Abstract
Arthropods show two kinds of developmental mode. In the so-called long germ developmental mode (as exemplified by the fly Drosophila), all segments are formed almost simultaneously from a preexisting field of cells. In contrast, in the so-called short germ developmental mode (as exemplified by the vast majority of arthropods), only the anterior segments are patterned similarly as in Drosophila, and posterior segments are added in a single or double segmental periodicity from a posterior segment addition zone (SAZ). The addition of segments from the SAZ is controlled by dynamic waves of gene activity. Recent studies on a spider have revealed that a similar dynamic process, involving expression of the segment polarity gene (SPG) hedgehog (hh), is involved in the formation of the anterior head segments. The present study shows that in the myriapod Glomeris marginata the early expression of hh is also in a broad anterior domain, but this domain corresponds only to the ocular and antennal segment. It does not, like in spiders, represent expression in the posterior adjacent segment. In contrast, the anterior hh pattern is conserved in Glomeris and insects. All investigated myriapod SPGs and associated factors are expressed with delay in the premandibular (tritocerebral) segment. This delay is exclusively found in insects and myriapods, but not in chelicerates, crustaceans and onychophorans. Therefore, it may represent a synapomorphy uniting insects and myriapods (Atelocerata hypothesis), contradicting the leading opinion that suggests a sister relationship of crustaceans and insects (Pancrustacea hypothesis). In Glomeris embryos, the SPG engrailed is first expressed in the mandibular segment. This feature is conserved in representatives of all arthropod classes suggesting that the mandibular segment may have a special function in anterior patterning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A characteristic hallmark of the arthropods is that their bodies are composed of serially homologous units, the segments. In most arthropods, the so-called short germ developing species, which include all chelicerates, myriapods, and crustaceans and the majority of insects, anterior segments are patterned and formed from a preexisting field of cells. Posterior segments are added from a posterior located segment addition zone (SAZ) in a single or double segmental periodicity (Chipman et al. 2004a; Schoppmeier and Damen 2005; Janssen et al. 2011a; Sarrazin et al. 2012). Only in some derived groups of insects, including the model arthropod Drosophila melanogaster, are all segments patterned from a preexisting field of cells and these animals therefore lack a classical SAZ (e.g. Davis and Patel 2002; Damen 2007). It has been suggested that the anterior patterning system may have extended successively towards posterior and eventually replaced the SAZ in long germ arthropods (Peel and Akam 2003).
Patterning of the Drosophila embryo is achieved by the action of the well-understood hierarchic segmentation gene cascade in which gradients of maternal effect genes control the spatial expression of gap genes; these then activate pair rule genes in a double segmental pattern. Different combinations of pair rule genes then activate the segment polarity genes (SPGs) that are expressed in a segmental pattern and define segment polarity and boundaries (St. Johnston and Nüsslein-Volhard 1992; Akam 1987). The regulatory network and function of the SPGs were shown/suggested to be conserved also in other arthropods (e.g. Oppenheimer et al. 1999; Damen 2002; Miyawaki et al. 2004; Sánchez et al. 2008; Farzana and Brown 2008; Janssen et al. 2008). Despite the fact, however, that comparative data on SPG expression and function are available from numerous arthropods including insects, crustaceans, chelicerates and myriapods, comprehensive data sets of SPGs are restricted to few model organisms such as the fly Drosophila (e.g. Sanson 2001), the beetle Tribolium (e.g. Farzana and Brown 2008), the spiders Achaearanea (Schwager et al. 2009; Pechmann et al. 2009; Akiyama-Oda and Oda 2010; Kanayama et al. 2011) and Cupiennius (Damen 2002; Prpic and Damen 2005; Prpic et al. 2003) and the myriapod Glomeris (Janssen et al. 2004, 2008; Prpic et al. 2005). In most cases, data are restricted to the expression of engrailed (en) and wingless (wg), the two most intensively studied SPGs (e.g. Hughes and Kaufman 2002; Chipman et al. 2004b; Prpic 2008; O’Donnell and Jockusch 2010). The vast majority of work concerns the expression and function of SPGs during terminal addition rather than anterior patterning. Similarly, only few data are available on the early expression of hedgehog (hh), which is an important component of the conserved SPG network. Most data are restricted to later stages and the appearance of hh expression during posterior segment addition, or in long germ insects at later developmental stages (e.g. Simonnet et al. 2004; Janssen et al. 2004; Dearden et al. 2006). Alternatively, they are inconclusive with respect to the precise spatiotemporal appearance within the anterior embryo. Our knowledge of SPG expression and function during anterior body patterning in arthropods is thus generally lagging behind the profound knowledge that has been gathered on SPGs’ action during terminal addition of segments. Two recent studies, however, investigated the early expression of hh in the anterior head region of the spider Achaearanea (Pechmann et al. 2009; Kanayama et al. 2011). Interestingly, it was found that hh is expressed in a dynamic pattern in the anterior head. This represented a novel feature not reported previously for any SPG in the anterior “preexisting field of cells” in any arthropod. It thus described a new developmental mode in anterior patterning. The splitting of the most anterior segments and the dynamic expression patterns involved are at least similar to posterior patterning of the segments that are generated from the SAZ.
The delayed expression of en in the rudimentary intercalary/premandibular (=tritocerebral) segment in insects and Glomeris already implies that this segment is likely not patterned together with the ocular region and the first antennal segment as, in contrary, is the case for spiders (e.g. Janssen et al. 2004; Chipman et al. 2004b; Miyawaki et al. 2004; O’Donnell and Jockusch 2010). However, in the anterior head segments of Drosophila and other arthropods, hh expression precedes expression of en (and wg) (e.g. Mohler 1995; Brown et al. 1994; Kanayama et al. 2011). This leaves the possibility that the most anterior head segments in Glomeris may be patterned via hh but without having an effect upon the conservative stripe-by-stripe appearance of other SPGs.
A specific intent of this work was therefore to investigate the early expression of hh during the patterning of the anterior head segments in a basally branching mandibulate arthropod to reveal whether the recently discovered anterior patterning mechanism in a spider may represent an ancestral feature of arthropods. Another, more general goal was to conduct an exhaustive investigation of the spatiotemporal expression of SPG in the anterior blastoderm of a non-insect arthropod. Special focus concerned the spatial patterning of the reduced premandibular (tritocerebral) segment. It appears that all SPGs are expressed with delay in this segment, a feature that is shared with the insects. This finding is discussed with respect to arthropod phylogeny.
Material and methods
Species husbandry, gene cloning, in situ hybridization, nuclei staining and documentation techniques
The handling of Glomeris marginata is described by Janssen et al. (2004). After oviposition, embryos were allowed to develop at room temperature. Staging follows Dohle (1964) and Janssen et al. (2004). The developmental stage of all embryos was determined by using 4′-6-diamidino-2-phenylindole (DAPI). Cloning and sequence analysis of the Glomeris segment polarity genes has been described by Janssen et al. (2004, 2008, 2010). Single whole mount in situ hybridization was performed as described in Prpic and Tautz (2003). Embryos were analyzed under a Leica dissection microscope equipped with either an Axiocam (Zeiss) or a Leica DC100 digital camera. Brightness, contrast and colour values were corrected (linear transformations only) in all images using the image processing software Adobe Photoshop CS2 (version 9.0.1 for Apple Macintosh).
Results
Early expression of hedgehog and patched in the regio germinalis
In Glomeris, all anterior segments including the first trunk segment (T1) are formed from a preexisting field of cells that is recruited from the blastoderm. This is the so-called regio germinalis. All segments posterior to T1 are added sequentially as single segments from the posterior segment addition zone (SAZ) and that despite the presence of so-called diplosegments in the posterior of the embryo (Dohle 1964; Janssen 2011).
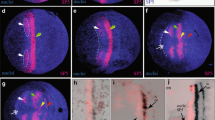
At stage 0 (blastoderm stage), Glomeris hedgehog is expressed in the anlagen of the hindgut in the very posterior of the developing embryo (Fig. 1c). Shortly later, hh appears as a broad band covering the anlagen of the future ocular region and the antennal segment. At the same time, expression appears as a single stripe in the mandibular segment (Fig. 1a, c). Note that at the same developmental stage engrailed (en) is not expressed in a comparable anterior domain, but that its most anterior expression lies in the mandibular segment (Fig. 1b) (Janssen et al. 2004). Soon after its appearance in the broad ocular/antennal domain, hh expression is cleared from the centre of this domain, but expression at the anterior and posterior rim of the domain is enhanced (Fig. 1d). This enhanced expression represents the remaining expression as seen at stage 0.2 in the ocular region and the antennal segment, respectively (Fig. 1e). At the same time, de novo expression appears in the maxillary and the first trunk segment (T1). The latter represents the last segment of the regio germinalis, the anterior field of cells. At stages 0.3, the postmaxillary stripe and the T2 stripe appear together with weak expression in the prospective hindgut (Fig. S1). This expression becomes stronger in the subsequent stage 0.4 (Fig. 1f). Note the gap of expression between the two gut primordia (white arrowhead in Fig. 1f). Slightly later at stage 0.5, the premandibular stripe of hh expression becomes first detectable (Fig. 1g). At the same time, a third trunk stripe (T3) forms. At stage 1.2, a fourth trunk stripe appears, and in the anterior embryo, de novo expression appears in a region that will later give rise to the labrum. At this stage, expression of the prospective gut primordia has fused (Fig. 1h).
Early expression of Glomeris hedgehog and engrailed. a Stage 0.1. Expression of hh in a solid broad anterior domain corresponding to the future ocular and antennal regions (cf. Fig. 1d showing expression in a slightly older embryo), and the mandibular (md) segment. Expression in the future hindgut (hg). b Stage 0.1. At the same stage when hh is expressed in a broad anterior domain, engrailed expression is restricted to the mandibular segment, and weakly to the first trunk segment (T1). c Blastoderm stage (stage 0). Glomeris hh is exclusively expressed posterior to the SAZ in the future hindgut (hg). d Stage 0.1. hh is now expressed also in a broad domain corresponding to the ocular region (oc) and the antennal segment (an), and in the mandibular segment (md). Note that in the shown embryo the anterior domain is already in the process of splitting. Expression in the ocular (anterior) region of the domain disappears ventrally (black arrowhead). e Stage 0.2. Expression in the maxillary segment (mx) and first trunk segment (T1) appears. The oc/an stripe(s) are fully separated. f Stage 0.4. Expression appears in the postmaxillary segment (pmx). The future T2 and T3 segments segregate from the SAZ and express hh. Expression in the invaginating hindgut (asterisk) and the anal rim (white arrowhead). g Stage 0.5. Not before now hh appears in the premandibular segment (pmd) (arrow). Expression in T3. h Stage 1.2. All anterior segments now express hh. Arrow as in e. Additionally, hh is expressed in an anterior median position where the labrum will form (asterisk). c′–h′ DAPI stainings of the embryos shown in c to h
Expression of patched (ptc) occurs in the same spatiotemporal order as hh in the different segmental primordia of the regio germinalis: at stage 0.1, expression is in a broad domain covering the ocular region and the antennal segment. A transverse stripe is visible in the mandibular segment and the hindgut anlagen (Fig. 2a). At stage 0.2, expression appears in the maxillary and T1 segments (and very faintly also in T2), and expression in the ocular/antennal region disappears ventrally (Fig. 2b). Already at this time, the characteristic double stripes form in each segment by the disappearing of expression from the centre of the developing segmental stripes (cf. Janssen et al. 2008). At stage 0.4, all stripes in the regio germinalis, except the premandibular stripe, have formed; the T2 stripe has now clearly appeared (Fig. 2c). At the subsequent stage 0.5, the premandibular stripe appears. Notably, this expression does not appear as a broad stripe that later splits, but directly as two stripes (Fig. 2d). Somewhat later, this (double) stripe is strongly expressed (Fig. 2e).
Early expression of Glomeris patched. All embryos are oriented with anterior to the left. a Stage 0.1. Like hh, also ptc is expressed strongly in a broad anterior domain corresponding to the ocular (oc) and antennal (an) segments, and weakly also in the mandibular (md) segment and in the posterior most area of the germ band that will later invaginate and form the hindgut (hg). b Stage 0.2. The broad oc + an-expressing domain splits and the ptc-typical segmental double-stripes form. Expression in the maxillary (mx) segment and the first trunk segment (T1) appears. c Stage 0.4. The postmaxillary (pmx) stripe and the T2 stripe appear. Note that the premandibular (pmd) stripe has not yet appeared (asterisk). d Stage 0.5. Faint expression in the pmd appears (arrow). The T3 stripe has appeared. e Stage 1. Expression in the pmd is now clearly visible. Arrow as in d
Early expression of wingless, cubitus interruptus and Notum in the regio germinalis
Compared to hh and ptc, the appearance of wingless (wg) is delayed in the anterior segments (Fig. 3). At stage 0.1, the earliest expression is in a ring around the anlagen of the hindgut, the later anal valves (Fig. 3a). At stage 0.2, expression appears in the ocular region; this expression is not in one transverse stripe but as two distinct patches in the developing eyes (Fig. 3b). At stage 0.3, faint expression of wg appears in the mandibular segment and in the developing antennae (Fig. 3c). At stage 0.5, expression appears in the maxillary segment (Fig. 3d), and at stage 1, expression appears in the postmaxillary, T1 and T2 segments (Fig. 3e). Finally, at stage 1.1, expression appears in the premandibular segment and weakly also in T3 (Fig. 3f).
Early expression of Glomeris wingless. All embryos are oriented with anterior to the left. a Stage 0.1. Expression is in a ring in the future anal valves (av) surrounding the hindgut primordium. b Stage 0.2. Expression appears in the ocular region (oc). c Stage 0.3. Expression in the ocular region (oc), the antennal primordia (an) and the mandibular segment (md). d Stage 0.5. No expression in the premandibular segment (black asterisk), but in the anterior adjacent antennal (an) segment and the posterior adjacent mandibular (md) and maxillary (mx) segments. The white asterisk marks a piece of vitelline membrane (non-specifically stained). e Stage 1. All segments of the regio germinalis, except the premandibular segment (asterisk) express wg. f Stage 1.1. Now expression is detectable also in the premandibular segment (pmd) (arrow)
Expression of Glomeris cubitus interruptus (ci) is difficult to document at very early stages because it is almost below detectable levels. At approximately stage 0.1, expression is detectable as a transverse stripe in the ocular region and two broader transverse stripes corresponding to the premandibular + mandibular (pmd + md) and maxillary + postmaxillary (mx + pmx) segments. Expression in T1 is in a single-spaced transverse stripe (Fig. 4a). Very faint expression is detectable in the SAZ. Slightly later expression in the ocular region disappears from ventral tissue. Expression in T2 appears (Fig. 4b). The double stripes corresponding to pmd + md and mx + pmx begin to split at stage 0.4 (Fig. 4c), and soon after appear as single stripes (Fig. 4d). At this point, expression is present in both the antennal segment and the ocular region (Fig. 4d); the former is likely to have appeared de novo as indicated by the space seen at earlier stages between the most anterior expression (in the ocular region) and the splitting pmd + md stripe (cf. Fig. 4c, d).
Early expression of Glomeris cubitus interruptus. All embryos are oriented with anterior to the left. Note that staging of early-stage ci-stained embryos is difficult due to quenching of the DAPI signal by the fuzzy expression of ci. a Approximately stage 0.1 embryo. Very faint expression is in all future segments of the regio germinalis except the antennal segment (white asterisk), and the segment addition zone (SAZ). Black asterisk and black filled circle mark future premandibular + mandibular (pmd + md) and maxillary + postmaxillary (mx + pmx) tissue, respectively. b Approximately stage 0.3. Clear expression is now in the ocular region (oc), and the first two trunk segments (T1 and T2). Weak expression in broad domains corresponding to pmd + md and mx + pmx tissue. c Approximately stage 0.5. Intensity of expression increases, and former broad domains begin to split into distinct stripes. d The complete set of single-segmental stripes has formed
Notum expression appears simultaneously in the antennal primordia, the mandibular segment, the maxillary segment and weakly in the postmaxillary segment, and T1 at approximately stage 0.4 (Fig. 5a). At the subsequent stage, the premandibular stripe appears, and expression in all segments except the postmaxillary segment becomes clearer. Additionally, expression is now in the forming hindgut (Fig. 5b). At stage 1, expression in the postmaxillary segment becomes stronger, and expression in T2 appears (Fig. 5c).
Early expression of Glomeris Notum. All embryos are oriented with anterior to the left. a Approximately stage 0.4. Notum is expressed in the primordia of all anterior segments except the premandibular segment (asterisk). b Approximately stage 0.5. Expression appears in the premandibular segment (arrow) and the hindgut (arrowhead). c Stage 1. an antennal segment, md mandibular segment, mx maxillary segment, oc ocular region, pmx postmaxillary segment, T1 first trunk segment
Discussion
Splitting of a broad anterior hedgehog-positive domain is involved in the generation of the ocular region and the antennal segment
Two recent publications on hh signaling in the spider Achaearanea tepidariorum described dynamic expression of hh during patterning of the three most anterior head segments (regions), the ocular region, the chelicerae-bearing segment and the pedipalpal segment (Pechmann et al. 2009; Kanayama et al. 2011). The latter two segments are homologous to the first antennal and intercalary/premandibular segment in mandibulate arthropods (Telford and Thomas 1998; Damen et al. 1998; Janssen and Damen 2006). Dynamic gene expression including splitting domains and wave-front like translocation of expression domains has not previously reported for any segment polarity gene (SPG) during patterning of the anterior segments. The new data reveal that a similar pattern of splitting of a broad anterior hh stripe is also present in the millipede Glomeris (Fig. 6). However, in the spider, but not in Glomeris, the initial domain of hh-positive cells undergoes two splitting events; the first gives rise to the pedipalpal hh expression, and the second results in one stripe of expression in the cheliceral segments and one stripe of expression in the ocular region. In contrast, in Glomeris, a broad anterior stripe of hh splits only once giving rise to single stripes in the ocular region and the antennal segment (Fig. 6). Another striking difference is that in the spider, but not in the millipede, the anterior domain of hh appears in the very anterior of the developing embryo and later shifts to a more posterior position, while in Glomeris, the anterior domain directly appears in some distance from the anterior pole of the germ band.
Schematic representation of anterior hh stripe formation in the spider A. tepidariorum and the millipede Glomeris marginata. Grey circles represent embryos of Achaearanea (upper row) and Glomeris (lower row). Anterior is up. Dark grey represents the segment addition zone (SAZ). Black lines in embryos mark hh expression; red lines in embryos mark expression of hh in the pedipalpal (in the spider) and premandibular (in the millipede) segments. Rows of pointing down arrowheads represent direction and mechanism of splitting off of hh stripes from a broader domain of expression. a Broad anterior expression of hh corresponding to the later ocular region (oc), the chelicerae-bearing segment (ch) and the pedipalpal segment (pp). b The pp stripe splits off from the broad domain. c A new broad domain forms corresponding to future oc and ch. d The ch stripe splits off from the broad anterior domain resulting in three distinct stripes of hh expression in oc, ch and pp (not shown). e Broad anterior expression of hh corresponding to the later ocular region (oc) and antennal segment (an). f The anterior domain splits into two. g Two distinct stripes of hh in oc and an. h De novo appearance of hh expression in the premandibular segment. L1–L4 primordia of the four walking limb-bearing segments, md-T1 primordia of the mandibular to first trunk segments, pmd-T1 primordia of the premandibular to first trunk segments
Spatiotemporal appearance of Glomeris engrailed (en), hedgehog (hh), patched (ptc) and wingless (wg) in the regio germinalis. Expression in the stages 0 to 0.5 is shown. en is in red, hh is in blue, ptc is in green and wg is in yellow. Note that expression of en appears very early in the mandibular (md) segment (red asterisk) (cf. Fig. S2). Expression of wg in the premandibular (pmd), the postmaxillary (pmx) and first trunk (T1) segments is not shown as a bar, but the stage is given at which expression of this gene appears first. an antennal segment primordium, mx maxillary segment primordium, oc ocular region
Until now, informative data on the early expression of hh in the most anterior head segments were restricted to the insects Drosophila (Mohler and Vani 1992; Tabata et al. 1992; Tashiro et al. 1993), Tribolium (Farzana and Brown 2008) and Gryllus (Miyawaki et al. 2004), and indeed the spider Achaearanea (Pechmann et al. 2009; Kanayama et al. 2011). In Drosophila, a broad (two to three cells wide) anterior domain of hh expression covers the region where the anlagen of the ocular region and the antennal segment lie (Jürgens et al. 1986; Mohler and Vani 1992; Tabata et al. 1992; Tashiro et al. 1993). Despite the unfortunate circumstance that the earliest expression of Drosophila hh is not described in great detail, position and width (two to three cells compared to the other hh stripes that are only one cell wide) of the anterior stripe suggest that it may represent later expression in the eyes and antennae (discussed in Ntini and Wimmer 2011a). For both short germ insects, Tribolium and Gryllus, de novo appearance of the antennal hh stripe is described after the initial appearance of hh in the ocular region (Farzana and Brown 2008; Miyawaki et al. 2004). However, the presented data on Gryllus hh give rise to the impression that the earliest expression domain is indeed corresponding to both the ocular region and the prospective antennal primordium (but note that the authors (Miyawaki et al. 2004) interpret their data differently and suggest that the antennal hh stripe appears later and de novo). The same may be true for hh expression in Tribolium. Although the antennal hh stripe is described as being formed de novo, it forms (or splits off from an oc/an stripe?) in very close proximity to the antennal stripe, and is even connected to the former at some points (Farzana and Brown 2008 (their Fig. 1a, b)). Taken together, these data suggest that the early double-segment wide expression of hh may be a conserved character in at least myriapods and insects. To further explore the evolution of anterior SPG patterning, it will be necessary to re-investigate early hh expression in insects and crustaceans. It will be interesting to see if the ocular + antennal domain of hh is also conserved in crustaceans. If not, it may represent a possible synapomorphy supporting the traditional Atelocerata hypothesis that unites insects and myriapods (discussed below). Furthermore, hh expression must be studied in the closest relatives of the extant arthropods, the tardigrades and onychophorans (e.g. Dunn et al. 2008; Edgecombe 2010; Rota-Stabelli et al. 2010; Campbell et al. 2011). At least with respect to engrailed and wingless expression, a splitting mechanism is apparently not conserved in onychophorans (Eriksson et al. 2009). Here, the earliest expression is in the jaw and slime papilla-bearing segments, homologs of the mandibulate first antenna and intercalary/premandibular segment (Eriksson et al. 2010). Unfortunately, data on the more interesting (in this context) hh gene are neither available for any onychophoran nor tardigrade species.
Does delayed SPG patterning of the tritocerebral segment in insects and a myriapod represent a synapomorphy for the Atelocerata, or a case of convergent evolution?
In Drosophila, the most anterior segments are not under the control of the hierarchic segmentation gene cascade (St. Johnston and Nüsslein-Volhard 1992), but are instead regulated by an anterior gap gene-like system (e.g. Cohen and Jürgens 1990; Mohler 1995) So-called second-order regulatory genes, such as collier, act to transmit positional information from the head gap genes to regulate SPGs (Crozatier et al. 1999; Ntini and Wimmer 2011a, b). Despite some functional differences (Schinko et al. 2008), the expression patterns of head gap genes and collier are widely conserved in insects and a myriapod suggesting at least some degree of functional conservation in the process of head segmentation (Economou and Telford 2009; Schaeper et al. 2010; Janssen et al. 2011b, c; Birkan et al. 2011). It was recently suggested that the genetic patterning of the tritocerebral segment in insects and myriapods, but not crustaceans and chelicerates, involves the early action of collier (Janssen et al. 2011b; Schaeper et al. 2010). This implied either that the expression of collier in the tritocerebral segment in insects and myriapods is a result of convergent evolution, or that it represents a true synapomorphy for a group uniting insects and myriapods (Janssen et al 2011b).
In order to shed further light on this controversial topic, the early expression of SPGs (and associated factors) was investigated in Glomeris. Earlier work reported on the delayed expression of the SPG engrailed (en) in the tritocerebral segment in Glomeris (Janssen et al. 2004), the centipede Strigamia maritima (Chipman et al. 2004b) and insects (e.g. Peterson et al. 1998; Patel et al. 1989; Miyawaki et al. 2004; Posnien and Bucher 2010, O’Donnell and Jockusch 2010), but not crustaceans (Scholtz et al. 1994; Browne et al. 2005; Alwes and Scholtz 2005) and chelicerates (Schwager et al. 2009; Pechmann et al. 2009; Kanayama et al. 2011).
As expected from the fact that the SPG network is highly conserved even beyond the arthropods (Eriksson et al. 2010; Dray et al. 2010), all SPGs (and associated factors) are expressed in conserved patterns during the process of segment formation in Glomeris (Janssen et al. 2004, 2008, this study). And expression of all investigated factors is delayed in the tritocerebral segment.
The finding that delayed SPG patterning is also, like the expression of collier, conserved only in insects and myriapods raises the question on how likely convergent evolution may be. Therefore, one goal for the future must therefore be to understand the apparent co-evolution (independent recruitment) of genetic networks such as the SPG system in insects and myriapods.
SPG expression in the premandibular segment is associated with the fading of sloppy paired expression
In insects such as Drosophila and Tribolium, pair rule genes regulate the expression of SPGs (e.g. Choe et al. 2006; Akam 1987; Pankratz and Jäckle 1993). The conserved intrasegmental expression patterns of the pair rule gene orthologs in Glomeris (Janssen et al. 2011a; Janssen et al. 2012) suggest that this interaction may at least be partially conserved.
It is eye-catching that in Glomeris, expression of the SPGs does not start in the premandibular (tritocerebral) segment before the clearance of the pair rule gene sloppy paired (slp) from the same region (Janssen et al. 2012). In all segments, slp is expressed anterior and adjacent to en/hh (Janssen et al. 2011a; Janssen et al. 2012). Also, in Drosophila and Tribolium slp acts as a regulator of the SPGs (Cadigan et al. 1994a, b; Choe and Brown 2007, 2009). It has been shown, for example, that the early expression of slp in the head segments in Drosophila represses other pair rule genes and thus sets their anterior borders, which are required for the proper activation of en in the mandibular segment (Andrioli et al. 2004). Therefore, it may be that the delayed SPG patterning of the tritocerebral segment is a result of the segment-spanning expression of slp. If this is the case, the delayed SPG patterning of the tritocerebral segment may represent a conserved trait. In Drosophila, slp is directly involved in the development of this segment, and in a double mutant of the two slp paralogs (slp1 and slp2), en is de-repressed in the intercalary segment (Cadigan et al. 1994a, b). Furthermore, it has been shown that slp represses other pair rule genes in Drosophila (Andrioli et al. 2004), and this may also be the case in Glomeris where the orthologs of the primary pair rule gene runt (run) and even-skipped (eve) are absent from the border between the mandibular and the premandibular segment until slp begins to disappear from this region (Janssen et al. 2012). In Tribolium, however, slp is not expressed comparably early but appears delayed in the tritocerebral segment (Posnien and Bucher 2010). Unfortunately, functional methods have yet not been established for any myriapod species, so that testing of this hypothesis on the possible function of slp in Glomeris must wait until this obstacle has been overcome.
Engrailed expression first appears in the mandibular segment: a conserved trait in arthropod development
The new results on the earliest expression of engrailed (en) in Glomeris show that it appears first in the mandibular segment (Figs. S2 and 7). This finding is complemented by data from the insect Tribolium, where en is expressed first in the mandibular segment (Brown et al. 1994). Furthermore, also in the amphipod crustacean Parhyale, En protein is first expressed in the mandibular segment (Scholtz et al. 1994). Also, in the spider Achaearanea, en is first expressed in the corresponding homologous segment, which is the first walking leg-bearing segment (L1) (Schwager et al. 2009; Kanayama et al. 2011). It appears thus that en is first expressed in the mandibular segment (L1 segment in chelicerates) in representatives of all arthropod classes (no data are available from pycnogonids which may represent a fifth class (Dunlop and Arango 2005)). Notably, however, in the model arthropod Drosophila it is not the mandibular segment that expresses en first, but the posterior adjacent maxillary segment (DiNardo et al. 1985). On account of the derived developmental mode (long germ vs short germ developmental mode), the distant position in phylogenetic trees and the available data from other arthropods, the situation in Drosophila must be considered derived. Although the exact order of appearance of En stripes is unclear in the wasp Nasonia, another species with a long germ band mode of development, the mandibular stripe appears earlier than the maxillary stripe (Pultz et al. 1999).
Despite the fact that data from arthropod sister groups, i.e. a tardigrade and an onychophoran, show that the early expression of en in the mandibular segment is not conserved outside the Arthropoda (Gabriel and Goldstein 2007; Eriksson et al. 2009), it appears that the available data are sufficient to bring the idea forward that earliest expression of en in the mandibular segment indeed represents an ancestral feature of the arthropods.
References
Akam M (1987) The molecular basis for metameric pattern in the Drosophila embryo. Development 101:1–22
Akiyama-Oda Y, Oda H (2010) Cell migration that orients the dorsoventral axis is coordinated with anteroposterior patterning mediated by Hedgehog signaling in the early spider embryo. Development 137:1263–1273
Alwes F, Scholtz G (2005) Stages and other aspects of the embryology of the parthenogenetic Marmorkrebs (Decapoda, Reptantia, Astacida). Dev Genes Evol 216:169–184
Andrioli LP, Oberstein AL, Corado MS, Yu D, Small S (2004) Groucho-dependent repression by sloppy-paired 1 differentially positions anterior pair-rule stripes in the Drosophila embryo. Dev Biol 276:541–551
Birkan M, Schaeper ND, Chipman AD (2011) Early patterning and blastodermal fate map of the head in the milkweed bug Oncopeltus fasciatus. Evol Dev 13:436–447
Brown SJ, Patel NH, Denell RE (1994) Embryonic expression of the single Tribolium engrailed homolog. Dev Genet 15:7–18
Browne WE, Price AL, Gerberding M, Patel NH (2005) Stages of embryonic development in the amphipod crustacean, Parhyale hawaiensis. Genesis 42:124–149
Cadigan KM, Grossniklaus U, Gehring WJ (1994a) Functional redundancy: the respective roles of the two sloppy paired genes in Drosophila segmentation. Proc Natl Acad Sci U S A 91:6324–6328
Cadigan KM, Grossniklaus U, Gehring WJ (1994b) Localized expression of sloppy paired protein maintains the polarity of Drosophila parasegments. Genes Dev 8:899–913
Campbell LI, Rota-Stabelli O, Edgecombe GD, Marchioro T, Longhorn SJ, Telford MJ, Philippe H, Rebecchi L, Peterson KJ, Pisani D (2011) MicroRNAs and phylogenomics resolve the relationships of Tardigrada and suggest that velvet worms are the sister group of Arthropoda. Proc Natl Acad Sci U S A 108:15920–15924
Chipman AD, Arthur W, Akam M (2004a) A double segment periodicity underlies segment generation in centipede development. Curr Biol 14:1250–1255
Chipman AD, Arthur W, Akam M (2004b) Early development and segment formation in the centipede, Strigamia maritima (Geophilomorpha). Evol Dev 6:78–89
Choe CP, Miller SC, Brown SJ (2006) A pair-rule gene circuit defines segments sequentially in the short-germ insect Tribolium castaneum. Proc Natl Acad Sci U S A 103:6560–6564
Choe CP, Brown SJ (2007) Evolutionary flexibility of pair-rule patterning revealed by functional analysis of secondary pair-rule genes, paired and sloppy-paired in the short-germ insect, Tribolium castaneum. Dev Biol 302:281–294
Choe CP, Brown SJ (2009) Genetic regulation of engrailed and wingless in Tribolium segmentation and the evolution of pair-rule segmentation. Dev Biol 325:482–491
Cohen SM, Jürgens G (1990) Mediation of Drosophila head development by gap-like segmentation genes. Nature 346:482–485
Crozatier M, Valle D, Dubois L, Ibnsouda S, Vincent A (1999) Head versus trunk patterning in the Drosophila embryo; collier requirement for formation of the intercalary segment. Development 126:4385–4394
Damen WG, Hausdorf M, Seyfarth E-A, Tautz D (1998) A conserved mode of head segmentation in arthropods revealed by the expression pattern of Hox genes in a spider. Proc Natl Acad Sci U S A 95:10665–10670
Damen WG (2002) Parasegmental organization of the spider embryo implies that the parasegment is an evolutionary conserved entity in arthropod embryogenesis. Development 129:1239–1250
Damen WG (2007) Evolutionary conservation and divergence of the segmentation process in arthropods. Dev Dyn 236:1379–1391
Davis GK, Patel NH (2002) Short, long and beyond: molecular and embryological approaches to insect segmentation. Annu Rev Entomol 47:669–699
Dearden PK, Wilson MJ, Sablan L, Osborne PW, Havler M, McNaughton E, Kimura K, Milshina NV, Hasselmann M, Gempe T, Schioett M, Brown SJ, Elsik CG, Holland PW, Kadowaki T, Beye M (2006) Patterns of conservation and change in honey bee developmental genes. Genome Res 16:1376–1384
DiNardo S, Kuner JM, Theis J, O'Farrell PH (1985) Development of embryonic pattern in D. melanogaster as revealed by accumulation of the nuclear engrailed protein. Cell 43:59–69
Dohle W (1964) Die Embryonalentwicklung von Glomeris marginata (Villers) im Vergleich zur Entwicklung anderer Diplopoden. Zool Jahrb Anat 81:241–310
Dray N, Tessmar-Raible K, Le Gouar M, Vibert L, Christodoulou F, Schipany K, Guillou A, Zantke J, Snyman H, Behague J, Vervoort M, Arendt D, Balavoine G (2010) Hedgehog signaling regulates segment formation in the annelid Platynereis. Science 329:339–342
Dunlop JA, Arango CP (2005) Pycnogonid affinities: a review. J Zool Syst Evol Res 43:8–21
Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA, Seaver E, Rouse GW, Obst M, Edgecombe GD, Sörensen MV, Haddock SH, Schmidt-Rhaesa A, Okusu A, Kristensen RM, Wheeler WC, Martindale MQ, Giribet G (2008) Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452:745–749
Economou AD, Telford MJ (2009) Comparative gene expression in the heads of Drosophila melanogaster and Tribolium castaneum and the segmental affinity of the Drosophila hypopharyngeal lobes. Evol Dev 11:88–96
Edgecombe GD (2010) Arthropod phylogeny: an overview from the perspectives of morphology, molecular data and the fossil record. Arthropod Struct Dev 39:74–78
Eriksson BJ, Tait NN, Budd GE, Akam M (2009) The involvement of engrailed and wingless during segmentation in the onychophoran Euperipatoides kanangrensis (Peripatopsidae: Onychophora) (Reid 1996). Dev Genes Evol 219:249–264
Eriksson BJ, Tait NN, Budd GE, Janssen R, Akam M (2010) Head patterning and Hox gene expression in an onychophoran and its implications for the arthropod head problem. Dev Genes Evol 220:117–122
Farzana L, Brown SJ (2008) Hedgehog signaling pathway function conserved in Tribolium segmentation. Dev Genes Evol 218:181–192
Gabriel WN, Goldstein B (2007) Segmental expression of Pax3/7 and engrailed homologs in tardigrade development. Dev Genes Evol 217:421–433
Hughes CL, Kaufman TC (2002) Exploring myriapod segmentation: the expression patterns of even-skipped, engrailed, and wingless in a centipede. Dev Biol 247:47–61
Janssen R, Prpic NM, Damen WG (2004) Gene expression suggests decoupled dorsal and ventral segmentation in the millipede Glomeris marginata (Myriapoda: Diplopoda). Dev Biol 268:89–104
Janssen R, Damen WG (2006) The ten Hox genes of the millipede Glomeris marginata. Dev Genes Evol 216:451–465
Janssen R, Budd GE, Damen WG, Prpic NM (2008) Evidence for Wg-independent tergite boundary formation in the millipede Glomeris marginata. Dev Genes Evol 218:361–370
Janssen R, Le Gouar M, Pechmann M, Poulin F, Bolognesi R, Schwager EE, Hopfen C, Colbourne JK, Budd GE, Brown SJ, Prpic NM, Kosiol C, Damen WG, Balavoine G, McGregor AP (2010) Conservation, loss, and redeployment of Wnt ligands in protostomes: implications for understanding the evolution of axis elongation and segmentation. BMC Evol Biol 10:374
Janssen R (2011) Diplosegmentation in the pill millipede Glomeris marginata is the result of dorsal fusion. Evol Dev 13:477–487
Janssen R, Budd GE, Prpic NM, Damen WG (2011a) Expression of myriapod pair rule gene orthologs. EvoDevo 2:5
Janssen R, Damen WG, Budd GE (2011b) Expression of collier in the premandibular segment of myriapods: support for the traditional Atelocerata concept or a case of convergence? BMC Evol Biol 11:50
Janssen R, Budd GE, Damen WG (2011c) Gene expression suggests conserved mechanisms patterning the heads of insects and myriapods. Dev Biol 357:64–72
Janssen R, Damen WG, Budd GE (2012) Expression of pair rule gene orthologs in the blastoderm of a myriapod: evidence for pair rule-like mechanisms? BMC Dev Biol 12:15
Jürgens G, Lehmann R, Schardin M, Nüsslein-Volhard C (1986) Segmental organization of the head in the embryo of Drosophila melanogaster. Roux´s Arch Dev Biol 195:359–377
Kanayama M, Akiyama-Oda Y, Nishimura O, Tarui H, Agata K, Oda H (2011) Travelling and splitting of a wave of hedgehog expression involved in spider-head segmentation. Nat Commun 2:500
Miyawaki K, Mito T, Sarashina I, Zhang H, Shinmyo Y, Ohuchi H, Noji S (2004) Involvement of Wingless/Armadillo signaling in the posterior sequential segmentation in the cricket, Gryllus bimaculatus (Orthoptera), as revealed by RNAi analysis. Mech Dev 121:119–130
Mohler J, Vani K (1992) Molecular organization and embryonic expression of the hedgehog gene involved in cell–cell communication in segmental patterning of Drosophila. Development 115:957–971
Mohler J (1995) Spatial regulation of segment polarity gene expression in the anterior terminal region of the Drosophila blastoderm embryo. Mech Dev 50:151–161
Ntini E, Wimmer EA (2011a) Unique establishment of procephalic head segments is supported by the identification of cis-regulatory elements driving segment-specific segment polarity gene expression in Drosophila. Dev Genes Evol 221:1–16
Ntini E, Wimmer EA (2011b) Second order regulator Collier directly controls intercalary-specific segment polarity gene expression. Dev Biol 360:403–414
O’Donnell BC, Jockusch EL (2010) The expression of wingless and Engrailed in developing embryos of the mayfly Ephoron leukon (Ephemeroptera: Polymitarcyidae). Dev Genes Evol 220:11–24
Oppenheimer DI, MacNicol AM, Patel NH (1999) Functional conservation of the wingless-engrailed interaction as shown by a widely applicable baculovirus misexpression system. Curr Biol 9:1288–1296
Pankratz MJ, Jäckle H (1993) Blastoderm segmentation. In: Bate M, Martinez Arias A (eds) The development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 467–516
Patel NH, Kornberg TB, Goodman CS (1989) Expression of engrailed during segmentation in grasshopper and crayfish. Development 107:201–212
Pechmann M, McGregor AP, Schwager EE, Feitosa NM, Damen WG (2009) Dynamic gene expression is required for anterior regionalization in a spider. Proc Natl Acad Sci U S A 106:1468–1472
Peel A, Akam M (2003) Evolution of segmentation: rolling back the clock. Curr Biol 13:R708–R710
Peterson MD, Popadic A, Kaufman TC (1998) The expression of two engrailed-related genes in an apterygote insect and a phylogenetic analysis of insect engrailed-related genes. Dev Genes Evol 208:547–557
Posnien N, Bucher G (2010) Formation of the insect head involves lateral contribution of the intercalary segment, which depends on Tc-labial function. Dev Biol 338:107–116
Prpic NM, Janssen R, Wigand B, Klingler M, Damen WG (2003) Gene expression in spider appendages reveals reversal of exd/hth spatial specificity, altered leg gap gene dynamics, and suggests divergent distal morphogen signaling. Dev Biol 264:119–140
Prpic NM, Tautz D (2003) The expression of the proximodistal axis patterning genes Distal-less and dachshund in the appendages of Glomeris marginata (Myriapoda: Diplopoda) suggests a special role of these genes in patterning the head appendages. Dev Bio 260:97–112
Prpic NM, Janssen R, Damen WG, Tautz D (2005) Evolution of dorsal–ventral axis formation in arthropod appendages: H15 and optomotor-blind/bifid-type T-box genes in the millipede Glomeris marginata (Myriapoda: Diplopoda). Evol Dev 7:51–57
Prpic NM, Damen WG (2005) A homolog of the hydrolase Notum is expressed during segmentation and appendage formation in the Central American hunting spider Cupiennius salei. Naturwissenschaften 92:246–249
Prpic NM (2008) Parasegmental appendage allocation in annelids and arthropods and the homology of parapodia and arthropodia. Front Zool 5:17
Pultz MA, Pitt JN, Alto NM (1999) Extensive zygotic control of the anteroposterior axis in the wasp Nasonia vitripennis. Development 126:701–710
Rota-Stabelli O, Kayal E, Gleeson D, Daub J, Boore JL, Telford MJ, Pisani D, Blaxter M, Lavrov DV (2010) Ecdysozoan mitogenomics: evidence for a common origin of the legged invertebrates, the Panarthropoda. Genome Biol Evol 2:425–440
Sánchez L, Chaouiya C, Thieffry D (2008) Segmenting the fly embryo: logical analysis of the role of the segment polarity cross-regulatory module. Int J Dev Biol 52:1059–1075
Sanson B (2001) Generating patterns from fields of cells. Examples from Drosophila segmentation. EMBO Rep 2:1083–1088
Sarrazin AF, Peel AD, Averof M (2012) A segmentation clock with two-segment periodicity in insects. Science 336:338–341
Schaeper ND, Pechmann M, Damen WG, Prpic NM, Wimmer EA (2010) Evolutionary plasticity of collier function in head development of diverse arthropods. Dev Biol 344:363–376
Schinko JB, Kreuzer N, Offen N, Posnien N, Wimmer EA, Bucher G (2008) Divergent functions of orthodenticle, empty spiracles and buttonhead in early head patterning of the beetle Tribolium castaneum (Coleoptera). Dev Biol 317:600–613
Scholtz G, Patel NH, Dohle W (1994) Serially homologous engrailed stripes are generated via different cell lineages in the germ band of amphipod crustaceans (Malacostraca, Peracarida). Int J Dev Biol 38:471–478
Schoppmeier M, Damen WG (2005) Expression of Pax group III genes suggests a single-segmental periodicity for opisthosomal segment patterning in the spider Cupiennius salei. Evol Dev 7:160–167
Schwager EE, Pechmann M, Feitosa NM, McGregor AP, Damen WG (2009) Hunchback functions as a segmentation gene in the spider Achaearanea tepidariorum. Curr Biol 19:1333–1340
Simonnet F, Deutsch J, Queinnec E (2004) hedgehog is a segment polarity gene in a crustacean and a chelicerate. Dev Genes Evol 214:537–545
St. Johnston D, Nüsslein-Volhard C (1992) The origin of pattern and polarity in the Drosophila embryo. Cell 68:201–219
Tabata T, Eaton S, Kornberg TB (1992) The Drosophila hedgehog gene is expressed specifically in posterior compartment cells and is a target of engrailed regulation. Genes Dev 6:2635–2645
Tashiro S, Michiue T, Higashijima S, Zenno S, Ishimaru S, Takahashi F, Orihara M, Kojima T, Saigo K (1993) Structure and expression of hedgehog, a Drosophila segment-polarity gene required for cell–cell communication. Gene 124:183–189
Telford MJ, Thomas RH (1998) Expression of homeobox genes shows chelicerate arthropods retain their tritocerebral segment. Proc Natl Acad Sci USA 95:10671–10675
Acknowledgments
I would like to thank two anonymous reviewers for their helpful comments on the manuscript and John Peel for proofreading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: S. Roth
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Expression of Glomeris hedgehog at stage 0.3. Embryos are oriented with anterior to the left. a Stage 0.3. Same complement of hh stripes as in a slightly older stage 0.4 embryo (cf. Fig. 1d). a′ DAPI counterstaining of the same embryo shown in a (JPEG 153 kb)
Fig. S2
Additional aspects of engrailed expression. Embryos are oriented with anterior to the left. a Embryo of intermediate stage between stage 0 and stage 0.1 (cf. Janssen et al. (2004)). Weak expression of en which is in the mandibular segment primordium (arrowhead) precedes expression in the other segment primordia. a′ Same embryo as shown in a. DAPI counterstaining. Arrowhead as in a (JPEG 150 kb)
Rights and permissions
About this article
Cite this article
Janssen, R. Segment polarity gene expression in a myriapod reveals conserved and diverged aspects of early head patterning in arthropods. Dev Genes Evol 222, 299–309 (2012). https://doi.org/10.1007/s00427-012-0413-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-012-0413-9