Abstract
The expression of the segment polarity genes wingless (wg) and engrailed (en) is highly conserved across arthropods, and these genes play a crucial role in patterning of the segmental body plan. Investigations of the expression and function of wg and en have focused primarily upon holometabolous insects, with the notable exception of recent detailed work in Oncopeltus (Hemiptera), Schistocerca, and Gryllus (Orthoptera). An increase in the phylogenetic breadth of our understanding of molecular patterning is crucial to ascertain the extent of conservation and divergence in molecular patterning mechanisms during insect embryogenesis. We examined the expression of wg mRNA transcripts and localization of En protein during embryogenesis in the mayfly Ephoron leukon (Ephemeroptera: Polymitarcyidae). These data represent one of the first embryonic gene expression pattern data for a mayfly, a lineage that may be the sister group to all other winged insects. Many aspects of wg and En expression are highly conserved, notably their expression in juxtaposed stripes in each parasegment, as well as expression domains in the procephalon, mouthparts, thoracic limbs, and nervous system. Future work in mayflies can be used to determine if conservation extends to other components of the segmentation hierarchy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The role of the segment polarity genes wingless (wg) and engrailed (en) in insect development has been studied intensively, and accumulating evidence suggests that parasegments demarcated by wg and en expression were present in the most recent common ancestor of all arthropods (Damen 2002; reviewed recently by Damen 2007; Hughes and Kaufman 2002a) as well as in the sister group to arthropods, the onychophorans (Eriksson et al. 2009). In Drosophila, wg and en are expressed in adjacent stripes that delineate the posterior and anterior regions of each parasegment along the main body axis (Baker 1987, 1988; Fjose et al. 1985; Kornberg et al. 1985). wg (or wnt-1) encodes a secreted glycoprotein that functions as a signaling factor and is part of the large family of Wnt genes (Nusse and Varmus 1992), with nine paralogues recently isolated from Tribolium (Bolognesi et al. 2008b). en encodes a homeobox-containing transcription factor (Desplan et al. 1985) that activates the diffusible signaling protein encoded by hedgehog (Zecca et al. 1995) which maintains the expression of wg in neighboring posterior cells (Ingham and Hidalgo 1993). Similarly, wg maintains expression of en in the adjacent anterior cells of most parasegments (Klingensmith and Nusse 1994; Nusse and Varmus 1992). Drosophila mutant for wg or en produce embryonic phenotypes with deleted regions of segments (Nüsslein-Volhard and Wieschaus 1980).
Our understanding of body plan formation, segmentation, and appendage patterning in insects is based primarily on data from Drosophila melanogaster and Tribolium castaneum, which are holometabolous insects (those developing with a pupal stage). For insects outside of Holometabola, wg and/or en expression has been documented in Thermobia (Thysanura), Periplaneta (Blattodea), Tenodera (Mantodea), Gryllus, Acheta, Schistocerca (Orthoptera), and Oncopeltus (Hemiptera; Angelini and Kaufman 2005a; Dearden and Akam 2001; Giorgianni and Patel 2004; Jockusch and Ober 2004; Mahfooz et al. 2004; Miyawaki et al. 2004; Patel et al. 1989; Peterson et al. 1998; Rogers and Kaufman 1996). In Tribolium, ectopic expression of wg leads to increased en expression (Oppenheimer et al. 1999), and knockdown of wg leads to loss of most En expression (Bolognesi et al. 2008a; Ober and Jockusch 2006). Tribolium and Oncopeltus embryos in which wg expression has been knocked down have malformed segment boundaries (Angelini and Kaufman 2005a; Bolognesi et al. 2008a; Ober and Jockusch 2006). Although segment defects were not observed in Gryllus embryos in which wg was knocked down, gene knockdowns targeting components of the Wnt signaling cascade or multiple Wnt paralogues led to severely truncated embryos lacking posterior segments in Gryllus (Miyawaki et al. 2004), Tribolium (Bolognesi et al. 2008b), and Oncopeltus (Angelini and Kaufman 2005a). Posterior segments were also deleted in Oncopeltus with reduced expression of en (Angelini and Kaufman 2005a).
Currently available data suggest that the expression and function of segment polarity genes are relatively conserved across the pool of insect species studied to date (see above references), yet this conclusion is based upon data drawn from a limited sample of the total number of lineages circumscribed by Insecta (only about a third of the thirty orders of insects have been sampled). An expanded and inclusive set of focal organisms used in studies of evolutionary developmental biology is necessary to make well-supported inferences about ancestral states and the sequence of evolutionary transformations (see similar themes explored in Jenner and Wills 2007 and Travis 2006). Mayflies (Ephemeroptera) are an evolutionarily important lineage for comparative studies of molecular development as they constitute the presumed sister group (perhaps along with Odonata, the dragonflies and damselflies) to all or most other winged insects (Gullan and Cranston 2005; Kjer 2004; Regier et al. 2010; Simon et al. 2009; Willman 2004; Zhang et al. 2008). Thus, information on developmental mechanisms in mayflies will help clarify the ancestral state for processes of segmentation and appendage development in winged insects. In this study, we document the expression of wg mRNA transcripts and En protein localization during embryogenesis in a mayfly species, Ephoron leukon (Polymitarcyidae). Our data show that the expression of wg and En in the procephalon, body segments, and appendage primordia is relatively conserved in this species. Additionally, this work establishes that field collection of gravid females of E. leukon leads to a tractable system for developmental studies of mayfly embryogenesis.
Materials and methods
Embryo collection and fixation
Eggs of E. leukon were field collected from females on the Housatonic River (near Cornwall, Connecticut, USA) from 2001 to 2005. Species identification was confirmed by consultation with Steve Burian (Southern Connecticut State University, personal communication). Emergence of E. leukon occurs over several days in late July or early August and is typified by extremely dense and synchronized hatches of the aquatic nymphs into winged adults. Mating swarms occur over a period of 1–1.5 h directly after dusk, and females typically mate immediately after hatching to the winged stage. To collect fertilized eggs, a black light was positioned on the bank of the river and females were captured, held by the wings and the abdomen immersed into water to induce oviposition. A single female may deposit more than a thousand eggs, each of which is approximately 300 µm along the long axis, including the polar cap (O’Donnell, unpublished). Thus, it is possible to collect hundreds of thousands of fertilized eggs for laboratory culturing from a single evening’s hatch.
Eggs were transported back to the laboratory and placed in small glass bowls filled with filtered river water. Eggs were allowed to develop at room temperature (RT) in ambient light conditions with periodic recharging with fresh filtered river water for 4–6 weeks or until eyespots/ocelli developed in a majority of the eggs (the signal of the onset of embryonic diapause in this species). These collection and maintenance procedures mirror those used for Ephoron virgo (Olivier) in the Netherlands (Greve et al. 1999), and we have used them to obtain and raise eggs from several different mayfly species (B. O’Donnell, unpublished data).
From our in-lab cultures, we collected embryos of a variety of developmental stages: from the heart stage/torpedo stage through the onset of diapause. Development is arrested shortly after the late post-elongated germ band (EGB) stage and resumes only after extended exposure to cold temperatures, as in several other species of Ephoron which undergo an obligate diapause (Britt 1962; Edmunds et al. 1956; Giberson and Galloway 1985, Greve et al. 1999; Watanabe and Takao 1991). Watanabe (1998) and Giberson and Galloway (1985) documented the effects of temperature upon embryonic development and egg hatching rate in the congenerics Ephoron shigae and Ephoron album, respectively. Several studies have demonstrated the ultimate impacts of temperature on nymphal development, timing of emergence, and duration of the emergence period in E. leukon, E. album, and E. shigae (Giberson and Galloway 1985; Snyder et al. 1991; Watanabe and Ohkita 2000; Watanabe and Takao 1991). The seasonal diapause is quite useful for providing late-stage embryos and early nymphal stages throughout the year as embryos can be maintained in this state for over a year. Embryos held in diapause can be kept in the lab year round, and clutches of eggs can be brought to room temperature for rearing of late-stage embryos through hatching and early nymphal stages.
Eggs were subjected to a 6-min soak in 50% fresh bleach solution to soften the chorion and then fixed for 30–50 min (older stage embryos were fixed for longer times) in 6% formaldehyde in phosphate-buffered saline + 0.1% Tween (PBTw). Heptanes were added (2:1 ratio of fixative to heptanes) and eggs were agitated on a platform shaker at high speed. Eggs were rinsed in PBTw and stored in absolute methanol at −20°C.
Gene amplification
RNA was extracted from a mixture of embryonic stages of freshly collected embryos of E. leukon using a Trizol-based method (Gibco BRL). cDNA was synthesized using MulV reverse transcriptase and an oligo dT primer with incubations of 5 min at 65°C, 1 h at 37°C, and 10 min at 75°C. wg was amplified from E. leukon (GenBank accession number EU931674) using the degenerate primers 5′wg1: GARTGYAARTGYCAYGGYATGTCTGG and 3′wg2: ACTICGCRCACCARTGGAATGTRC (Brower and DeSalle 1998). PCR reaction conditions were 94°C/1 min for denaturation, 56°C/1 min for annealing, and 72°C/1 min for extension for 36 cycles with a final extension step of 5 min at 72°C. A band of the appropriate size was excised from the gel, cleaned, and cloned into a dual-promoter vector (TOPO TA Cloning Kit, Invitrogen). Colonies were picked directly into a PCR mixture, and their inserts were then amplified with M13 forward and M13 reverse primers; inserts of the appropriate size were cleaned and cycle-sequenced. wg was amplified from several other mayfly species from a total of six different families: Paraleptophlebia (Leptophlebiidae), Baetisca (Baetiscidae), Ephemerella (Ephemerellidae), Siphlonurus (Siphlonuridae), Caenis (Caenidae), and Ephemera (Ephemeridae; GenBank accession numbers: EU931673, EU931675–EU931679) using genomic DNA obtained via a CTAB extraction (modified from Murray and Thompson 1980). wg bands of the appropriate size from the additional taxa were excised from 1% agarose gels, cleaned, and cycle-sequenced using standard cycle sequencing recipes and reaction conditions. Cycle sequencing used ABI BigDye, version 1.1, and resulting products were electrophoresed on an ABI3100 automated DNA sequencer.
Riboprobe synthesis and in situ hybridization
DIG-labeled riboprobes for E. leukon were synthesized with the DIG RNA Labeling Kit (Roche BioChemicals, Germany). Probes were hydrolyzed using 0.2 M sodium carbonate and 0.2 M sodium bicarbonate solution (2:3 ratio) at 60°C for 10 min, precipitated with 7.5 M ammonium acetate, and resuspended in hybridization buffer (see below). Probe strength was assessed by the dot-blot method (Huang et al. 1998) with reference to known standards.
Fixed eggs of E. leukon were placed in a small glass cuvette containing 1 ml of PBTw and dipped 5–30 times (dependent upon developmental stage) in a sonicator (FS30 Ultrasonic Cleaner, Fisher Scientific) to extract embryos from the egg membranes. Embryos were then rinsed several times in PBTw and pre-hybridized in buffer (50% formamide + 5XSSC (sodium chloride + sodium citrate solution) + 1X Denhardt’s reagent + 0.1% Tween + 0.1% CHAPS + 200 μg/ml herring sperm DNA (Nulsen and Nagy 1999) or dextran sulfate-enhanced hybridization buffer: 50% formamide + 4XSSC + 1X Denhardt’s reagent + 5% dextran sulfate + 0.1% Tween + 250 μg/ml tRNA (Broadus and Doe 1995) at 56°C for a minimum of 1 h up to overnight. Both hybridization buffers produced robust staining. Probes were subsequently added at a concentration ranging from 0.1 to 1 μg/ml, dependent upon the developmental stage. Incubation lasted >18 h at 56°C. Embryos were rinsed five times quickly and then for a total of four more washes spaced by at least 30 min between washes and then left overnight at 56°C in plain hybridization buffer (50% formamide + 5XSSC + 0.1% Tween).
The next day, embryos were rinsed in 2XSSC for 1.5 h at 56°C then in 1XSSC for an additional hour at 56°C. Next, embryos were rinsed twice in PBTw at RT and incubated in a 1:1,500 dilution of anti-digoxygenin-labeled Fab fragments (Roche BioChemicals) in 2% bovine serum albumin (Fisher Scientific) in PBTw for 2 h at RT. Embryos were then rinsed in PBTw every 10 min for a total of 1 h and equilibrated in alkaline phosphatase buffer (100 mM Tris–Cl, + 100 mM NaCl + 50 mM MgCl2 + 0.1% Tween, pH 9.5) for 15 min. The spatial distribution of wg mRNA transcripts was detected by development with the nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl phosphate-4-toluidine color reaction for up to 12 h at room temperature. Rinsing with PBTw stopped the color reaction, and embryos were counterstained with DAPI to label nuclei and stored in 80% glycerol at −20°C.
Immunohistochemistry
Fixed embryos were rinsed in phosphate-buffered saline + 0.1% Triton-X (PBTr) then incubated overnight at 4°C in a 1:50–1:400 dilution of En4F11 (Patel et al. 1989) in 2% bovine serum albumen in PBTr. The next day, embryos were rinsed every 10 min for a total of 1 h at RT then incubated with a peroxidase-conjugated secondary antibody (Jackson Immunochemicals) for 2 h at RT. Embryos were then rinsed in PBTr every 10 min for an hour before equilibration in 1X stable peroxidase substrate buffer (1XHP; Pierce, Rockford, IL, USA) then developed in a 1:10 dilution of metal-enhanced diaminobenzadine substrate (Pierce) in 1XHP buffer for 15 min at RT. Embryos were rinsed, stained with DAPI, and stored in 80% glycerol at −20°C. All embryos were viewed with a Zeiss Axioskop 2 Plus compound microscope and images were collected with an Olympus digital camera and image collection software (Magnafire: Meyer Instruments, Houston, TX, USA).
Results
Characterization of E. leukon wg
Amplification of a portion of the wg (Wnt-1) gene from E. leukon produced a 477-bp sequence, and its identity was confirmed by BLAST search results and by the presence of a number of conserved residues (n = 42) between the inferred E. leukon Wnt-1 protein sequence and other Wnt-1 protein sequences (Fig. 1a). Partial wg sequences from E. leukon plus six additional mayfly species ranged from 441 to 480 bp in length, owing to a variable insertion region (Fig. 1b). Protein-specific BLAST searches of the mayfly insertion region did not return any significant similarity to known proteins; thus, the functional significance of this region is unknown.
a Alignment of a portion of the inferred Wg (Wnt-1) protein sequence in Ephoron leukon, six additional mayfly species (mayfly species names are followed by asterisks), a firebrat (Thermobia domestica, GenBank accession number AF214035.1), grasshopper (Schistocerca, AAD37798.1), cricket (Gryllus, BAB19660.1), treehopper (Telamona, AAS57853.1), beetle (Tribolium, NM_001114350.1), moth (Bombyx, ABX57129.1), fruitfly (Drosophila, AAF52501.1), tadpole shrimp (Triops, AAC32377.1), spider (Achaearanea, BAD12586.1), millipede (Glomeris, CAE83648.1), onychophoran (Euperipatoides, EU347403), and lancelet (Branchiostoma, AAC86432). The blue highlighted region indicates the variable length insertion region (e.g., “49 a.a.” refers to an insertion of 49 amino acid residues). The specific amino acids for insertions of fewer than five amino acids are shown; all others are omitted for clarity. Forty-two amino acids were conserved across all included taxa and are denoted by gray shading. The ruler at the top of the alignment shows tick marks at every ten amino acids and does not count amino acids from the highlighted variable region. b Alignment of only the mayfly-specific insertion region of Wnt-1. Conserved residues are shaded in gray (n = 16). Dash indicates a gap in the alignment and question mark indicates missing data
Segmental expression of wg during E. leukon embryogenesis
During embryogenesis, E. leukon embryos add segments progressively to the posterior as is typical in short germ insects (Anderson 1972), and many of the embryonic stages are highly similar to those seen in other insects (Fig. 2). Embryogenesis in E. leukon proceeds in a manner highly similar to that seen in other burrowing mayfly species (e.g., Ephemera japonica, Tojo and Machida 1997, 1999), including the related polymitarcyid species, Tortopus incertus (Tsui and Peters 1974).
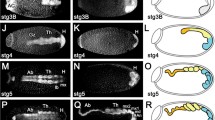
Select stages of E. leukon embryos stained with DAPI to highlight morphology. Ventral view (a, c, d) or lateral view (b); anterior is up for all embryos. a Elongating germ band stage. Two abdominal segments are clearly distinguishable. b Elongating germ band stage, slightly older than in a. Elongated thoracic limbs, seven of the eventual ten abdominal segments and the folding of the posterior abdomen (asterisk) are evident. c Post-EGB stage. The overall widening of the embryo in the post-EGB stages is shown here. d Post-EGB stage, slightly older than in c. Embryos at this stage have ventrally folded the posterior of the abdomen. Two lateral cerci (Ce) flank a median caudal filament (Cf). Ant antenna, La labrum, Mn mandible, Mx maxilla, Lb labium, T 1 thoracic segment 1, A 1 abdominal segment 1. Scale bars 200 μm (a, b, d), 250 μm (c)
Stripes of wg expression appear before segments are morphologically distinguishable (i.e., before lateral furrows are evident along the anteroposterior axis of the body). Stripes are added progressively from anterior to posterior (Fig. 3a–e), with the exception that the intercalary stripe appears midway through segmentation after the anterior-most abdominal stripes of wg expression have already formed (Fig. 3c). During E. leukon embryogenesis, this is the sole instance of a wg stripe appearing out of anterior to posterior order. wg stripes in each segment are positioned several rows anterior to the posterior edge of each segment.
Expression of wg in E. leukon through embryogenesis. Ventral view (a–k, m) or lateral view (l): Anterior is to the left (a–g, m) or up (h–l). a Torpedo stage. Two head spots and strong expression in the posterior growth zone are evident. The extra tissue beyond the posterior-most expression is an artifact of the preparation. b Early segmentation stage. Posterior-most region of the embryo is folded ventrally. Stomodeal (presumptive mouth) expression has appeared (arrow) directly between paired patches of wg in the antennae; labial expression (arrowhead) is weaker at the ventral midline. c Early abdominal segmentation stage. Intercalary expression is evident between the antennal and mandibular segments (arrow). Two abdominal wg stripes are present, with fainter expression in the more posterior stripe (asterisk). d Mid-abdominal segmentation stage. Stomodeal expression remains evident (arrow), while ocular expression has been subdivided into anterior and posterior sections (single bracket). The thoracic appendages are just beginning to elongate, five abdominal stripes are present, and the newest expression in abdominal segments four and five is composed of discrete clusters of wg-expressing cells (asterisks) separated by a region of lower expression at the ventral midline. e Late segmentation stage. wg expression is downregulated along the ventral body wall of anterior segments, while segmental expression remains strong in the appendages, the intercalary segment, the distal-most region of the mandibular segment, and the posterior growth zone also maintains strong wg expression (asterisk). f EGB stage (the phylotypic stage for insects). All body segments are visible: three gnathal, three thoracic, and ten abdominal. Ventral body wall expression has been downregulated in the anterior-most segments of the embryo. g Post-EGB stage. wg is expressed in the paired labral buds (black arrows) and along the ventral margin of the antennae. Paired crescents of expression are evident in the gnathal region (white arrows). Palp and endite regions of the maxilla and labium are distinct and expression has been downregulated distally in the maxillary endite (asterisk), but not in the labial endite. Additionally, punctate expression is visible in the abdominal segments (only the first three abdominal segments are visible in this specimen). h Anterior end of an elongating embryo (stage is between that seen in d and e). i Procephalon of an embryo just prior to the EGB stage. The labral lobe expresses wg in patches (white arrows). Expression of wg in the lateral cephalon is also visible (black arrows). j Gnathal and thoracic regions of the embryo in i showing detail of wg stripes across the segments and into the gnathal and thoracic appendages. k Anterior of a post-EGB stage embryo showing loss of segmental expression outside of the appendages. The labrum has two patches of expression (white arrows) and stomodeal staining is present. l Thoracic limbs of a post-EGB embryo showing that ventral stripes that do not extend to the very distal tips of the limbs. m Posterior of a post-EGB embryo showing two expression domains, one in the anterior hindgut (arrow) and another ring at the base of the cerci (Ce) and caudal filament (Cf), corresponding to the posterior hindgut
When segmental wg expression is initiated, it appears as two clusters separated by a small gap along the midline. Developmentally older and more anterior segments express wg in a continuous stripe across the midline (Fig. 3d, e). Just before the completion of segmentation, wg expression in the gnathal and thoracic segments begins to fade mid-ventrally (Fig. 3e), eventually resolving to weak or no expression ventrally by the EGB stage (Fig. 3f). At this stage, the abdominal segments retain wg across their midlines (Fig. 3f). Subsequently, wg is expressed in discrete clusters in the abdominal segments (Fig. 3g).
wg expression in the procephalon
wg expression in the procephalon is visible in the ocular region, as well as in the antennae, labrum, and stomodeum. In the earliest stages of development, wg is expressed strongly in the procephalon in two patches recessed slightly from the edges of the embryo (Fig. 3a). These patches of wg expression become positioned laterally (Fig. 3b–d) as the embryo elongates and segmentation continues. By about mid-segmentation, the wg patches become bisected into a large anterior and smaller posterior domain (Fig. 3d, e, h).
The antennae of E. leukon express wg very early in embryogenesis. Faint paired expression domains are visible as segmentation commences (Fig. 3b). During segmentation, antennal wg stripes extend along the ventral margin of the elongating structures (Fig. 3c–e), but expression never extends across the ventral midline of this region. After the completion of segmentation, wg expression becomes faint and somewhat discontinuous along the ventral antennal margin (Fig. 3f, g).
wg is expressed in post-EGB embryos in two patches at the middle and anterior-most region of the procephalon (Fig. 3g), corresponding to the labral buds, which will eventually move medially and fuse to form the mature labrum—this is the first obvious expression of wg in the labral region (Fig. 3i, k). The stomodeum (the presumptive mouth and anterior limit of the gut) is positioned directly posterior to the labrum and expresses wg early in embryogenesis as a faint patch directly between the nascent antennae (Fig. 3b). Stomodeal expression remains apparent in the pre- and post-EGB stages (Fig. 3c–e, g, k).
Expression of wg in the mouthparts and thoracic legs
The expression of wg in the developing postoral appendages of E. leukon (mandibles, maxillae, labium, and thoracic legs) is inherited from the original segmental stripes. During the early segmenting stages, wg initially extends in a stripe along the ventral margin of each of the elongating appendages (Fig. 3d–h, j–l). Later, at the EGB stage, mandibular expression becomes restricted to two patches near the distal tip of the appendages (Fig. 3f). In contrast, maxillary and labial wg expression at the EGB stage consists of continuous stripes across these appendages out to the distal-most tips (Fig. 3f). In early post-EGB stage embryos (Fig. 3k), maxillary expression first becomes relegated to two domains, and older post-EGB stages (Fig. 3g) express wg in three distinct patches, including one patch at the tip of the elongating palp. Late-stage wg expression in three discrete regions of the maxillae may result from downregulation in a medial region that will give rise to the endites, or branches, as well as from downregulation along the ventral edge of the palp (Fig. 3g). In contrast, labial expression in the post-EGB stages consists of a single stripe that does not extend to the distal most tip of the limb (Fig. 3g, k).
Additional regions of wg expression
The earliest expression of wg in E. leukon embryos occurs in the most posterior region of the embryo, the so-called proliferative zone (Fig. 3a). A posterior patch of wg is visible throughout segmentation (Fig. 3b–e), but is absent by the EGB stage (Fig. 3f). At the post-EGB stages, wg patches occur in each abdominal segment (Fig. 3g). Additionally, in post-EGB embryos, wg is expressed at the base and along the inner margins of the cerci, along the caudal filament and in the posterior region of the hindgut (Fig. 3m). We did not obtain robust staining in the oldest embryonic stages and in young nymphs. This is likely due to a combination of factors: (1) decreased penetration of riboprobes and antibodies that leads to no visible signal or (2) increased “stickiness” of tissue that creates dark, non-specific staining.
Segmental localization of En during E. leukon embryogenesis
En expression in the segments occurs progressively over early embryogenesis, paralleling the pattern seen for wg. Like wg stripes, segmentally repeated stripes of En appear prior to the development of morphologically distinguishable segments and are added in an anterior to posterior progression (Fig. 4a–d). Comparison of wg and En expression patterns following the appearance of segmental boundaries suggests that En stripes are situated just posterior to the wg stripes; confirmation of this pattern requires double stains for wg mRNA and En protein. During limb elongation phases, En staining does not extend fully to the distal-most reach of the appendages (Fig. 4c, d). However, post-EGB stage embryos express En in stripes that extend to the lateral edges of each segment (Fig. 4e).
Localization of the En antibody 4F11 during E. leukon embryogenesis. Ventral view, anterior is to the left in a–g. a Heart stage, slightly younger stage than the torpedo stage seen in Fig. 3a. En expression is strongest in the posterior of the embryo. b Early abdominal segmentation stage; note that the posterior abdomen is missing. A stripe of intercalary expression is first evident at this stage (asterisk). Antennal expression is visible (black arrow). En stripes are situated at the very posterior of each segment and in contrast to wg (and En in later stages); expression extends to the lateral edges of each segment. c, d Mid-abdominal segmentation stages showing four (c) and five (d) abdominal stripes of En. In c and d, intercalary expression is indicated (asterisk) and expression in abdominal segment 4 is marked with an arrow. During limb elongation, En expression terminates before reaching the distal tips of the appendages. e Post-EGB stage. The posterior of the body is folded up against the ventrum (abdominal segments are labeled to illustrate folding); see Fig. 2d for clarity. Two faint patches of En are visible in the cephalon (white arrows) and the antennae express En distally (white asterisk). Stripes of En extend to the very edges of the segments (two black asterisks at top of panel). f Post-EGB stage embryo with posterior region unfolded. En expression in the first and second limbs is marked with arrowheads. Punctate staining in the most posterior abdominal segments is indicated (arrows). g Anterior of a late stage embryo. Punctate stain in the gnathal and thoracic segments near the ventral midline is indicated (arrow). An arrowhead indicates En expression in the first thoracic limb, which is aligned parallel to the main body axis, with the very distal tip curved inward. Scale bar 100μm (a, b), 200μm in (c–g)
Localization of En in the procephalon, mouthparts, thoracic legs, and ventral region
En localization in the procephalon of E. leukon is evident in the ocular and antennal regions. Ocular expression is evident at the EGB stage (Fig. 4e). Later expression, if present, is obscured. Antennal expression occurs in paired stripes in the head during segmentation (Fig. 4b–d), but by the post-EGB stage, the antennae express En only in their distal tips (Fig. 4e). Labral expression of En is not observed at any of the stages of embryogenesis that we examined.
Developing appendages of E. leukon inherit En expression from the segmental expression initiated early in embryogenesis, as is also seen for wg expression. As appendages elongate, En expression extends along the ventral margins of the limbs (Fig. 4c, d) and out to the tips of the limbs in post-EGB stages (Fig. 4f, g). En expression in the mouthparts and thoracic legs of E. leukon remains relatively unchanged in the late stages we have examined, including the post-EGB stage (Fig. 4e–g), indicating that downregulation of wg is not attributable to loss of En expression in these regions. Late in embryogenesis, clusters of cells along the mid-ventrum express En in each segment (Fig. 4e–g); these are presumed to belong to the nervous system.
Discussion
Expression of wg in mayflies relative to other arthropods
wg expression in the segmenting embryos of E. leukon is similar to that of numerous arthropods. Both the sequential appearance of segmentally reiterated stripes and the out-of-sequence appearance of the intercalary expression are highly conserved across insects (Dearden and Akam 2001; Giorgianni and Patel 2004; Jockusch and Ober 2004; Jockusch et al. 2004; Miyawaki et al. 2004; Nagy and Carroll 1994; Niwa et al. 2000; Ober and Jockusch 2006). Striped expression has also been observed in several crustacean, myriapod, and chelicerate taxa (Damen 2002; Duman-Scheel et al. 2002; Hughes and Kaufman 2002a; Janssen et al. 2004; Nulsen and Nagy 1999; Prpic 2004; Williams and Nagy 1996).
Variation in the intensity of wg expression along the dorsoventral axis occurs in many taxa. Lower levels of wg expression ventrally, as seen in E. leukon (Fig. 2g, j, k), are also seen in the myriapods Glomeris marginata (in which wg expression is also absent dorsally) and Lithobius atkinsoni (Hughes and Kaufman 2002a; Janssen et al. 2004) as well as the mysid shrimp Mysidium columbiae where lateral expression is consistently more robust than ventral expression (Duman-Scheel et al. 2002). In the spider Cupiennius salei, wg is only expressed in the appendages and never extends across segments (Damen 2002). Downregulation of expression of wg along the dorsoventral axis of each segment occurs in the embryos of Drosophila and Tribolium, resulting in discrete patches of expression (Baker 1987, 1988; Nagy and Carroll 1994), and in Drosophila, the differential regulation of wg along the dorsoventral axis has been characterized (Bejsovec and Martinez Arias 1991). Thus, while segmentally reiterated expression of wg is a conserved component of arthropod segmentation, this perceived conservation masks considerable evolutionary variation in the dorsoventral extent and dynamics of segmental expression across taxa. The functional significance of this variation is not yet clear. Janssen et al. (2008) have argued that in millipedes, wg is required only for the patterning of ventral segment boundaries, while dorsal segment boundary formation is independent of wg.
Strong wg expression in two patches in the procephalon occurs in regions that likely correspond to the “head blobs” described in Drosophila (Liu et al. 2006; Schmidt-Ott and Technau 1992) as well as the dual patches in the cephalon of Tribolium and Schistocerca embryos (Dong and Friedrich 2005; Liu et al. 2006). Bisection of the wg domains in the procephalon of E. leukon embryos closely parallels the splits seen in Tribolium and Schistocerca (Dong and Friedrich 2005; Liu et al. 2006). In these two insects, this subdivision has been implicated in the partitioning of dorsal and ventral protocerebral neuroectoderm into subcomponents of the visual system and the protocerebrum, respectively (Liu et al. 2006). The division of an originally large patch of wg expression in the procephalon into subdomains has been suggested to be associated with the development of stemmata in Tribolium and compound eyes in Schistocerca, as contrasted with the derived condition of Drosophila larvae which do not manifest this subdivision (Baker 1988; Liu et al. 2006; Schmidt-Ott and Technau 1992).
In first-instar hatchlings of E. leukon, five pigmented spots on the dorsum of the head are visible: two lateral pairs (one anterior and one posterior pair) and a single crescent-shaped structure at the midline which correspond to the ocelli or light-sensing organs. Mayflies including E. leukon hatch without compound eyes. As documented in several heptageniid mayflies, the compound eyes of mayfly nymphs originate from the posterior pair of ocelli as a single ommatidium (Needham et al. 1935), with additional ommatidia added progressively in late nymphal development (Clifford et al. 1979; Needham et al. 1935). Further investigation of the molecular patterning of the compound eyes in E. leukon requires chronicling wg expression over late embryogenesis and through nymphal development, complemented with expression and functional data for genes involved in retinal determination, e.g., eyeless, sine oculis, and eyes absent (Bonini et al. 1993; Cheyette et al. 1994; Quiring et al. 1994).
As is true for segmental stripes, wg expression along the ventral edges of gnathal appendages is highly conserved across arthropods (Angelini and Kaufman 2005b). At the EGB stage, E. leukon expresses wg in two patches in the mandibles and in continuous stripes across the maxillary and labial appendage primordia, similar to the expression patterns reported for Tribolium and Schistocerca (Giorgianni and Patel 2004; Jockusch et al. 2004). The mandibles of young E. leukon nymphs are composed of an inner toothed region (the canines and molar surface) plus “tusks” that are oriented at a right angle to the toothed region; these mandibular tusks are unique to mayflies and are found in several burrowing species (McCafferty 1975). In E. leukon, the tusks first appear as small nubs in nymphs past the second instar (O’Donnell 2009). In Drosophila, wg acts cooperatively with decapentaplegic (dpp) to upregulate the expression of Distal-less and consequently promote the outgrowth of appendages (Lecuit and Cohen 1997). Determining whether wg plays a role in regulating the outgrowth of the mandibular tusks in E. leukon will require a study of the post-embryonic stages of development. Specifically of interest is whether a novel patch of wg is expressed at the frontal margin of the mandible prior to tusk outgrowth, as would be expected in a Drosophila-based spatial reiteration model of mouthpart branching (Panganiban et al. 1995). Data drawn from E. leukon will add important information to our understanding of the origin and modification of mouthparts with a secondary axis.
Expression of En in mayflies relative to other arthropods
The segmental expression of En in E. leukon is highly similar to that of several other arthropods and invertebrates (Abzhanov and Kaufman 2000; Patel et al. 1989; Prud’homme et al. 2003). The absence of labral En expression is typical of all insects examined except Drosophila (e.g., Patel et al. 1989; Peterson et al. 1998; Rogers and Kaufman 1996). Similarly, clusters of En-expressing cells in the ventral region have been documented in a host of arthropods including several crustaceans (Procambarus clarkii, Porcellio scaber; Abzhanov and Kaufman 2000), Marmokrebs (Sintoni et al. 2007), and insects (Thermobia domestica: Peterson et al. 1998; Schistocerca: Boyan and Williams 2002; Siegler et al. 2001; Tribolium: Patel et al. 1989; Drosophila: DiNardo et al. 1985). Mutational analyses in D. melanogaster suggest that En functions in neural specification during embryogenesis (Brower 1986; DiNardo et al. 1985). In addition, studies in Schistocerca and Marmorkrebs have implicated En in neuronal patterning of the cephalon and segmental neuromeres (Boyan and Williams 2002; Siegler et al. 2001; Sintoni et al. 2007). Definitive evidence as to whether or not the ventral clusters of En expression in late stage E. leukon embryos precede neuronal structures of the nymphs awaits functional work as our data provide purely correlative data in terms of timing and location of En expression.
Engrailed paralogues
En expression in E. leukon as revealed with the En4F11 antibody may have provided only a partial picture of expression as two copies of En have been isolated from the mayfly Ephemera vulgata (Ephemeridae; and a host of other insects (see Peel et al. 2006)). This suggests the strong likelihood that E. leukon has at least two copies of En in its genome. We do not know whether En4F11 recognizes one or both En paralogues in E. leukon. Expression of the two paralogues in Schistocerca embryos is highly similar, with differences reported only in the timing of expression of each gene copy in the mandibles and antennae (Peel et al. 2006). Minor differences in expression of engrailed paralogues have also been noted in Thermobia (Peterson et al. 1998), Periplaneta (Marie and Bacon 2000) and Drosophila (Coleman et al. 1987). However, in two crustaceans, Abzhanov and Kaufman (2000) documented divergent expression during late embryogenesis of the two En paralogues in tandem with two different antibodies (4D9 and 4F11). Cloning and expression details for each copy of En are essential to determine whether the En4F11-based expression profile reflects a composite or partial picture of En expression in E. leukon. The En4D9 antibody did not produce discernible signal in E. leukon embryos, while En4F11 worked robustly. A lack of signal may reflect a mismatch between the homeodomain epitope recognized by the 4D9 antibody (ELGLNEAQIKI; Serrano et al. 1995) and the sequence of the homologous region of mayfly En. In E. vulgata, both En paralogues have several amino acid mutations in this region (En-1: DLGLHENQIKI, En-2: ELKLNESQIKI; Peel et al. 2006; underlined residues indicate divergent sites between the epitope region of the 4D9 antibody and the mayfly paralogues), which may account for the lack of signal when E. leukon embryos were exposed to En4D9.
Mayflies as a developmental model
Some components of the molecular mechanisms underlying the patterning of the segmental body plan of arthropods and their close relatives are highly conserved, including the widely documented expression of abutting stripes of wg and en in parasegments (Damen 2002; Eriksson et al. 2009). Expression data from the mayfly E. leukon documented here provide additional evidence for this conservation across insects. In addition, this work provides a starting point for elevating a mayfly species onto the stage for expanded study of molecular development, along with recent expression data from the mayfly Ephoron eophilum documented by Niwa et al. (2010). Future work will focus on genes that have been documented to have divergent expression and function across arthropods, including those that lie upstream and downstream of the segment polarity genes as well as genes implicated in branched appendage patterning (e.g., in Tribolium, Oncopeltus, and Gryllus; Angelini and Kaufman 2004, 2005a; Choe and Brown 2007, 2009; Miyawaki et al. 2004). Data from other components of the segmentation cascade (e.g., pair rule and Hox genes) and particular appendage patterning genes (e.g., decapentaplegic) in E. leukon will be helpful to calibrate the character and timing of evolutionary transitions for the majority of insect lineages (Angelini and Kaufman 2005b; Hughes and Kaufman 2002b). In addition, paralogues of wg and en have been isolated across insects (Bolognesi et al. 2008b; Peel et al. 2006), and functional studies in Tribolium demonstrated that knockdown of wg/Wnt-1 alone has only a limited ability to disrupt segmental patterning (Bolognesi et al. 2008a). Therefore, screening of the E. leukon genome for additional members of the Wnt and en families followed by careful documentation of expression is critical to more fully understand the roles of different members of these gene families in E. leukon. Lastly, gene expression data provide one type of data to understand the developmental basis of morphology. In turn, developing RNA interference (RNAi) methods for mayflies is of interest to probe the functional effects of important patterning genes. The recent demonstration of working RNAi protocols in thysanuran embryos (Ohde et al. 2009) is a promising development for the tractability of the development of functional assays in a greater number of non-model organisms.
Recently, Sommer (2009) argued that evo–devo should proceed by concentrating on a small number of model systems and investing heavily in the development of functional tools for these systems. While we agree that it is important to be able to study some systems in depth, we believe that studying the full phylogenetic breadth contributed by the “evo” component of evo–devo is equally important. One major reason that breadth is required is that accurate inferences of ancestral states are critically dependent on taxon sampling. Failure to sample key lineages can lead to ill-informed assumptions about ancestral conditions (see Jenner 2006). Comparisons across deep phylogenetic distances, such as between major lineages of arthropods or between arthropods and vertebrates, rely implicitly on inferring the morphology and development of the most recent common ancestor of each group. A good example of this comes from the recent debate about whether segmentation is ancestral for bilaterians, and the recent discovery that Notch and Delta, which are required for segmentation in vertebrates, are also used in segmentation in a diversity of arthropods (Damen 2007; Pueyo et al. 2008; Stollewerk et al. 2003). As far as is known, these genes are not required for segmentation in the major insect models Drosophila and Tribolium, so prior to the availability of data from other taxa, there was no reason to argue against the conclusion that segmentation evolved independently in different segmented lineages. As this example shows, restriction of studies to only a few taxa will necessarily inhibit our ability to infer ancestral states or the sequence of evolutionary transformations that produced the current diversity of life.
Mayflies add a major new phylogenetic branch to the insect systems in which developmental patterning has been studied at the molecular level. Because of their phylogenetic position, developmental data from mayflies are especially important for accurate inferences about the most recent common ancestor of winged insects. Data from representatives of a small number of orders have identified major developmental shifts that have occurred during the evolution of insects. One well known example is the conversion of Hox3 from a canonical hox gene to a gene involved in extraembryonic patterning, which occurred after the most recent common ancestor of branchiopod crustaceans and insects and before the divergence of orthopterans from their sister group (Hughes and Kaufman 2002b; Papillon and Telford 2007). Similarly, alterations in the C-terminal end of the Hox gene Ultrabithorax which cause transcriptional repression of Distal-less and suppression of appendage development occurred after the divergence of collembolans from other hexapods and preceding the diversification of holometabolous insects (Galant and Carroll 2002; Ronshaugen et al. 2002). The large phylogenetic gaps during which these transitions occurred reflect the poor sampling of taxa in this portion of the tree. Developmental data from mayflies and other unsampled (or poorly sampled) lineages that diverged during these time periods can be used to further pinpoint when major developmental transitions such as these occurred. On the other hand, data from mayflies also offer the opportunity to study the origin of specialized mayfly traits, like asymmetric mouthparts (e.g., mandibles in nymphal stages) and the structurally and functionally diverse abdominal gills.
References
Abzhanov A, Kaufman TC (2000) Evolution of distinct expression patterns for engrailed paralogues in higher crustaceans (Malacostraca). Dev Genes Evol 210:493–506
Anderson DT (1972) The development of hemimetabolous insects. In: Counce SJ, Waddington CH (eds) Developmental systems: insects. Academic, London, pp 96–165
Angelini DR, Kaufman TC (2004) Functional analyses in the hemipteran Oncopeltus fasciatus reveal conserved and derived aspects of appendage patterning in insects. Dev Biol 271:306–321
Angelini DR, Kaufman TC (2005a) Functional analyses in the milkweed bug Oncopeltus fasciatus (Hemiptera) support a role for Wnt signaling in body segmentation but not appendage development. Dev Biol 283:409–423
Angelini DR, Kaufman TC (2005b) Insect appendages and comparative ontogenetics. Dev Biol 286:57–77
Baker NE (1987) Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J 6:1765–1773
Baker NE (1988) Localization of transcripts from the wingless gene in whole Drosophila embryos. Development 103:289–298
Bejsovec A, Martinez Arias A (1991) Roles of wingless in patterning the larval epidermis of Drosophila. Development 113:471–485
Bolognesi R, Farzana L, Fischer TD, Brown SJ (2008a) Multiple Wnt genes are required for segmentation in the short-germ embryo of Tribolium castaneum. Curr Biol 18:1624–1629
Bolognesi R, Beermann A, Farzana L, Wittkopp N, Lutz R, Balavoine G, Brown SJ, Shroder R (2008b) Tribolium Wnts: evidence for a larger repertoire in insects with overlapping expression patterns that suggest multiple redundant functions in embryogenesis. Dev Genes Evol 218:193–202
Bonini NM, Leiserson WM, Benzer S (1993) The eyes absent gene: genetic control of cell survival and differentiation in the developing Drosophila eye. Cell 72:379–395
Boyan G, Williams L (2002) A single cell analysis of engrailed expression in the early embryonic brain of the grasshopper Schistocerca gregaria: ontogeny and identity of the secondary head spots. Arth Struct Dev 30:207–218
Britt NW (1962) Biology of two species of Lake Erie mayflies: Ephoron album and Ephemera simulans. Bull Ohio Biol Surv 1:1–70
Broadus J, Doe CQ (1995) Evolution of neuroblast identity: seven-up and prospero expression reveal homologous and divergent neuroblast fates in Drosophila and Schistocerca. Development 121:3989–3996
Brower D (1986) engrailed gene expression in Drosophila imaginal discs. EMBO J 5:2649–2656
Brower AVZ, DeSalle R (1998) Patterns of mitochondrial versus nuclear DNA sequence divergence among nymphalid butterflies: the utility of wingless as a source of characters for phylogenetic inference. Insect Mol Biol 7:73–82
Cheyette BN, Green PJ, Martin K, Garren H, Hartenstein V, Zipursky SL (1994) The Drosophila sine oculis locus encodes a homeodomain-containing protein required for the development of the entire visual system. Neuron 12:977–996
Choe CP, Brown SJ (2007) Evolutionary flexibility of pair-rule patterning revealed by functional analysis of secondary pair-rule genes, paired and sloppy-paired in the short-germ insect, Tribolium castaneum. Dev Biol 302:281–294
Choe CP, Brown SJ (2009) Genetic regulation of engrailed and wingless in Tribolium segmentation and the evolution of pair-rule segmentation. Dev Biol 325(2):482–491
Clifford HF, Hamilton H, Killins BA (1979) Biology of the mayfly Leptophlebia cupida (Say) (Ephemeroptera: Leptophlebiidae). Can J Zool 57:1026–1045
Coleman KG, Poole SJ, Weir MP, Soeller WC, Kornber T (1987) The invected gene of Drosophila: sequence analysis and expression studies reveal a close kinship to the engrailed gene. Genes Dev 1:19–28
Damen WGM (2002) Parasegmental organization of the spider embryo implies that the parasegment is an evolutionary conserved entity in arthropod embryogenesis. Development 129:1239–1250
Damen WGM (2007) Evolutionary conservation and divergence of the segmentation process in arthropods. Dev Dynam 236:1379–1391
Dearden PK, Akam M (2001) Early embryo patterning in the grasshopper, Schistocerca gregaria: wingless, decapentaplegic and caudal expression. Development 128:3435–3444
Desplan C, Theis J, O'Farrell PH (1985) The Drosophila developmental gene, engrailed, encodes a sequence-specific DNA binding activity. Nature 318:630–635
DiNardo S, Kuner JM, Theis J, O'Farrell PH (1985) Development of embryonic pattern in Drosophila melanogaster as revealed by accumulation of the nuclear engrailed protein. Cell 43:59–69
Dong Y, Friedrich M (2005) Comparative analysis of Wingless patterning in the embryonic grasshopper eye. Dev Genes Evol 215:177–197
Duman-Scheel M, Pirkl N, Patel NH (2002) Analysis of the expression pattern of Mysidium columbiae wingless provides evidence for conserved mesodermal and retinal patterning among insects and crustaceans. Dev Genes Evol 212:114–123
Edmunds GF Jr, Nielson LT, Larsen JR (1956) The life history of Ephoron album (Ephemeroptera: Polymitarcidae). Wasmann J Biol 14:145–153
Eriksson BJ, Tait NN, Budd GE, Akam M (2009) The involvement of engrailed and wingless during segmentation in the onychophoran Euperipatoides kanangrensis (Peripatopsidae: Onychophora) (Reid 1996). Dev Genes Evol 219:249–264
Fjose A, McGinnis WJ, Gehring WJ (1985) Isolation of a homeobox containing gene from the engrailed region of Drosophila and the spatial distribution of its transcripts. Nature 313:284–289
Galant R, Carroll SB (2002) Evolution of a transcriptional repression domain in an insect Hox protein. Nature 415:910–913
Giberson DJ, Galloway TD (1985) Life history and production of Ephoron album (Say) (Ephemeroptera: Polymitarcidae) in the Valley River, Manitoba. Can J Zool 63:1668–1674
Giorgianni MW, Patel NH (2004) Patterning of the branched head appendages in Schistocerca americana and Tribolium castaneum. Evol Dev 6:402–410
Greve GD, Van der Geest HG, Stuijfzand SC, Kraak MHS (1999) Development and validation of an ecotoxicity test using field collected eggs of the riverine mayfly Ephoron virgo. Proc Exp Appl Entomol 10:105–112
Gullan PJ, Cranston PS (2005) The insects: an outline of entomology. Wiley-Blackwell, Massachusetts
Huang C-Y, Kasai M, Buetow DE (1998) Extremely-rapid RNA detection in dot blots with digoxigenin-labeled RNA probes. Genet Anal Biomol Eng 14:109–112
Hughes CL, Kaufman TC (2002a) Exploring myriapod segmentation: the expression patterns of even-skipped, engrailed and wingless in a centipede. Dev Biol 247:47–61
Hughes CL, Kaufman TC (2002b) Hox genes and the evolution of the arthropod body plan. Evol Dev 4(6):459–499
Ingham PW, Hidalgo A (1993) Regulation of wingless transcription in the Drosophila embryo. Development 117:283–291
Janssen R, Prpic N, Damen WGM (2004) Gene expression suggests decoupled dorsal and ventral segmentation in the millipede Glomeris marginata (Myriapoda: Diplopoda). Dev Biol 268:89–104
Janssen R, Budd GE, Damen WGM, Prpic N-M (2008) Evidence for Wg-independent tergite boundary formation in the millipede Glomeris marginata. Dev Genes Evol 218:361–370
Jenner RA (2006) Unburdening evo–devo: ancestral attractions, model organisms, and basal baloney. Dev Genes Evol 216:385–394
Jenner RA, Wills MA (2007) The choice of model organisms in evo–devo. Nature Rev Genet 8:311–319
Jockusch EL, Ober KA (2004) Hypothesis testing in evolutionary developmental biology: a case study from insect wings. J Hered 95:382–396
Jockusch EL, Williams TA, Nagy LM (2004) The evolution of patterning of serially homologous appendages in insects. Dev Genes Evol 214:324–338
Kjer KM (2004) Aligned 18S and insect phylogeny. Syst Biol 53:506–514
Klingensmith J, Nusse R (1994) Signaling by wingless in Drosophila. Dev Biol 166:396–414
Kornberg T, Siden I, O'Farrell PH, Simon M (1985) The engrailed locus of Drosophila: in situ localization of transcripts reveals compartment-specific expression. Cell 40:45–63
Lecuit T, Cohen SM (1997) Proximal–distal axis formation in the Drosophila leg. Nature 388:139–145
Liu Z, Yang X, Dong Y, Friedrich M (2006) Tracking down the “head blob”: comparative analysis of wingless expression in the developing insect procephalon reveals progressive reduction of embryonic visual system patterning in higher insects. Arth Struct Dev 35:341–356
Mahfooz NS, Li H, Popadic A (2004) Differential expression patterns of the hox genes are associated with differential growth of insect hind legs. PNAS 101:4877–4882
Marie B, Bacon JP (2000) Two engrailed-related genes in the cockroach: cloning, phylogenetic analysis, expression and isolation of splice variants. Dev Genes Evol 210:436–448
McCafferty WP (1975) The burrowing mayflies of the United States (Ephemeroptera: Ephemeroidea). Trans Am Entomol Soc 101(3):447–504
Miyawaki K, Mito T, Sarashina I, Zhang H, Shinmyo Y, Ohuchi H, Noji S (2004) Involvement of Wingless/Armadillo signaling in the posterior sequential segmentation in the cricket, Gryllus bimaculatus (Orthoptera), as revealed by RNAi analysis. Mech Dev 121:119–130
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucl Acids Res 8:4321–4325
Nagy LM, Carroll S (1994) Conservation of wingless patterning functions in the short-germ embryos of Tribolium castaneum. Nature 367:460–463
Needham JG, Traver JR, Hsu YC (1935) The biology of mayflies with a systematic account of North American species. Comstock, New York
Niwa N, Inoue Y, Nozawa A, Saito M, Misumi Y, Ohuchi H, Yoshioka H, Noji S (2000) Correlation of diversity of leg morphology in Gryllus bimaculatus (cricket) with divergence in dpp expression pattern during leg development. Development 127:4373–4381
Niwa N, Akimoto-Kato A, Niimi T, Tojo K, Machida R, Hayashi S (2010) Evolutionary origin of the insect wing via integration of two developmental modules. Evol Dev 12:168–176
Nulsen C, Nagy LM (1999) The role of wingless in the development of multibranched crustacean limbs. Dev Genes Evol 209:340–348
Nusse R, Varmus HE (1992) Wnt genes. Cell 69:1073–1087
Nüsslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila. Nature 287:795–801
O’Donnell BC (2009) Early nymphal development in Ephoron leukon (Ephemeroptera: Polymitarcyidae) with particular emphasis on mouthparts and abdominal gills. Ann Entomol Soc Am 102(1):128–136
Ober KA, Jockusch EL (2006) The roles of wingless and decapentaplegic in axis and appendage development in the red flour beetle, Tribolium castaneum. Dev Biol 294:391–405
Ohde T, Masumoto M, Yaginuma T, Niimi T (2009) Embryonic RNAi analysis in the firebrat, Thermobia domestica: Distal-less is required to form caudal filament. J Insect Biotechnol Sericology 78:99–105
Oppenheimer DI, MacNicol AM, Patel NH (1999) Functional conservation of the wingless-engrailed interaction as shown by a widely applicable baculovirus misexpression system. Curr Biol 9:1288–1296
Panganiban G, Sebring A, Nagy L, Carroll S (1995) The development of crustacean limbs and the evolution of arthropods. Science 270:1363–1366
Papillon D, Telford MF (2007) Evolution of Hox3 and ftz in arthropods: insights from the crustacean Daphnia pulex. Dev Genes Evol 4:315–322
Patel NH, Martin-Blanco E, Coleman KG, Poole SJ, Ellis MC, Kornberg TB, Goodman CS (1989) Expression of engrailed proteins in arthropods, annelids and chordates. Cell 58:955–968
Peel AD, Telford ML, Akam M (2006) The evolution of hexapod engrailed-family genes: evidence for conservation and concerted evolution. Proc R Soc B 273:1733–1742
Peterson MD, Popadic A, Kaufman TC (1998) The expression of two engrailed-related genes in an apterygote insect and a phylogenetic analysis of insect engrailed-related genes. Dev Genes Evol 208:547–557
Prpic N (2004) Homologs of wingless and decapentaplegic display a complex and dynamic expression profile during appendage development in the millipede Glomeris marginata (Myriapoda: Diplopoda). Front Zool 1:1–12
Prud’homme B, de Rosa R, Arendt D, Julien J-F, Pajaziti R, Dorresteijn AWC, Adoutte A, Wittbrodt J, Balavoine G (2003) Arthropod-like expression patterns of engrailed and wingless in the annelid Platynereis dumerilii suggest a role in segment formation. Curr Biol 13:1876–1881
Pueyo JI, Lanfear R, Couso JP (2008) Ancestral Notch-mediated segmentation revealed in the cockroach Periplaneta americana. PNAS 105(43):16614–16619
Quiring R, Walldorf U, Kloter U, Gehring WJ (1994) Homology of the eyeless gene of Drosophila to the Small eye gene in mice and Aniridia in humans. Science 265:785–789
Regier JC, Shultz JW, Swick A, Hussey A, Ball B, Wetzer R, Martin JW, Cunningham CW (2010) Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 463:1079–1083
Rogers BT, Kaufman TC (1996) Structure of the insect head as revealed by the EN protein pattern in developing embryos. Development 122:3419–3432
Ronshaugen M, McGinnis N, McGinnis W (2002) Hox protein mutation and macroevolution of the insect body plan. Nature 415:914–917
Schmidt-Ott U, Technau GM (1992) Expression of en and wg in the embryonic head and brain of Drosophila indicates a refolded band of seven segment remnants. Development 116:111–125
Serrano N, Brock HW, Demeret C, Dura J-M, Randsholt NB, Kornberg TB, Maschat F (1995) polyhomeotic appears to be a target of Engrailed regulation in Drosophila. Development 121:1691–1703
Siegler MVS, Pankhaniya RR, Jia XX (2001) Pattern of expression of engrailed in relation to gamma-aminobutyric acid immunoreactivity in the central nervous system of the adult grasshopper. J Comp Neurol 440:85–96
Simon S, Strauss S, von Haeseler A, Hadrys H (2009) A phylogenomic approach to resolve the basal pterygote divergence. Mol Biol Evol 26:2719–2730
Sintoni S, Fabritius-Vilpous K, Harzsch S (2007) The Engrailed-expressing secondary head spots in the embryonic crayfish brain: examples for a group of homologous neurons in Crustacea and Hexapoda? Dev Genes Evol 217:791–799
Snyder CD, Willis LD, Hendricks AC (1991) Spatial and temporal variation in the growth and production of Ephoron leukon (Ephemeroptera: Polymitarcyidae). JN Am Benthol Soc 10:57–67
Sommer RJ (2009) The future of evo–devo: model systems and evolutionary theory. Nature Rev Genet 10:416–422
Stollewerk A, Schoppmeier M, Damen WG (2003) Involvement of Notch and Delta genes in spider segmentation. Nature 423:863–865
Tojo K, Machida R (1997) Embryogenesis of the mayfly Ephemera japonica McLachlan (Insecta: Ephemeroptera, Ephemeridae), with special reference to abdominal formation. J Morphol 234:97–107
Tojo K, Machida R (1999) Early embryonic development of the mayfly Ephemera japonica McLachlan (Insecta: Ephemeroptera, Ephemeridae). J Morphol 238:327–335
Travis J (2006) Is it what we know or who we know? Choice of organism and robustness of inference in ecology and evolutionary biology. Am Nat 167:303–314
Tsui PTP, Peters WL (1974) Embryonic development, early instar morphology, and behavior of Tortopus incertus (Ephemeroptera: Polymitarcidae). Fla Entomol 57(4):349–356
Watanabe NC (1998) Geographical variation in Japan in egg development of the mayfly, Ephoron shigae (Ephemeroptera: Polymitarcyidae). Freshwater Biol 40:245–254
Watanabe NC, Ohkita A (2000) Life cycle and synchronization of nymphal development of the mayfly Ephoron shigae in Japan (Ephemeroptera: Polymitarcyidae). Aquat Insects 22:108–121
Watanabe NC, Takao S (1991) Effect of a low temperature period on the egg hatching of the Japanese burrowing mayfly, Ephoron shigae. In: Alba-Tercedor J, Sánchez-Ortega A (eds) Overview and strategies of Ephemeroptera and Plecoptera. Sandhill Crane Press, Florida, pp 439–445
Williams TA, Nagy LM (1996) Comparative limb development in insects and crustaceans. Semin Cell Dev Biol 7:615–628
Willman R (2004) Phylogenetic relationships and evolution of insects. In: Cracraft J, Donoghue MJ (eds) Assembling the tree of life. Oxford University Press, New York, pp 330–344
Zecca M, Basler K, Struhl G (1995) Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development 121:2265–2278
Zhang J, Zhou C, Gai Y, Song D, Zhou K (2008) The complete mitochondrial genome of Parafronurus youi (Insecta: Ephemeroptera) and the phylogenetic position of the Ephemeroptera. Gene 424:18–24
Acknowledgments
Janine Caira and Steve Burian contributed feedback on early drafts, and the comments of two anonymous reviewers greatly improved this manuscript. Karen Ober and Dave Angelini provided critical feedback on troubleshooting protocols for microscopy, in situ hybridization, and protein localization. Special thanks to Karen Ober and Katie Rose Boissonneault for their assistance with imaging of DAPI-stained embryos and to Nipam Patel for generously donating the En4F11 antibody.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Simpson
Rights and permissions
About this article
Cite this article
O’Donnell, B.C., Jockusch, E.L. The expression of wingless and Engrailed in developing embryos of the mayfly Ephoron leukon (Ephemeroptera: Polymitarcyidae). Dev Genes Evol 220, 11–24 (2010). https://doi.org/10.1007/s00427-010-0324-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-010-0324-6