblast: analysis of CD34 expression in benign, in situ, and invasive breast lesions. J Clin Pathol 56:271–276
Chesney J, Bacher M, Bender A, Bucala R (1997) The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A 94:6307–6312
Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R (1998) Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol 160:419–425
Hagedorn H, Elbertzhagen A, Ruoss I, Sauer U, Nerlich AG (2001) Immunohistochemical analysis of major TGF-beta isoforms and their receptors in laryngeal carcinomas. Virchows Arch 439:531–539
Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN (2001) Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J 15:2215–2224
Humphreys TR, Monteiro MR, Murphy GF (2000) Mast cells and dendritic cells in basal cell carcinoma stroma. Dermatol Surg 26:200–203
Kirchmann TT, Prieto VG, Smoller BR (1994) CD34 staining pattern distinguishes basal cell carcinoma from trichoepithelioma. Arch Dermatol 130:589–592
Koliopanos A, Friess H, di Mola FF, Tang WH, Kubulus D, Brigstock D, Zimmermann A, Buchler MW (2002) Connective tissue growth factor gene expression alters tumor progression in esophageal cancer. World J Surg 26:420–427
Nakayama H, Enzan H, Miyazaki E, Kuroda N, Naruse K, Hiroi M (2000) Differential expression of CD34 in normal colorectal tissue, peritumoral inflammatory tissue, and tumour stroma. J Clin Pathol 53:626–629
Nakayama H, Enzan H, Miyazaki E, Kuroda N, Naruse K, Kiyoku H, Toi M, Hiroi M (2001) CD34 positive stromal cells in gastric adenocarcinomas. J Clin Pathol 54:846–848
Natsugoe S, Xiangming C, Matsumoto M, Okumura H, Nakashima S, Sakita H, Ishigami S, Baba M, Takao S, Aikou T (2002) Smad4 and transforming growth factor beta1 expression in patients with squamous cell carcinoma of the esophagus. Clin Cancer Res 8:1838–1842
Ramaswamy A, Moll R, Barth PJ (2003) CD34+ fibrocytes in tubular carcinomas and radial scars of the breast. Virchows Arch 443:536–540
Soma L, LiVolsi VA, Baloch ZW (2001) Dendritic interstitial and myofibroblastic cells at the border of salivary gland tumors. Arch Pathol Lab Med 125:232–236
Welker P, Grabbe J, Zuberbier T, Grutzkau A, Henz BM (2001) GM-CSF downmodulates c-kit, FcεRIα and GM-CSF receptor expression as well as histamine and tryptase levels in cultured human mast cells. Arch Dermatol Res 293:249–258
Zidar N, Gale N, Kambic V, Fischinger J (2002) Proliferation of myofibroblasts in the stroma of epithelial hyperplastic lesions and squamous carcinoma of the larynx. Oncology 62:381–385
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barth, P.J., Schenck zu Schweinsberg, T., Ramaswamy, A. et al. CD34+ fibrocytes, α-smooth muscle antigen-positive myofibroblasts, and CD117 expression in the stroma of invasive squamous cell carcinomas of the oral cavity, pharynx, and larynx. Virchows Arch 444, 231–234 (2004). https://doi.org/10.1007/s00428-003-0965-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-003-0965-1