Abstract
In the present study, we analyzed tumor associated stromal remodeling with special respect to SPARC (secreted protein acid rich in cysteine) expression. 25 invasive ductal carcinomas of the breast and corresponding tumor-free breast tissue were studied immunohistochemically (CD34, α-SMA, SPARC and TGFβ-R1). Tumor associated stroma was characterized by a loss of CD34 expression, paralleled by a gain in α-SMA. While SPARC expression was virtually absent from normal stromal cells in the tumor stroma, strong cytoplasmic SPARC reactivity was found in the majority of stromal cells. The TGFβ-R1 also showed stronger expression in the tumor stroma compared to that of the normal breast. Stromal response to antecedent core needle biopsy was similar to that observed in the tumor stroma. We conclude that SPARC overexpression is a constant and functionally important feature of invasive ductal carcinomas, since SPARC mediates stromal de-adhesion crucial for local tumor invasion and systemic spread, respectively. When considering changes of the stromal phenotype (normal: CD34+α-SMA−SPARC− vs. carcinoma: CD34−α-SMA+SPARC+) as a tool in distinguishing benign from malignant breast lesion one has to keep in mind that the phenotype of granulation tissue in areas of antecedent biopsy resembles that of tumor stroma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stromal remodeling precipitated by invasive carcinomas is independent of the histologic type and primary tumor site, and shows uniform histological features as CD34 expression is lost in stromal cells, paralleled by a gain of α-SMA expression [3–6, 22]. CD34 is mainly found on hemopoetic precursor cells and endothelial cells [16]. In the microvasculature CD34 serves as an L-selectin ligand regulating leukocyte rolling and emigration into tissue [16]. On the other side, the function and significance of CD34 on stromal cells referred to as CD34+ fibrocytes [8, 11] has up to now rarely been addressed and therefore remains enigmatic. However, CD34+ fibrocytes constitute an important element of the connective tissue of various organs [3–6, 18, 19, 22, 27]. Although it remains to be clarified whether all CD34+ fibrocytes histologically detected in the connective tissue derive from circulating cells, it appears reasonable to assume that stromal CD34+ fibrocytes are at least closely related to circulating CD34+ fibrocytes originally described by Bucala and coworkers in 1995 [8]. CD34+ fibrocytes are capable of tissue matrix synthesis [11, 14] and have been shown to be potent antigen-presenting cells [10]. A loss of CD34+ fibrocytes as described in most carcinomas might therefore favor local tumor invasion and subsequent systemic spread.
Stromal remodeling in invasive carcinomas is certainly an important prerequisite of local tumor invasion and distant spread. TGFβ has been reported to induce α-SMA up-regulation in fibrocytes, and might therefore play a role in tumor associated stromal remodeling [1]. SPARC (secreted protein acid rich in cysteine) also devised as osteonectin or BM-40 belongs to the group of matricellular proteins and has been claimed as a member of the invasion associated cluster [15, 24]. We therefore undertook the present study to relate TGFβ-R and SPARC expression in stromal cells to the phenotypic alterations in malignancy induced stromal remodeling.
Materials and methods
The present study comprises a total of 25 breast biopsies obtained exclusively from women with proven invasive ductal carcinoma. The epidemiological data of the patients are summarized in Table 1, together with the major tumor characteristics.
After resection, tissues were fixed in a 10% formalin solution, embedded in paraffin, cut and stained H&E and PAS for routine purposes. In all cases tumor free tissue from resection margins was available for comparison. The site of previous core needle biopsy was included in the specimens in 14 cases.
Immunohistochemistry
CD34, α-SMA, SPARC and the TGFβ-R1 were detected immunohistochemically by means of the standard avidin biotin complex (ABC)-peroxidase method (ABC Elite Kit; Vector, Burlingame, Calif., USA) using 3,3′-diaminobenzidine (DAB) as chromogen. The primary antibodies and type of tissue pre-treatment are listed in Table 2. Microwave pretreatment was performed by heating the deparaffinized and rehydrated sections, immersed in 10 mM sodium citrate buffer (pH 6.0), in a microwave oven at 600 W for 3 or 4 times 5 min (see Table 2).
Double labeling of CD34 and TGFβ-R
For double labeling of CD34 and TGFβ-R1 sections were pre-treated by micro-wave heating for 4 times 5 min as described above. Thereafter TGFβ-R1 was detected by means of the ABC-alkaline phosphatase method (ABC-AP Kit; Vector, Burlingame, CA). As primary antibody, a monoclonal antibody against TGFβ-R1 (IgM, Novocastra, dilution 1:10 in PBS containing 1% horse serum) was used. Thereafter, sections were incubated with a biotinylated horse anti mouse IgM antibody (1:100 in PBS) followed by incubation with the avidin-biotin alkaline phosphatase complex. Histo Red (Linaris, Wertheim-Bettingen, Germany) was used as chromogen according to the instructions of the manufacturer. After rinsing in PBS for 5 min, CD34 was detected according to the ABC-peroxidase method using the Qbend10 (IgG1a) antibody at a dilution of 1:40 as primary antibody. Thereafter a biotinylated horse anti mouse IgG1 (1:100 in PBS) was applied and finally sections were incubated with the ABC peroxidase complex (VECTASTAIN Elite ABC Kit, Vector). Histo Green (Linaris) was used as chromogen according to the instructions of the manufacturer and resulted in a green color reaction, whereas the TGFβ-R1 stained red.
Semi-quantitative assessment of immunohistochemistry
Immunoreactivity of CD34, α-SMA and SPARC was assessed semi-quantitatively in the tumor free tissue, in the center and periphery of the tumor. The percentage of stromal cells expressing the respective antigen was graded as “0”, “+”, “++” and “+++” when up to 5%, more than 5% and up to 25%, more than 25% and up to 50% or more than 50% of stromal cells, respectively, disclosed immunoreactivity. Percentages were assessed by two independent observers, assuming that a microscopic high power field (objective 40x, microscopic magnification: ×400) harbors 100 stromal cells (range: 75–150). Immunohistochemical expression of TGFβ-R1 was assessed qualitatively as positive or negative.
Statistical analyses
Comparison of groups (tumor-free versus tumor periphery; tumor periphery versus tumor border) was performed using the χ2-test defining P<0.01 to be statistically significant.
Results
Tumor free breast tissue
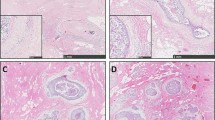
In the tumor free breast tissue CD34+ fibrocytes were predominantly found in the lobular stroma, forming a dense network that surrounded ducts and acini (Fig. 1a). Most CD34+ fibrocytes showed bipolar slender cytoplasmic processes which appeared to communicate with neighbouring CD34+ fibrocytes. In the extralobular stroma, CD34+ fibrocytes were found in the adventitia of small arteries and arterioles and few CD34+ fibrocytes were detected in the collagen rich stroma remotely located from vessels. The tumor free stroma was devoid of α-SMA positive myofibroblasts. Immunohistochemically SPARC expression was found in myoepithelial cells and in endothelial cells of arterioles and capillaries (Fig. 1b, Table 3). In nine cases, a small population of stromal cells making up less then 5% of stromal cells were weakly positive for SPARC, in the remaining cases, stromal cells showed no SPARC immunoreactivity. TGFβ-R1 immunoreactivity was strong in lymphocytes, myoepithelial cells, a subpopulation of ductal and acinar epithelia and few intralobular fibrocytes (Fig. 1c). Double labeling of the TGFβ-R1 and CD34 showed the bipolar processes of CD34+ fibrocytes to be reactive with CD34, whereas the body of the cells exhibited positivity for TGFβ-R1 (Fig. 1d).
The tumor-free lobular stroma consists of densely packed CD34+ fibrocytes, the extralobular stroma discloses scattered CD34+ fibrocytes (a). SPARC is immunohistochemically expressed in myoepithelial cells, few stromal cells and endothelial cells (b). TGFβ−R1 immunohistochemistry shows membrane staining of acinar epithelia and intra- and extralobular stromal cells (c). Double labeling shows co-expression of CD34 (green) and TGFβ−R1 (red, see arrows) in intralobular fibrocytes (d). The tumor associated stroma contains α-SMA positive myofibroblasts (e) that also show cytoplasmic SPARC immunoreactivity (f). Double labeling of the tumor tissue shows CD34 positivity (green) of endothelial cells, a loss of CD34+ fibrocytes and strong TGFβ−R1 immunoreactivity (red) (g)
Invasive ductal carcinomas
The tumor associated stroma was virtually free of CD34+ fibrocytes and the interface between tumor-free and tumor associated stroma showed an abrupt loss of CD34+ fibrocytes. CD34+ fibrocytes at the tumor border were morphologically identical to those located remotely from the tumor. In four cases, few residual CD34+ fibrocytes were found in the tumor periphery, making up less than 5% of the stromal cells. The tumor associated stroma consisted of plump myofibroblasts showing intense cytoplasmic α-SMA immunoreactivity mostly displaying a tram track pattern (Fig. 1e). In all cases, stromal cells also showed strong cytoplasmic staining for SPARC (Fig. 1f). Comparison of the tumor center and periphery disclosed no statistically significant differences in α-SMA- and SPARC expression, respectively (Table 3). TGFβ-R1 immunoreactivity of tumor associated myofibroblasts was more intense compared to that observed in CD34+ fibrocytes of the tumor free stroma (Fig. 1g).
In areas of antecedent biopsy, the granulation tissue showed a phenotype similar to that of the tumor stroma as the stromal cells showed strong expression of α-SMA and SPARC accompanied by a negative reaction of CD34.
Discussion
Porter and coworkers were the first to report SPARC expression in tumor associated stromal cells and assumed that SPARC expression “might contribute to some aspects of tumor progression” [21]. In the present study, stromal remodeling associated with invasive breast cancer, characterized by a loss of CD34 expression paralleled by a gain of α-SMA, is strongly related to an increased expression of SPARC (secreted protein acid rich in cysteine). The regulation of SPARC synthesis and secretion is elusive, but recent investigations have shown that TGFβ1 increases the synthesis of SPARC in stromal cells [2, 23] while SPARC vice versa interferes with the TGFβ1 signaling system [25]. These findings suggest a close functional relation of SPARC and TGFβ1 which may in part be reflected by a similar cellular distribution pattern observed in the present study in that, SPARC and the TGFβ-R1 are predominantly found in myoepithelial cells and few lobular stromal fibrocytes of the normal breast.
SPARC exerts three major functions: anti-proliferation, de-adhesion and regulation of ECM synthesis [7]. De-adhesion of the tumor associated stroma is defined as an intermediate state of adhesion caused by restructuring of focal adhesions and stress fibers mediated by matricellular proteins such as SPARC [17] and constitutes a prerequisite for tumor cell migration and tissue invasion [13]. Moreover, de-adhesion leads to alterations of the cytoskeleton in part characterized by synthesis of stress fibers, which histologically is reflected by the abundance of α-SMA positive myofibroblasts. In addition, TGFβ1 has also been reported to directly increase α-SMA expression in fibrocytes [1]. On the other side, the loss of CD34 expression in stromal cells can less easily be explained. In primary cultures of fibrocytes, a progressive time dependent loss of CD34 expression was observed [12, 20, 26]. Moreover, in vitro differentiation of CD34+ fibrocytes from CD14+ monocytes requires direct T-cell interaction, indicating that cell-cell contacts are crucial for the development and maintenance of CD34 expression in fibrocytes [1]. Therefore it appears to be reasonable that the loss of CD34 expression in stromal cells is at least in part due to a reduction and modification of cell-cell contacts mediated by SPARC. This assumption might additionally explain the phenotypic similarity of tumor stroma and granulation tissue. Chauhan and coworkers were the first to point out that stromal remodeling in carcinomas closely resembles granulation tissue and woung healing observed at sites of antecedent biopsy [9]. This is in keeping with the findings of the present study. Moreover, benign complex lesions of the breast such as radial scars are characterized by a focal loss of CD34+ fibrocytes [22]. Thus, when considering the detection of CD34+ fibrocytes as a tool in distinguishing benign from malignant breast lesions, it has to be kept in mind that a loss of this cell population does not automatically indicate malignancy. On the other side, the presence of CD34+ fibrocytes excludes malignancy with a high degree of certainty [3, 9, 22].
References
Abe R, Donnelly SC, Peng T, Bucala R, Metz CN (2001) Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol 166:7556–7562
Abe K, Hibino T, Mishima H, Shimomura Y (2004) The cytokine regulation of SPARC production by rabbit corneal epithelial cells and fibroblasts in vitro. Cornea 23:172–179
Barth PJ, Ebrahimsade S, Ramaswamy A, Moll R (2002) CD34+ fibrocytes in invasive ductal carcinoma, ductal carcinoma in situ, and benign breast lesions. Virchows Arch 440:298–303
Barth PJ, Ebrahimsade S, Hellinger A, Moll R, Ramaswamy A (2002) CD34+ fibrocytes in neoplastic and inflammatory pancreatic lesions. Virchows Arch 440:128–133
Barth PJ, Ramaswamy A, Moll R (2002) CD34+ fibrocytes in normal cervical stroma, cervical intraepithelial neoplasia III, and invasive squamous cell carcinoma of the cervix uteri. Virchows Arch 441:564–568
Barth PJ, Schenck zu Schweinsberg T, Ramaswamy A, Moll R (2004) CD34+ fibrocytes, α–smooth muscle antigen-positive myofibroblasts, and CD117 expression in the stroma of invasive squamous cell carcinomas of the oral cavity, pharynx, and larynx. Virchows Arch 444:231–234
Bradshaw AD, Sage EH (2001) SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J Clin Invest 107:1049–1054
Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A (1994) Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1:71–81
Chauhan H, Abraham A, Phillips JR, Pringle JH, Walker RA, Jones JL (2003) There is more than one kind of myofibroblast: analysis of CD34 expression in benign, in situ, and invasive breast lesions. J Clin Pathol 56:271–276
Chesney J, Bacher M, Bender A, Bucala R (1997) The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA 94:6307–6312
Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R (1998) Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol 160:419–425
Espana EM, Kawakita T, Liu CY, Tseng SC (2004) CD-34 expression by cultured human keratocytes is downregulated during myofibroblast differentiation induced by TGF-beta1. Invest Ophthalmol Vis Sci 45:2985–2991
Framson PE, Sage EH (2004) SPARC and tumor growth: where the seed meets the soil? J Cell Biochem 92:679–690
Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN (2001) Fibrocytes induce an angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J 15:2215–2224
Iacobuzio-Donahue CA, Argani P, Hempen PM, Jones J, Kern SE (2002) The desmoplastic response to infiltrating breast carcinoma: gene expression at the site of primary invasion and implications for comparisons between tumor types. Cancer Res 62:5351–5357
Khan AI, Landis RC, Malhotra R (2003) L-Selectin ligands in lymphoid tissues and models of inflammation. Inflammation 27:265–280
Murphy-Ullrich JE (2001) The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest 107:785–790
Nakayama H, Enzan H, Miyazaki E, Kuroda N, Naruse K, Hiroi M (2000) Differential expression of CD34 in normal colorectal tissue, peritumoral inflammatory tissue, and tumour stroma. J Clin Pathol 53:626–629
Nakayama H, Enzan H, Miyazaki E, Kuroda N, Naruse K, Kiyoku H, Toi M, Hiroi M (2001) CD34 positive stromal cells in gastric adenocarcinomas. J Clin Pathol 54:846–848
Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM (2004) Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 114:438–446
Porter PL, Sage EH, Lane TF, Funk SE, Gown AM (1995) Distribution of SPARC in normal and neoplastic human tissue. J Histochem Cytochem 43:791–800
Ramaswamy A, Moll R, Barth PJ (2003) CD34+ fibrocytes in tubular carcinomas and radial scars of the breast. Virchows Arch 443:536–540
Reed MJ, Vernon RB, Abrass IB, Sage EH (1994) TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J Cell Physiol 158:169–179
Ryu B, Jones J, Hollingsworth MA, Hruban RH, Kern SE (2001) Invasion-specific genes in malignancy: serial analysis of gene expression comparisons of primary and passaged cancers. Cancer Res 61:1833–1838
Schiemann BJ, Neil JR, Schiemann WP (2003) SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-β-signaling system. Mol Biol Cell 14:3977–3988
Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S (2003) Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 171:380–389
Soma L, LiVolsi VA, Baloch ZW (2001) Dendritic interstitial and myofibroblastic cells at the border of salivary gland tumors. Arch Pathol Lab Med 125:232–236
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barth, P.J., Moll, R. & Ramaswamy, A. Stromal remodeling and SPARC (secreted protein acid rich in cysteine) expression in invasive ductal carcinomas of the breast. Virchows Arch 446, 532–536 (2005). https://doi.org/10.1007/s00428-005-1256-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-005-1256-9