Abstract
Main conclusion
A critical investigation into arsenic uptake and transportation, its phytotoxic effects, and defense strategies including complex signaling cascades and regulatory networks in plants.
Abstract
The metalloid arsenic (As) is a leading pollutant of soil and water. It easily finds its way into the food chain through plants, more precisely crops, a common diet source for humans resulting in serious health risks. Prolonged As exposure causes detrimental effects in plants and is diaphanously observed through numerous physiological, biochemical, and molecular attributes. Different inorganic and organic As species enter into the plant system via a variety of transporters e.g., phosphate transporters, aquaporins, etc. Therefore, plants tend to accumulate elevated levels of As which leads to severe phytotoxic damages including anomalies in biomolecules like protein, lipid, and DNA. To combat this, plants employ quite a few mitigation strategies such as efficient As efflux from the cell, iron plaque formation, regulation of As transporters, and intracellular chelation with an array of thiol-rich molecules such as phytochelatin, glutathione, and metallothionein followed by vacuolar compartmentalization of As through various vacuolar transporters. Moreover, the antioxidant machinery is also implicated to nullify the perilous outcomes of the metalloid. The stress ascribed by the metalloid also marks the commencement of multiple signaling cascades. This whole complicated system is indeed controlled by several transcription factors and microRNAs. This review aims to understand, in general, the plant–soil–arsenic interaction, effects of As in plants, As uptake mechanisms and its dynamics, and multifarious As detoxification mechanisms in plants. A major portion of this article is also devoted to understanding and deciphering the nexus between As stress-responsive mechanisms and its underlying complex interconnected regulatory networks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The metalloid arsenic (As) is a naturally occurring soil element with no proven beneficial physiological activity for plants (Abbas et al. 2018). Natural geologic processes and anthropogenic activities escalate the level of As in the drinking water and food chain thereby increasing the human exposure for As (Shri et al. 2019). Arsenic contamination in drinking water possesses serious health concerns in many parts of the world, especially in the regions of South and South-East Asia. A huge number of people in some parts of the Bengal delta consume drinking water that contains ≥ 50 μg L−1 of As which is way higher than the permissible limit (10 μg L−1) of the World Health Organization (WHO) (Zhao et al. 2010a; Islam et al. 2015). Moreover, As easily incorporated into plant-based diets such as cereals, vegetables, and fruits (Tripathi et al. 2007). A large portion of food grains such as rice is produced in countries like India, Bangladesh, Vietnam, and China where irrigation with As contaminated water and overuse of As-based agrochemicals is very common. As a result, rice grain produced in these regions is found to accumulate up to 2.24 mg kg−1 of As, whereas the threshold limit of As in rice grain stands at 0.40 μg kg−1 dry weight (Shri et al. 2019). Arsenic holds the top position in the hazardous substance priority list published by the agency for toxic substances and disease registry in 2019 (ATSDR 2019). Acute As poisoning leads to various diseases in humans like skin lesions, thickening of the skin, high blood pressure, blindness, reproductive disorders, partial paralysis, type 2 diabetes, and cardiovascular diseases. The metalloid is also classified as a group-1 human carcinogen by the International Agency for Research on Cancer (IARC) and is responsible for various kinds of cancer (skin, lung, liver, prostate, bladder, colorectal, and breast). Moreover, it affects the intellectual functioning, intelligence, and working memory of children (Das and Sarkar 2018; Rahman et al. 2020; Mondal et al. 2021).
In nature, As persists in both organic and inorganic forms. The inorganic species (iAs) such as arsenate [As(V)] and arsenite [As(III)] are predominant in terrestrial environments (Shri et al. 2019) (Fig. 1). Under aerobic soil conditions highly oxidized As(V) is more frequent, while in anaerobic conditions such as flooded rice fields, the reduced form As(III) is predominant (Finnegan and Chen 2012; Chen et al. 2017a; Tang and Zhao 2021). However, the abundance of organic species of As (oAs) in the soil is fairly low, and whatever minimal quantity found is largely due to the application of As-based agrochemicals in crop fields and/or microbial activity (Quaghebeur and Rengel 2005). In submerged paddy fields, the dynamics of oAs are largely controlled by microbial entities. In those conditions, sulfate-reducing bacteria and methanogens regulate As methylation and demethylation, respectively (Chen et al. 2019). Among oAs, monomethylarsonic acid (MMA), dimethylarsinic acid (DMA), and trimethylarsine oxide (TMAO) is mostly found in soils (Zhao et al. 2010a; Finnegan and Chen 2012). Besides, arsenobetaine and arsenosugar are also found in the soils of acidic fen and some coastal regions, respectively. Nevertheless, these oAs species are ultimately converted into DMA or another iAs (Zhao et al. 2010a). Recently, a new form of As species, thioarsenates has been uncovered from paddy soils (Fig. 1). This pentavalent As species formed due to interaction between As(III), zero-valent sulfur and sulfide (in case of inorganic thioarsenates), and MMA or DMA with sulfide (in case of methylated thioarsenates). The prerequisite for the occurrence of inorganic thioarsenates is neutral to alkaline soil (pH 6.5 and above) and zero-valent sulfur. On the other hand, neutral to acidic soil (below pH 7) and methylated oxyarsenates are essential for methylated thioarsenates. Thioarsenates can be observed throughout the cropping season and their concentrations are almost similar to that of methylated oxyarsenates (Wang et al. 2020). A widely used poultry feed additive roxarsone (3-nitro-4-hydroxy-phenylarsonic acid, ROX) can also act as a potent source of As in soil. The manure of animals that are fed with ROX contains multiple As species such as As(V), As(III), MMA, DMA, 3-amino-4-hydroxyphenylarsonic acid (3-AHPA), 4-hydroxy-phenylarsonic acid (4-HPA), and many other unknown As species which ultimately increases the concentration of As in plants (Yao et al. 2016).
As(V) being a phosphate analog enters plants through phosphate transporters (PHTs). As opposed to, As(III) and methylated As species were generally taken up by aquaglyceroporins (Li et al. 2016). Aquaglyceroporins or aquaporins are integral membrane proteins essentially utilized for water uptake and transit of certain molecules such as CO2, boron (B), silicon (Si), and ammonia (Gautam et al. 2020). Other such transporters involved in either As uptake, translocation, or detoxification include natural resistance-associated macrophage protein (NRAMP) transporters, C-type ATP-binding cassette (ABCC) transporters, inositol transporters, multidrug and toxic compound extrusion (MATE) transporters, auxin transporters (PIN-FORMED or PIN), arsenic compounds resistance (ACR), and vacuolar phosphate transporters (VPT) (Indriolo et al. 2010; Tiwari et al. 2014; Tang et al. 2017; Das et al. 2018; Luan et al. 2019; Ashraf et al. 2020). The metalloid exerts its toxic effect through the disruption of different metabolisms in plants manifested through various physiological, biochemical, and molecular attributes. The most pronounced physiological effects of As include leaf chlorosis, stunted growth, disruption of root architecture, and an overall reduction in growth and yield (Shri et al. 2009; Niazi et al. 2017; Ronzan et al. 2019). Among biochemical toxic effects, impairment in photosynthesis, nitrogen metabolism, ATP synthesis, and overproduction of various reactive oxygen species (ROS) is well documented (Bianucci et al. 2018; Ahmad et al. 2020). The outburst of As stress-induced ROS eventually jeopardizes the redox homeostasis of the cell. ROS causes irreparable damages to biomolecules such as carbohydrates, proteins, lipids, and DNA. To acclimatize with such adversity, plants came up with several strategies including metabolic adjustments. For example, elevated As efflux, reduced As uptake, formation of iron plaque, regulation of As transporters and phytochelatin (PC) and/or glutathione-mediated chelation followed by vacuolar sequestration of the metalloid. Besides, the antioxidant machinery of plants helps immensely in reducing ROS-induced oxidative damage. The antioxidant machinery is usually comprised of both enzymatic and non-enzymatic components. The enzymatic components include superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), guaiacol peroxidase (GOPX), glutathione reductase (GR), glutathione peroxidase (GPX), monodehydroascorbate reductase (MDHAR), and dehydroascorbate reductase (DHAR); the non-enzymatic counterparts include ascorbic acid (AsA), reduced glutathione (GSH), α-tocopherol, carotenoids, flavonoids, phenolics, proline, etc. (Mishra et al. 2019). However, higher doses of the viperous metalloid coupled with other environmental stress factors often limit plants’ stress-responsive mechanisms which in turn hinder their growth and sometimes even lead to cell death.
Arsenic stress-responsive mechanisms in plants cannot be simply elucidated as a mere detoxification process or combination of a few detoxification processes to counter the metalloid toxicity. The underlying mechanism behind these detoxification processes is complicated and orchestrated by diverse signaling cascades that lead to up-regulation and/or down-regulation of As stress-responsive genes. Calcium signaling, phytohormone signaling, mitogen-activated protein kinase (MAPK) signaling, ROS/oxidative stress, and reactive nitrogen species (RNS) -mediated signaling are suspected to be operated during As stressed conditions. These signaling cascades are often found to be interconnected and amplify stress signals to promote As stress-related gene expression. Several transcription factors (TFs) from different families often serve as a downstream target of these signaling pathways which can further modulate the expression of As stress-responsive genes. Recent shreds of evidence also suggested the involvement of microRNAs or miRNAs in such kind of regulatory processes.
Arsenic is a toxicant that some way or other affects plant systems drastically and by doing so, it also increases the health risks of a large number of human populations. In this context, elimination of such a potent toxin from plant tissue is a major research goal and for which we need to better understand the interaction between plants and As in field conditions. Although a considerable amount of progress has been made to unravel the interaction between plants and As, a substantial knowledge gap still prevails. In this review, a molecular insight has been given in the light of recent development on transporters involved in different As species uptake, its long-distance transport, phytotoxic effects, detoxification mechanisms, underlying complex signaling cascades, regulation of As stress-responsive gene expression via TFs and miRNAs, and limitations of plant detoxification mechanisms.
Arsenic uptake in plants
Plants receive As mostly in their inorganic form through various transporter proteins exploiting the concentration gradient between source and sink (Abbas et al. 2018). The As(V) concentration in soil solution is typically below 1 μM except for highly contaminated sites. The reason behind the low availability of As(V) in the soil is probably due to its strong absorption by different minerals particularly iron oxides/hydroxides in the soil solid phase. In submerged field conditions such as in flooded paddy soil As exists mainly in its trivalent form probably due to reduction of As(V) into As(III) and subsequent reductive dissolution of iron oxides/hydroxides. The As(III) concentration in such conditions ranges from sub μM to over 100 μM (Tang and Zhao 2021). The oAs concentration (e.g., MMAs and DMAs) in paddy fields typically ranges between 0–0.1 μM and 0–2.5 μM respectively (Chen et al. 2019). Besides, the total thioarsenate concentration in soil is said to be < 2 μM as observed exclusively in rice fields (Wang et al. 2020). The transport of As from soil to plants largely depends on some determinants such as rhizospheric oxygen level, the redox status of the soil, numerous root factors, and mineral nutrients like iron (Fe), Sulfur (S), Phosphorus (P), and Si (Vithanage et al. 2012). Primarily, As is taken up by plants through root absorption except for a few submerged plants that utilize their leaves for As absorption (Li et al. 2016). Active transport, passive transport, and direct transcellular transport are the three main mechanisms that are utilized by plants to take up As from the surrounding environment (Vithanage et al. 2012).
Phosphate transporter-mediated arsenate uptake
The oxyanions (H2AsO4− or HAsO42−) of most As acid (H3AsO4)/As(V) are chemically analogous to inorganic phosphate (Pi) and, therefore, As(V) easily enters plant root via PHTs (Fig. 2) (Zhao et al. 2010a; Li et al. 2016). The PHT transporters are mostly unidirectional (Abbas et al. 2018) and plants contain five phylogenetically distinct sub-families of PHTs (PHT1-5). Among them, the PHT1 sub-family was found responsible for phosphate uptake from soil (Li et al. 2019a, b). This PHT1 sub-family comes under major facilitator superfamily (MFS) contains a conserved sequence and acts as a Pi/H+ symporter localized mainly on the plasma membrane (Nussaume et al. 2011; Li et al. 2019a, b).
Diagrammatic representation of transporters involved in arsenic uptake, translocation, and basic detoxification mechanism found in different plant species. In root cells, arsenite [As(III)] and methylated arsenic species are taken up through aquaporins like nodulin26-like intrinsic proteins (NIPs), plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), etc. while Pi transporters (PHTs) are responsible for arsenate [As(V)] intake. Once inside, As(V) is reduced to As(III) by arsenate reductases (HACs) and glutaredoxins (GRXs) using glutathione (GSH) as a reducing agent. As(III) frequently form complexes with GSH, phytochelatins (PCs), and metallothionines (MTs) before being sequestered into vacuoles via ATP-binding cassette (ABCC) transporters or arsenical compound resistance (ACR) transporters. An auxin transporter PIN FORMED 2 (PIN2) and NIPs are associated with As(III) efflux. The ABCC7, ACR3, and low silicon 2 (Lsi2) transporters are probably involved in the xylem loading of As(III) and As(III)-PC complexes. Multidrug and toxic compound extrusion (MATE) transporters, inositol (INT) transporters, and natural resistance associated macrophage protein (NRAMP) transporters also assist in long-distance transport of As(III). Putative peptide transporters (PTRs) are responsible for the long-distance transport of methylated arsenic species and their accumulation in reproductive tissues
In Arabidopsis thaliana, there are altogether six PHT1 transporters that are known to transport As(V) (Li et al. 2016) (Table1). Among them, AtPHT1;1 and AtPHT1;4 are high-affinity Pi transporters generally expressed in root cells and mediate acquisition of Pi and As(V) from both high and low Pi environments (Shin et al. 2004; Catarecha et al. 2007). AtPHT1;5 plays a pivotal role in the translocation of Pi from source to sink organs. Loss of function mutation in AtPHT1;5 results in the altered allocation of Pi between root and shoot. The study also reveals moderate to weak tolerance to As(V) in mutant AtPht1;5, suggesting the transporter’s influence in both Pi and As(V) uptake (Nagarajan et al. 2011). The other transporters include AtPHT1;7, AtPHT1;8, and AtPHT1;9 whose primary role is to uptake Pi in Pi deprived condition (Remy et al. 2012; LeBlanc et al. 2013). In Oryza sativa, high-affinity PHT1 transporters were found to be engaged in As(V) uptake via roots. One of them is OsPHT1;8 (OsPT8) expressed in both root and shoot tissues has a high affinity for Pi uptake regardless of Pi concentration (Jia et al. 2011). The overexpression of OsPT8 was found to raise As(V) influx by 3- to 5- fold (Wu et al. 2011). This is in turn can affect root elongation (Wang et al. 2016). OsPHT1;1(OsPT1) is another such Pi transporter constitutively expressed in both root and shoot tissues independent of Pi supply condition (Sun et al. 2012) and is also involved in As(V) uptake from soil or apoplast (Kamiya et al. 2013). Knocking out of OsPHT1;4 (OsPT4) decreased iAs accumulation in O. sativa grains confirming its involvement in As(V) uptake and transport (Cao et al. 2017; Ye et al. 2017).
As-hyperaccumulating Pteris vittata also possesses quite a few PHT transporters that are associated with As(V) uptake. Among them, PvPHT1;1 and PvPHT1;2 shares 98.5% similarity in amino acid composition and thus can be considered as one. PvPHT1;1/2 also shares 72% identity with PvPHT1;3. Surprisingly, PvPHT1;1/1;2 show little or no transport activity for As(V) unlike other Pi transporters (Cao et al. 2019). On the flip side, PvPHT1;3 have a similar affinity for both Pi and As(V), unlike other PHT transporters that prefer Pi over As(V) (DiTusa et al. 2016). Heterologous expression of high-affinity As(V) transporter PvPHT1;3 suggested its efficient role in the translocation of As(V) in P. vittata (Cao et al. 2019). A newly discovered transporter PvPHT1;4 was also shown to have substantial As(V) and Pi transport activity (Sun et al. 2019).
Aquaporin-mediated arsenite uptake
Plant aquaporins belong to the ancient superfamily of major intrinsic proteins (MIPs) and in higher plants, they are categorized into five major subfamilies: the plasma membrane intrinsic proteins (PIPs), the nodulin26-like intrinsic proteins (NIPs), the tonoplast intrinsic proteins (TIPs), the small basic intrinsic proteins (SIPs) and the uncategorized (X) intrinsic proteins (XIPs) (Maurel et al. 2015). Aquaporins are also known to transport essential nutrients like Si, selenium (Se), and B alongside As(III) (Pommerrenig et al. 2015; Kumar et al. 2019). The NIP subfamily is further subdivided into three subgroups, NIP I-III (with exceptions), mainly based on the constriction region known as aromatic/arginine (ar/R) selectivity filter (Mitani-Ueno et al. 2011; Pommerrenig et al. 2015; Yamaji and Ma 2021). Apart from water, the NIP I subgroup is permeable to arsenous acid and antimonous acid, while NIP II subgroup is permeable to boric acid in addition to other elements transported through NIP I. On the other hand, NIP III subgroup is permeable to all elements transported through NIP I and NIP II. Besides, it is also permeable to silicic acid, selenous acid, and germanic acid (Pommerrenig et al. 2015). The ar/R selectivity filter represents the narrowest part of the channel pore-forming a size exclusion barrier that plays a critical role in substrate selectivity for As, B, and Si (Mitani-Ueno et al. 2011).
NIPs are bidirectional and can move As(III) in both directions between plant cell and growth medium depending upon concentration difference (Abbas et al. 2018). Numerous NIPs from different plant species have been identified to date (Table 1). Such as AtNIP1;1, AtNIP1;2, AtNIP3;1, AtNIP5;1, AtNIP6;1, AtNIP7;1 (from A. thaliana); OsNIP1;1, OsNIP2;1, OsNIP2;2; OsNIP3;1; OsNIP3;2, OsNIP3;3 (from O.sativa); LjNIP5;1, LjNIP6;1 (from Lotus japonicus), HvNIP1;2 (from Hordeum vulgare) (Fig. 2) (Takano et al. 2006; Bienert et al. 2008; Isayenkov and Maathuis 2008; Ma et al. 2008; Li et al. 2009; Kamiya et al. 2009; Zhao et al. 2010b; Katsuhara et al. 2014; Xu et al. 2015; Lindsay and Maathuis 2016; Chen et al. 2017b; Sun et al. 2018). These are all associated with either As(III) uptake or translocation (Table 1). OsNIP2;1 (OsLsi1) primarily is a Si transporter localized at the distal side of both exodermis and endodermis cells of rice roots found to mediate the influx of silicic acid and As(III). Whereas OsLsi2 tend to localize on the proximal side of exodermis and endodermis cells of rice roots and is probably not involved in the influx of As(III) rather it is associated with As(III) efflux, xylem loading, and As accumulation in rice grains (Ma et al. 2008). OsNIP1;1, OsNIP3;1 and OsNIP2;2 (OsLsi6) also possesses As(III) uptake capability but their expression pattern is weak when compared to OsLsi1 (Ma et al. 2008).
The shared transporters between As(III) and essential nutrients like B, Se, and Si include AtNIP5;1 [As(III) and B], AtNIP6;1 [As(III) and B], AtNIP7;1 [As(III) and B], OsNIP2;1 [As(III), B, Se, and Si], OsNIP2;2 [As(III) and Si], OsNIP3;1 [As(III) and B]. The NIP-mediated uptake pathways of these nutrients ensure a sufficient supply of these elements to plants. But, while doing so the adventitious uptake of the toxic metalloid As(III) can not be avoided. The surplus As(III) is particularly troublesome for gramineous plants which express high levels of NIPs to ensure adequate Si supply to plants. Plant species such as Brassica crops which rely heavily on NIP-mediated boric acid uptake can accumulate higher levels of As(III) in B depleted and As(III) enriched soil environments (Pommerrenig et al. 2015). The similar geochemistry of As(III) and Si may drive competition for the adsorption sites in field conditions (Saifullah et al. 2018; Hussain et al. 2021). Moreover, Si transporters can also create competition between As(III) and Se(IV) adsorption (Saifullah et al. 2018; Boorboori et al. 2021). It is noteworthy to mention that, the polar localization of transporters is pivotal in directional uptake and efficient distribution of metalloids (Yamaji and Ma 2021). Progress has been made to decipher the mechanism of polar localization of A. thaliana AtNIP5;1, located preferentially on the soil side of the plasma membrane of root cells, predominantly a boric acid channel which is also permeable to As(III). The findings suggest the polar localization of AtNIP5;1 in root epidermal and endodermal cells is maintained by clathrin-mediated endocytosis which is dependent upon phosphorylation of Thr residues in the conserved ThrProGly (TPG) repeat in the N-terminal region of AtNIP5;1 (Wang et al. 2017). Therefore, a possible entrance pathway for As into plant cells via endocytosis cannot be ruled out. Clathrin-mediated endocytosis is also responsible for polar localization of the A. thaliana borate exporter AtBOR1 (Yoshinari et al. 2019).
Apart from NIPs, PIP aquaporins also showed promising outcomes about As(III) uptake (Table1). Heterologous expression study of OsPIP2;4, OsPIP2;6 and OsPIP2;7 in Xenopus laevis oocytes manifested rise in As(III) uptake. On the other hand, overexpression of OsPIP2;4, OsPIP2;6 and OsPIP2;7 in A. thaliana portrays exaggerated As(III) tolerance and higher biomass accumulation (Mosa et al. 2012). Additionally, TIP aquaporin of P.vittata (PvTIP4;1) may also involve in As(III) uptake (He et al. 2016).
Methylated arsenic species uptake
For a long period, it was believed that organic arsenicals are less toxic. Although pentavalent forms of MMA and DMA are less toxic than their inorganic counterparts, the trivalent forms of MMA and DMA are way more toxic than iAs species (Costa de Oliveira et al. 2020). Besides, DMA is supposed to be the causal agent for straight head disease in O. sativa (Tang et al. 2020). It has been already discussed that the presence of oAs such as MMA or DMA in the soil is a rarity. The minimal amount of such species traced in soils is largely due to the use of arsenical pesticides/herbicides or may be synthesized by soil microorganisms (Li et al. 2016; Kumar et al. 2019). It is believed that MMA or DMA is taken up by plants more slowly and inefficiently than that of iAs (Zhao et al. 2009). But surprisingly, a significant amount of methylated As has been detected in plant saps probably due to the efficient root-to-shoot translocation ability of some oAs like DMA (Lomax et al. 2012). In a study, O. sativa grains were shown to accumulate twice as much DMA than that of iAs when exposed to identical concentrations of the two As species. The study further reveals the differential accumulation strategy of more toxic methylated As within reproductive tissues in O. sativa (Zheng et al. 2013). However, the source of the methylated As in plant tissues is still unclear, and whether plants methylate As by themselves or take up microbially produced methylated As is a matter of debate (Kumar et al. 2019). A study conducted by Lomax et al. (2012) suggested that plants generally take up methylated As produced by soil microorganisms. Some microorganisms possess arsM gene that codes for an enzyme S-adenosylmethionine methyltransferase (ArsM) that enables microbes to convert As(III) into mono-, di-, or tri- methyl As species. Higher plants usually lack ArsM and are, therefore, unable to methylate As(III) (Lomax et al. 2012). However, reduction of MMA(V) to MMA(III) followed by possible subsequent vacuolar sequestration of MMA(III) via thiol-rich PCs in O. sativa root was reported previously (Li et al. 2009). The O. sativa aquaporin channel OsNIP2;1 is found to be associated with methylated As species uptake. MMA(V) and DMA(V) are said to be taken up by OsLsi1 in a pH-dependent manner. The lsi1 mutant showed a significant reduction in uptake of MMA(V) (80%) and DMA(V) (50%) than the wild type (Li et al. 2009).
Root-to-shoot translocation of arsenic
Along with nutrients, arsenic transportation may occur via apoplastic (through cell walls), symplastic (through cellular connections), or through a coupled trans-cellular pathway involving polarized influx and efflux carriers. The same routes are associated with the As translocation to the aerial parts of the plants (Zhao and Wang 2020; Pan et al. 2021). The presence of numerous efficient As efflux and influx transporters localized at specific locations (such as exodermis and endodermis cells) facilitates root-to-shoot translocation of different As species. Both iAs and oAs including As(III), As(V), DMA, and MMA have been detected in xylem sap as well as in phloem sap (Awasthi et al. 2017). However, oAs have a better mobility rate than their inorganic counterparts (Zhao et al. 2009; Awasthi et al. 2017). In xylem sap As(III) is dominant over other As species (Zhao et al. 2009). Along with As uptake, PHT and NIP transporters are also involved in xylem loading and phloem loading of As. Peptide transporters, NRAMP1, and inositol transporters also perform a critical role in long-distance transport of As (Table 1). In O. sativa, OsLsi2 (OsNIP2;1) is devoted to xylem loading of As(III) and mutation in OsLsi2 significantly reduced As(III) concentration in xylem sap (Ma et al. 2008). Besides, OsNRAMP1, generally involved in Fe uptake displayed their engagement in As(III) transport (Tiwari et al. 2014). Overexpression studies suggested that OsPHT1;8 initially loads As(V) into the xylem that later converted into As(III) (Wu et al. 2011). Putative peptide transporters OsPTR7 (OsNPF8.1), AtPTR1, and AtPTR5 also influence the long-distance transport of DMA and its accumulation in reproductive parts (Tang et al. 2017). AtPTR1 was initially found to mediate the uptake of di- and tri-peptides into root cells and long-distance transport of organic nitrogen whereas the AtPTR5 mediates the uptake of peptides during pollen germination and transports nitrogen during ovule and early seed development (Dietrich et al. 2004; Komarova et al. 2008). In A. thaliana, AtNIP7;1 and AtNIP3;1 were involved in root-to-shoot translocation of As(III) (Xu et al. 2015; Lindsay and Maathuis 2016). Moreover, two H+-coupled symporters in A. thaliana (AtINT2 and AtINT4) originally meant for loading inositol into phloem are believed to load As(III) as well (Fig. 2) (Duan et al. 2015). More recently, a transporter belonging to the MATE family of transporters that are usually known to carry metabolites and/or xenobiotic compounds, OsMATE2 found to have a role in As(III) translocation in O. sativa grains. Endosperm-specific silencing of OsMATE2 reduced As(III) accumulation within O. sativa grains. However, evidence of direct transport of As(III) via OsMATE2 has not been disclosed yet (Das et al. 2018). An ABCC transporter, OsABCC7, strongly expressed in xylem parenchyma cells, is found to be involved in root-to-shoot transport of As(III) in a conjugated manner either with PC or GSH (Tang et al. 2019). The overexpression of PvACR3 in A. thaliana greatly increased As(III) translocation from roots to shoots, suggesting its involvement in xylem loading performing the role of a plasma membrane efflux transporter (Wang et al. 2018a).
Phytotoxic effects of arsenic
Arsenic gravely influences plant growth and metabolism and can be observed through different physiological, biochemical, as well as molecular parameters. Leaf chlorosis, and significant diminution in growth with visible toxicity symptoms (purplish leaf color) caused from As were observed in Brassica juncea and B. napus upon As(V) treatment (50 and 75 mg kg−1). Besides, overall height, leaf area, the number of leaves and shoot and root dry weight were also reduced (Niazi et al. 2017). Both As(V) and As(III) seem to be toxic for germination of O. sativa seedlings and at higher doses [As(III)—50 and 100 μM; As(V)—100 and 500 μM], no root formation was observed (Shri et al. 2009). Loss of dry weight and fresh weight of root and shoot tissues, reduced yield, impaired fruit production, and other morphological changes are widely reported in plants grown on As contaminated soils (Garg and Singla 2011). The metalloid was further found to alter adventitious root growth, lateral root primordia organization, and development thereby affecting root architecture. Arsenate (at 50 μM concentration) negatively affects IAA biosynthetic (OsASA2 and OsYUCCA2) and transporter gene (AUX1 and PIN5b) expression disrupting IAA biosynthesis, transport, and localization (Ronzan et al. 2019). One of the most detrimental effects of the toxic metalloid is the reduction in photosynthesis rate upon As exposure [6.6–52.8 μmol L−1 As(III) and As(V)] (Gusman et al. 2013a). The pernicious effects of As including malformation of chloroplast ultrastructure, interruption in chlorophyll biosynthesis pathway, and promotion of chlorophyll degradation ultimately inhibit photosynthesis to a certain level (Farnese et al. 2017; Chandrakar et al. 2018). Reduction in chlorophyll-a, chlorophyll-b, chlorophyll-a/b ratio, and total chlorophyll content has already been narrated in many plants like Zea mays [150 μM As(III)], Vigna radiata [22.5 mg kg−1As(V)], O. sativa [2.5 mM As(V)], Cicer arietinum [0.3–0.5 M As(V); 250 μg mL−1As(III)], Triticum aestivum [5 μM As(III)], and Vicia faba [10 and 20 μM As(III)] upon As exposure (Anjum et al. 2017; Das and Sarkar 2018; Ghosh et al. 2018; Adhikary et al. 2019; Maglovski et al. 2019; Ahmad et al. 2020). Arsenic is known for imposing iron deficiency in plants which in turn can interfere with chlorophyll biosynthesis (Shaibur et al. 2009a; Das and Sarkar 2018). Reduced generation of intermediates of chlorophyll biosynthesis pathway and/or chlorophyll degradation under As exposure has also been reported (Maglovski et al. 2019; Ahmad et al. 2020). An analysis on precursors of chlorophyll and degradation metabolites upon As stress revealed that the altered chlorophyll concentration observed is largely due to hindered biosynthesis. The precursor metabolite coproporphyrinogen III could not be detected after 0.5 μM As(V) exposure. Further, the levels of subsequent precursor metabolites like protoporphyrin IX, Magnesium-protoporphyrin (Mg-protoporphyrin), Mg-protoporphyrin methyl ester, and divinyl protochlorophyllide were also significantly decreased at the said concentration which indicates pathway blockage upstream of tetrapyrrole synthesis (Mishra et al. 2016). Accumulation of As beyond threshold level may be correlated with the substitution of central Mg atom of chlorophyll. The alteration in chlorophyll-a/b ratio may be due to the reorganization of the pigment-protein complexes of the photosynthetic apparatus (Maglovski et al. 2019). Besides, the metalloid was also found to manipulate photosynthetic rate [50–200 μM As(III)], stomatal conductance, intercellular CO2 concentration, transpiration rate [50–200 μM As(III); 6.6–52.8 μmol L−1 As(III) and As(V)], water use efficiency [6.6–52.8 μmol L−1 As(III) and As(V)], light-saturated net CO2 assimilation, photochemical efficacy of PS-II, quantum yield of electron transport, non-photochemical quenching coefficient [1.5 mg L−1 As(V)], etc. in plants like B. napus, Lactuca sativa, and Pistia stratiotes (Gusman et al. 2013a; Farnese et al. 2014, 2017; Farooq et al. 2016a).
Roots are the first contact site for As just like the other essential mineral nutrients; therefore, As plays an influential role in maintaining the mineral nutrition homeostasis of the plants. The toxicity of As can alter the cell membrane permeability and selectivity resulting in lower nutrient uptake (Gusman et al. 2013b). Accumulation of As beyond threshold level manipulates uptake of several micro- and macronutrients mainly through competition for binding to transport proteins (Chandrakar et al. 2018). Arsenic reportedly limits the trace minerals like Se, Zinc (Zn), and Nickel (Ni) in O. sativa upon 10–20 mg kg−1 As(V) treatment (Williams et al. 2009). Variable doses of As(III) [0–67 μmol L−1] reduced the accumulation of macronutrients [Calcium (Ca), potassium (K), Mg, and P] and micronutrients [copper (Cu), manganese (Mn), and Zn] with a concomitant increase of As concentrations in H. vulgare (Shaibur et al. 2009b). The majority of mineral elements found roots such as Ca, chlorine (Cl), Cu, Fe, K, S, and Si found to be diminished in As(III) treated [60 μM] roots of O. sativa (Singh et al. 2018). Similarly, in P. stratiotes, As(V) [1.5 mg L−1] was found to affect the concentrations of Fe, Mg, Mn, and P negatively (Farnese et al. 2014). The contents of Ca, Fe, and P were found to decrease greatly in seeds of C. arietinum grown in As(V) contaminated soils (5 mg kg−1 of dry soil) (Malik et al. 2011). The ionic concentrations of Ca, Cu, Fe, K, Mg, rubidium (Rb), Si, strontium (Sr), and Zn decreased in Helianthus annuus upon As(V) treatment (30 and 60 mg kg−1) (Gunes et al. 2010). In some cases, uptake of essential nutrients like Ca, P, and Mg was found to be increased in metalloid-exposed plants and is considered to be a common defense response. It has been assumed that an alteration in the vacuole and apoplast pools, the deposition site for the majority of the toxic elements, induces the uptake of Ca, K, Mg, and P, so they can form aggregates with the toxic elements. Some studies correlated low As concentration to an increase of P uptake by plants, due to a P deficiency driven by As(V). The presence of P strongly suppresses the As(V) adsorption. However, it causes an increase of As mobility in soil and a subsequent increase in As accumulation in plants. Therefore, it has been suggested that the interactions of P with As(V) are growth condition and concentration dependent (Duan et al. 2013; Gusman et al. 2013b). At resembling concentrations of P and As in soil, arsenic is more readily available for the uptake by the plants due to its smaller size, and the charge of P ions that binds with soil particles with a higher affinity than As(V). The lower soil uptake of P drives competition with As(V) over time. According to ligand exchange theory, stock charge hypothesis, and Steindorf–Rehbon–Shintoch equation, Pi is inclined to be replaced by As(V) in soil (Boorboori et al. 2021). Arsenic absorption compromises the cellular metabolism in plants that subsequently augment ROS production one way or other. In plants, As disrupts protein functions due to its high affinity towards sulfhydryl (–SH) groups. Moreover, it damages the plasma membrane through membrane lipid peroxidation by generating ROS, such as hydrogen peroxide (H2O2), superoxide radical (O2•−), and hydroxyl radicals (•OH), leading to apoptosis (Finnegan and Chen 2012; Jung et al. 2019). The ROS generated through As toxicity tend to accumulate within plant cells that can further deteriorate normal biological processes either by dismantling redox homeostasis or by impairing biosynthesis pathways of basic biomolecules required for plant growth, such as carbohydrates, proteins, fats, and nucleic acids (Jung et al. 2019). Elevated concentrations of hydrogen peroxide (H2O2) have been observed with increasing concentrations of As in horseradish and V. faba (Kofroňová et al. 2019; Ahmad et al. 2020). Similarly, As(III) aggravated H2O2 and superoxide radical (O2•−) levels in ryegrass and O. sativa (Jung et al. 2019; Li et al. 2019a, b). Membrane lipids are the most explicit target for ROS for their abundance (Demidchik 2015). Oxidation of lipids occurs mainly in its fatty acid moiety, particularly in polyunsaturated fatty acid (PUFA) (Mano 2012; Farmer and Mueller 2013). Peroxidation in PUFA occurs when a double bond in PUFA is oppressed by singlet oxygen (1O2) or a hydrogen atom is dispelled from a double bond by •OH or O2•− generating lipid hydroperoxide (LOOH). The LOOH can give rise to multiple radical species e.g., lipid alkoxy radicals (LO•), lipid peroxide radicals (LOO•), lipid radicals (L•), etc. Various carbonyl species (reactive carbonyl species or RCS) are generated through the spontaneous decomposition of these radical species (Mano 2012). Arsenic induces oxidative stress through elevated ROS production which in turn may induce peroxidation of PUFA in the membranes that can cause the emergence of malondialdehyde (MDA), a terminal product of membrane lipid peroxidation (Srivastava and Singh 2014). Increased level of MDA in As exposed plants is an indication of free radical formation in the cells which serve as determinative of lipid peroxidation. Arsenic-induced MDA production has also been reported in other plants like B. napus [50–200 μM As(III)], V. mungo [100 and 200 μM As(V)], C. arietinum [10–160 mg kg−1As(V)], O. sativa [15 μM As(III)], etc. by various authors (Farooq et al. 2016a; Srivastava et al. 2017; Adhikary et al. 2019; Jung et al. 2019). Arsenic incited membrane damage is also indicated by higher electrolyte leakage (EL) values which is an indication of higher membrane injury. Upon As treatment [10 and 20 μM As(III)], EL was found to increase by 100–300% in V. faba (Ahmad et al. 2020). Similar to lipid molecules, proteins are also susceptible to ROS attack. The ROS produced in response to As stress can modify the structural property of the proteins by adding carbonyl moieties, particularly on Arg, Cys, His, Lys, Met, Pro, Thr, and Trp residues directly or through RCS generated through lipid peroxidation conveying ROS signals to proteins. Protein carbonylation is often viewed as an indicator of protein oxidation in plants (Yadav et al. 2016; Mano et al. 2019).
One of the most drastic effects of As on plants is the replacement of Pi with As(V) in key metabolic processes like glycolysis, oxidative phosphorylation, RNA/DNA metabolism, lipid biosynthesis, protein phosphorylation/dephosphorylation, etc. The ability of As(V) to operate as a substrate in Pi requiring reactions such as the conversion of ADP to ATP through F0–F1 type ATP-synthase disrupts ATP synthesis and energy status of the cell (Finnegan and Chen 2012). Plants accumulate As(V) mainly through PHT channels and compete with Pi. A low affinity of As(V) towards PHT transporter means a reduction in As(V) uptake in adequate Pi conditions. However, in the Pi limiting environment, As(V) intake increases significantly (Abbas et al. 2018).
Arsenic was also found to affect the carbohydrate metabolism in plants. A sharp decline in the proportion of reducing to non-reducing sugars, inhibition of starch degrading enzymes (e.g., α- and β-amylase, starch phosphorylase, etc.), and activation of sucrose-hydrolyzing enzymes (e.g., acid invertase and sucrose synthase) are among the most frequently observed phenomena upon As exposure (Jha and Dubey 2004a; Roitsch and González 2004; Baud and Lepiniec 2009; Kaur et al. 2012).
Added further, As is well-known for its interference in the symbiotic N2 fixation and nitrogen assimilation mechanism (Bianucci et al. 2018). Arsenic caused a marked decline in nitrogen assimilatory enzymes viz. nitrate reductase, nitrite reductase, glutamine synthetase, and glutamate synthase (Jha and Dubey 2004b; Ghosh et al. 2013). The decline of the nitrogen assimilatory enzymes is accompanied by the decreased affinity for their substrates (Jha and Dubey 2004b). The total amount of soluble nitrogen and free amino acid contents were also adversely affected by As (Ghosh et al. 2013). A sharp decrease in total protein content and protease activity in plants is often observed with As contamination (Abbas et al. 2018; Ghosh et al. 2018).
The prolonged exposure to toxic quantities of As can induce genotoxicity in the forms of DNA–protein cross-links, breakage of chromatid/chromosome or their exchange, chromosomal aberrations due to segregational errors, creation of apurinic/apyrimidinic sites, chromosome deletion, aneuploidy, and/or polyploidy, sister chromatid exchange and micronuclei formation (Duquesnoy et al. 2010). DNA damage in leaves and root tips of V. faba upon As(V) exposure (5–10 μmolL−1) has been observed (Lin et al. 2007). Progressive enhancement in DNA damage has been reported in Solanum lycopersicum at 12.5 mg As(V)/250 kg soil exposure. The enhancement in DNA damage upon As(V) exposure is probably attributed to oxidative base damage due to the formation of DNA–protein adducts, chromosome breaks, inactivation of DNA repair enzymes, and generation of apurinic/apyrimidinic sites (Gupta and Seth 2019). Application of 75 μM As(V) was found to increase DNA oxidation (by 3569%), DNA fragmentation (by 2074%), and DNase activity (by 2692%) in Glycine max (Chandrakar et al. 2017).
Arsenic stress mitigation strategies in plants
Arsenic efflux from the cell
Arsenic efflux from root cells could be a potent metalloid stress amelioration strategy in plants. In the previous section, we came to know about a wide array of As transporters in plants that deal with different As species, expressed at a definite time and space, and serve specific functions. Among them, quite a few can extrude As(III) which ultimately helps to reduce the toxic cellular load of As. Cellular extrusion of As(III) as a detoxification strategy has already been found in prokaryotes and eukaryotes like Saccharomyces cerevisiae (Yan et al. 2019). Rice, tomato, and Arabidopsis roots were found to extrude As(III) when provided with As(V) and the export of As(III) accounts for 60–90% of the entire As(V) uptake (Zhao et al. 2010b; Tang and Zhao 2021). A mutant study using OsLsi1 confirmed the aquaporin OsNIP2;1 expressed mainly in exodermal and endodermal root cells (at the distal portion of the plasma membrane) is capable of extruding As(III) from root cell. However, the quantity of As(III) exported out accounts for only 15–20% of total As(III) efflux. Therefore, the likelihood of the existence of other As(III) efflux transporters is very much feasible (Zhao et al. 2010b). An auxin efflux protein AtPIN2 might be a potential candidate among others. It shares 35% similarity in structure with E. coli efflux transporter ArsB and functions as an auxin efflux transporter in lateral root cap, epidermal and cortical cells. Loss of PIN2 resulted As(III) hypersensitivity in A. thaliana root and the root apices accumulated up to 2–3 fold higher As concentration which sums up its role in the cellular efflux of As(III) (Ashraf et al. 2020). Additionally, many aquaporins like OsNIP1;1, OsNIP3;2; OsNIP3;3, LjNIP5;1, LjNIP6;1, OsPIP2;4, OsPIP2;6, OsPIP2;7, etc. demonstrate bidirectional activity for various As species that also might result in As efflux (Ashraf et al. 2020). In As hyperaccumulator P. vittata, higher doses of As resulted in As efflux not only from roots but also from fronds. For As tolerance, prokaryotes, and yeast utilize two well-established As(III) efflux transporter ArsB and ACR3 both from different sub-families of bile/arsenite/riboflavin transporter (BART) superfamily (Meng et al. 2004; Mansour et al. 2007). Heterologous expression of ScACR3 in A. thaliana and O. sativa enhanced their As tolerance by raising As(III) efflux (Ali et al. 2012; Duan et al. 2012). ACR3 is lost in flowering plants during evolution but it remains with duplication in P. vittata (Indriolo et al. 2010). When PvACR3 is engineered to A. thaliana, it is found to be localized on the plasma membrane which is different from its conventional localization site i.e. tonoplast in P. vittata. Moreover, transgenic A. thaliana expressing PvACR3 shown to efflux As(III) efficiently into the external medium (Chen et al. 2013).
Formation of iron plaque
Radial oxygen loss followed by iron plaque formation around the root surface also regulates As accumulation in plants. However, this strategy is only applicable to some selective plants such as O. sativa. Being submerged plant O. sativa is provided with aerenchyma tissues in its roots. This aerenchyma supposedly infiltrates oxygen from the shoot for respiratory purposes. Rice aerenchyma releases oxygen during the radial movement of oxygen. This released oxygen coupled with soil microbial activities oxidizes ferrous ion (Fe2+) to ferric ion (Fe3+) eventually forming iron plaques at the root surface. The iron plaques help to adsorb/co-precipitate As(V) (Awasthi et al. 2017; Shri et al. 2019). The iron plaque grown on the outer root surface is mainly composed of ferrihydrite (50–100%), goethite (0–22%), and lepidocrocite (0–29%) (Seyfferth et al. 2010). The iron plaque may restrict excessive uptake of Fe+/ Mn+ along with uptake of toxic substances like As into the root (Lakshmanan et al. 2015). The iron oxides/hydroxides are strong absorbents of As(V) and function as a natural sink for As. However, the role of iron plaque is controversial. While some postulated it as a barrier for As(V) uptake, others suggested that it might act as a source of As(V) for plants (Awasthi et al. 2017). Moreover, other factors like silica composition in soil, the microbial community in the rhizosphere, soil As content, and rice genotypes may also influence radial oxygen loss and subsequent iron plaque formation (Awasthi et al. 2017).
Regulation of arsenic transporters
Arsenic transporters are the most vital component in plant arsenic interaction which essentially serves as a central hub for As intake, transportation, and its further metabolic procedures. Unsurprisingly, these transporters are strictly regulated by plants at different levels (transcriptional, translational, and posttranslational) by TFs, regulatory proteins, miRNAs, and protein phosphorylation to manage the burden of As toxicity (Tang and Zhao 2021). The intake of As(V) is reportedly modulated by TF containing WRKYGQK domain and Zinc finger-like motif or WRKY TFs. An As responsive TF WRKY6 was found to repress As(V)/Pi transporter AtPHT1;1 expression whereas WRKY45 a Pi-starvation-responsive TF positively regulates AtPHT1;1 transcription (Castrillo et al. 2013; Wang et al. 2014). Another such TF is WRKY75 which primarily is a modulator of Pi starvation response and root development (Devaiah et al. 2007). The O. sativa OsWRKY28 was found to affect As(V)/Pi accumulation, root architecture, and fertility at an early developmental stage (Wang et al. 2018b). The expression of As(III) transporter genes is regulated by the R2R3 MYB transcription factor As(III) responsive MYB1 (OsARM1). It regulates the expression of key As(III) transporters like OsLsi1, OsLsi2, and OsLsi6 in O. sativa and AtNIP1;1, AtNIP3;1, and AtNIP5;1 in A. thaliana (Wang et al. 2017). OsARM1 weakly suppresses the expression of these transporter genes. Knocking out of OsARM1 improved As(III) tolerance and under low As(III) concentrations (2 μM) more As(III) was translocated from roots to shoots. On the other hand, overexpression lines of OsARM1 showed increased As(III) sensitivity and reduced As(III) translocation from roots to shoots upon high As(III) exposure (25 μM) (Wang et al. 2017). Similarly, OsPHR2, an MYB TF, involved in Pi starvation response signaling regulates As(V) uptake in O. sativa by positively regulating OsPHT1;8 (Wu et al. 2011).
AtPHF1 (Phosphate transporter traffic facilitator1) an ER-localized protein structurally related to plant-specific SEC12 protein of early secretory pathway found to regulate the localization of AtPHT1;1 in the plasma membrane (González et al. 2005). The regulatory protein is responsible for ER exit of three As(V) transporters (AtPHT1;1, AtPHT1;2, and AtPHT1;4) in A. thaliana (González et al. 2005; Bayle et al. 2011; Nussaume et al. 2011). A homolog of AtPHF1 in O. sativa, OsPHF1 modulates the ER retention of low and high-affinity Pi transporter OsPHT1;2 and OsPHT1;8 (Chen et al. 2011). An O. sativa mutant with defective OsPHF1 lost the ability to take up and translocate Pi and As(V) (Wu et al. 2011). A soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) protein in AtSYP51 was also found to regulate plasma membrane trafficking of AtNIP1;1 (Barozzi et al. 2019). Additionally, two regulatory proteins were also found to regulate different As transporters through protein–protein interaction. One of them is a Ca-dependent protein kinase AtCPK31 which acts as a positive regulator for As(III) uptake through modulating AtNIP1;1 (Ji et al. 2017). A RING-type ubiquitin ligase localized at plasma membrane physically interacts with OsPHT1;2 and OsPHT1;8 by degrading PHTs (Yang et al. 2017; Yue et al. 2017). Later, OsNLA1 was also found to be associated with As(V) uptake and tolerance in O. sativa (Xie et al. 2019). Several studies also revealed the involvement of miRNAs in the regulation of As transporters (Yu et al. 2012; Sharma et al. 2015). Protein phosphorylation and dephosphorylation, a common post-translational regulation also said to be involved in the regulatory process of transporters (Tang and Zhao 2021).
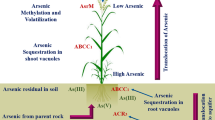
Chelation and vacuolar sequestration of arsenic
Chelation of As species followed by vacuolar sequestration is probably the most pronounced As detoxification mechanism in plants. In both hyperaccumulators as well as in non-hyperaccumulators, As(V) frequently reduced to As(III) by arsenate reductase followed by complexation with –SH-rich proteins like PCs, metallothioneins (MTs), reduced glutathione (GSH), etc. that leads to vacuolar sequestration of those complexes via vacuolar transporters (Fig. 2) (Xu et al. 2015; Shri et al. 2019). As(III) has a greater affinity for thiol groups and subsequently forms stable complexes with PCs and comparatively less stable complexes with As(III)-triglutathione (Tang and Zhao 2021). Numerous arsenate reductase (AR) genes have been identified from various plant species such as A. thaliana (AtACR2), O. sativa (OsACR2), P. vittata (PvACR2), and Holcus lanatus (HlASR) to name a few (Kumar et al. 2019). However, a study conducted by Chao et al. (2014) failed to observe any role of A. thaliana ACR2 in As metabolism or resistance in vivo. Rather they proposed an arsenate reductase called High Arsenic Content 1 (HAC1) through genome-wide association mapping. OsHAC1;1, OsHAC1;2, and OsHAC4 are also found to be involved in As(V) detoxification and As accumulation in O. sativa (Shi et al. 2016; Xu et al. 2017). Two rice glutaredoxins (OsGRx_C7 and OsGRx_C2.1) have also shown As(V) reductase activity (Verma et al. 2016).
PCs play a pivotal role in further detoxification of As specifically in crop plants (Li et al. 2016). PCs are synthesized non-ribosomally by PC synthase (PCS). Two such PCS enzymes in O. sativa are OsPCS1 and OsPCS2. However, the sensitivity of OsPCS1 towards As is greater than the other variants of PCS (Costa de Oliviera et al. 2019). An O. sativa chloroquine resistance transporter (CRT)-like transporter, OsCLT1 found to mediate the export of γ-glutamylcysteine and GSH from plastids to cytoplasm which in turn affects As detoxification in O. sativa (Yang et al. 2016). Finally, the reduced As(V) is sequestered into vacuole mainly through ABCC transporters. In A. thaliana, two GSH-conjugated organic molecule transporter, AtABCC1 and AtABCC2 also transports As(III)-PC complex and/or apo-PCs and is considered to be an important player in As(III) detoxification (Song et al. 2010). These two transporters (AtABCC1 and AtABCC2) of A. thaliana were initially reported to sequester GSH-conjugated organic molecules into the vacuoles (Wanke and Üner Kolukisaoglu 2010). The O. sativa homolog of AtABCC1, OsABCC1 localizes in the vacuolar membrane of the phloem region of the vascular bundle and is thought to sequester As(III)-PC complex into the vacuoles of phloem companion cell (Song et al. 2014). A PHT1 family transporter in A. thaliana, AtVPT1, mainly associated with vacuolar sequestration of Pi is found to be involved in As(V) tolerance. However, the As(V) transport activity of AtVPT1 is still not confirmed (Luan et al. 2019). The As hyperaccumulator P. vittata withstands a relatively higher degree of As toxicity than angiosperms. The capacity to tolerate such high As concentrations is probably attributed to its unique As transporters. One of them is, PvACR3, localized on the tonoplast of the gametophyte and is likely to be involved in vacuolar sequestration of As(III). Knocking out PvACR3 in gametophyte resulted in an As sensitive phenotype confirming its role in As detoxification (Indriolo et al. 2010). PvACR3;1 is another such transporter that stands responsible for vacuolar sequestration of As(III) in root cells and thereby decreasing As(III) translocation to shoots (Chen et al. 2017c).
Antioxidant-mediated detoxification
Enzymatic antioxidants
Production of ROS in plant cells is inevitable due to ongoing electron transfer processes in various organelles. Multiple abiotic stresses including As stress contribute significantly towards the native ROS pool which ultimately leads to oxidative damage. To mitigate such deleterious effect of ROS plants deploys their antioxidant machinery (Fig. 3). SOD constitutes the first line of defense and dismutates O2•− into H2O2. Based on the metal co-factors SOD are of three types—Cu/Zn SOD, Mn-SOD, and Fe-SOD located at various locations like chloroplast, mitochondria, peroxisome, cytosol, and even in the root nodules (Gratão et al. 2005; Sharma 2012). As induces SOD level directly through SOD gene expression or indirectly by overproducing O2•− (Tripathi and Tripathi 2019). The H2O2 generated by SOD is then metabolized subsequently by APX, GPX, CAT, or GOPX (Fig. 3) (Mishra et al. 2019). CAT scavenges H2O2 and converts it into O2 and H2O in an energy-efficient manner (Gratão et al. 2005; Sharma 2012). It is mostly found in peroxisomes but also reported from the cytosol, mitochondria, glyoxisomes, and root nodules (Sharma 2012). The H2O2 produced from the dismutation of O2•− in the chloroplast is further removed by the enzyme APX. APX is an essential component of the ascorbate–glutathione cycle and it reduces H2O2 while oxidizing ascorbate that subsequently generates monodehydroascorbate (MDHA) and dehydroascorbate (DHA). The MDHA and DHA are reduced back to ascorbate by MDHAR and DHAR utilizing NADPH and GSH as reducing equivalents, respectively (Fig. 3) (Gratão et al. 2005; Gill and Tuteja 2010; Sharma 2012; Mishra et al. 2019). GPX also reduces H2O2 using GSH as a reductant. GR is another component of the ascorbate–glutathione cycle, not only found mostly in the chloroplasts, but also present in mitochondria and cytosol in small quantities. It helps to maintain the GSH pool by reducing oxidized glutathione (GSSG) which is produced for the regeneration of ascorbate in an NADPH-dependent manner (Fig. 3) (Gill and Tuteja 2010; Sharma 2012). GOPX belongs to the large peroxidase family found mainly in the cytoplasm or cell wall-bound form. It also scavenges H2O2 generating GSSG as a bi-product which is further reduced to GSH by GR (Sharma 2012). The metabolic function of GOPX includes degradation of IAA, lignin biosynthesis, and protection against pathogens consuming H2O2 (Gratão et al. 2005; Gill and Tuteja 2010). Glutathione-S-transferases (GSTs) are a large group of enzymes that are known for catalyzing the conjugation of GSH (γ-glu-cys-gly) to several toxic compounds during their detoxification and is pivotal in As stress response of plants (Mishra et al. 2019). However, differential activities of antioxidants were evident upon As exposure. For example, in O. sativa, SOD, CAT, and APX upregulated in both roots and leaves in response to As(III). Conversely, AsA/DHA, and GSH/GSSG ratio were found to be decreased along with MDHAR, DHAR, and GR (Jung et al. 2019). Several other studies also reveal otherwise. A study reported by Ahmad et al. (2020) showed increased SOD, CAT, APX, GR, GSH, and GSSG activities upon As(III) treatment, whereas DHAR and MDHAR levels were said to be decreased. In another study, CAT levels were down while APX, SOD, and peroxidase (POX) levels were up (Srivastava et al. 2017). The CAT, GR, POX, and SOD levels were found to be declined in upland O. sativa and the decline was said to be due to different applications of As doses (Di et al. 2021). In general, it can be said that mild doses of As may trigger antioxidant activity significantly up to a level but as toxicity levels piled up, the activities were found to be declined (Gupta et al. 2009; Farooq et al. 2016a; Kofroňová et al. 2019). However, this is not true in all cases. The antioxidative defense system works differently in various plant species under As stress and their modulation is perhaps dose dependent. The statement is further justified by the fact that the peak of the enzymatic activity for different antioxidant enzymes is found at different As concentrations (Kofroňová et al. 2019).
Schematic outline of antioxidant defense machinery in plants that counter the negative effects of redox imbalance and oxidative stress caused by arsenic-induced reactive oxygen species (ROS) production. ROS is usually produced in organelles where electron transport chains are being operated due to partial reduction of O2 or on account of energy transfer to O2. Arsenic stress further contributes to the overall ROS pool of the cell. The superoxide radical (O2•−) dismutates into hydrogen peroxide (H2O2) by superoxide dismutase (SOD). The H2O2 is then further transformed into H2O either by catalase (CAT), guiacol peroxidase (GOPX), glutathione peroxidase (GPX), or ascorbate peroxidase (APX). Hydroxyl radical (•OH) is generated through the Fenton reaction or Haber–Weiss mechanism in presence of transition metals. The ascorbate–glutathione pathway is also operated to detoxify H2O2 involving various metabolites like ascorbate (AsA), monodehydroascorbate (MDHA), glutathione, and reduced nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) and the enzymes linking these metabolites such as glutathione reductase (GR) and dehydroascorbate reductase (DHAR). Proline helps to detoxify •OH. Glutathione-S-transferases (GSTs) are involved in the detoxification of arsenicals by conjugating them to glutathione
The increased activity of antioxidants is indicative of induced gene transcription, primarily through free radicals (Mishra et al. 2008). Microarray experiments showed induction of two different Cu/Zn SOD genes as well as SOD Cu chaperones. However, Fe-SODs are found to be strongly repressed in response to As(V) in A. thaliana (Abercrombie et al. 2008). Similarly, in B. juncea, transcriptome profiling showed induction of monothiol glutaredoxin 17 (GRXS17), glutathione peroxidase 6 (GPX6), monodehydroascorbate reductase (MDAR2), copper/zinc superoxide dismutase 1 (CSD1) in roots, and GPX3 and Fe SOD 2 (FSD2) in shoot upon As(V) exposure. Overexpression of GPX3 and GPX6 at different time points suggests their role in the regulation of ROS levels and stomatal opening under As stress. The up-regulation of SODs and MDAR under As stress indicates stimulation of antioxidant machinery for providing protection against oxidative damage (Srivastava et al. 2015). Several differentially expressed genes associated with antioxidant system like cytochrome P450, GRx, GST NADPH, oxidoreductase, peroxiredoxin, SOD, thioredoxin reductase were modulated under As(III) and As(V) treatments (Di et al. 2021). Differential expression of GST genes in As sensitive as well as As tolerant A. thaliana and O. sativa genotypes was also observed (Fu et al. 2014; Rai et al. 2015). Furthermore, alternative oxidase (AOX), DHAR, GRx, and POX genes were found exclusively downregulated in A. thalianas. Whereas AOX, GST, GR, class-III POX genes were found to be regulated with As treatment in O. sativa. Glutaredoxin (Grx) and thioredoxin (Trx) transcripts were particularly up-regulated in O. sativa roots upon exposure to As(V) (Huang et al. 2012). In As-sensitive accession of A. thaliana, a number of stress-responsive genes encoding Cyt P450 family members, GR1, GSTs, and HSPs were up-regulated, whereas As tolerant accession galactinol synthase 1 (AtGolS1) and members of peroxidase family were up-regulated and genes encoding allene oxidase cyclase, proline dehydrogenase 2, MDHAR, and other defense-related genes were down-regulated (Shukla et al. 2018).
Non-enzymatic antioxidants
GSH is the key element in the non-enzymatic antioxidant machinery and is considered as a redox buffer for maintaining the redox homeostasis of the cell. It is abundantly found in its reduced form (GSH) in various cellular compartments like cytosol, mitochondria, chloroplast, vacuole, endoplasmic reticulum, peroxisome, and even in the apoplast (Gill and Tuteja 2010). Inside the cell, GSH exists with its interconvertible form GSSG which is frequently generated when ROS oxidizes GSH. The fine balance between GSSG/GSH determines the redox state of the cell. It is important to retain proper GSH levels to maintain a reduced state of the cell to counteract the ROS-induced oxidative damage (Gill and Tuteja 2010). For this, GSSG is readily converted back to GSH with the help of GR using NADPH as a reductant (Mishra et al. 2019). GSH is the major participant of the ascorbate–glutathione cycle which primarily regenerates AsA. GSH also serves as a precursor for PCs that helps to sequesters As within the vacuole minimizing free As concentration within the cell. The other major function of GSH includes scavenging free radicals (1O2, H2O2 and •OH), regulation of sulfate transport; conjugation of metabolites; detoxification of xenobiotics via GST, signal transduction, and expression of stress-responsive genes (Gratão et al. 2005; Gill and Tuteja 2010; Mishra et al. 2019). AsA is probably the most abundant water-soluble antioxidant found in plants. It happens to be the most vital reducing substrate for H2O2 detoxification in plants. Apart from its role in the removal of H2O2, it protects membranes by regenerating membrane-bound carotenoids and α-tocopherol, preserves enzymatic activities of prosthetic transition metal ions. The reduced form of ascorbate reacts directly with free radicals like 1O2, O2•− and •OH. Ascorbate can be found in the stroma of chloroplast, mitochondria, apoplast, cytosol, and vacuole. A large amount of reduced ascorbate is kept in the stroma of the chloroplast due to its photoprotection efficacy (Gill and Tuteja 2010; Sharma 2012). Reduced ascorbate also functions as a co-factor of APX, which produces DHA (oxidized). The regeneration of ascorbate (reduced) is GSH dependent and catalyzed by DHAR (Sharma 2012). Carotenoids like β-carotene, zeaxanthin, and tocopherols that are widely found in photosynthetic organisms are fat-soluble pigments located in the thylakoid membrane of the chloroplast. The osmotic cytosolute proline also plays a pivotal role in As stress mitigation. It is reported that plants start to accumulate proline to counter the toxic effects of extremely toxic •OH and in vitro, it was found to scavenge •OH and 1O2 (Sharma 2012).
As hyperaccumulator P. vittata was found to have higher levels of carotenoids, ascorbate, glutathione, and their reduced/oxidized ratios when compared to P. ensiformis (As sensitive). This is also an indication of the involvement of the ascorbate–glutathione pathway in As detoxification in Pteris (Singh et al. 2006). Upon As(V) exposure decline in the ascorbate/DHA and GSH/GSSG ratios were noticed in Hydrilla verticillata (Srivastava et al. 2011). However, in O. sativa, besides maintaining ascorbate/DHA and GSH/GSSG ratios, PC and PCS activity was found to be elevated (Tripathi et al. 2012). Low concentration of As(V) and As(III) augmented thiols such as PC synthesis for metalloid detoxification in H. verticillata (Srivastava et al. 2007). In red clover, free polyamines such as spermidine, spermine, and putrescine increased even at low levels of As. As stress-mediated induction of PCs, GSH and other thiols have also been reported from Ceratophyllum sp., Bacopa sp., P. vittata, and Artemisia sp. (Cai et al. 2004; Mishra et al. 2008, 2013; Kumari et al. 2018). Increased accumulation of osmolyte proline in O. sativa upon As(V) exposure has also been reported (Ghosh et al. 2018).
Arsenic-induced signaling pathways
At least five types of signaling pathways are being operated by plants to combat As stress-calcium signaling, phytohormone signaling, mitogen-activated protein kinase (MAPK) signaling, ROS/oxidative stress, and reactive nitrogen species (RNS)-mediated signaling. Besides MAPK, other protein kinases like CT10 regulator of kinase (CRK), receptor-like cytoplasmic kinases (RLCKs), tyrosine kinase-like (TKL), and wall-associated kinases (WAK) are upregulated during As stress initiating the signaling cascade events (Nabi et al. 2021). All these pathways are vital for As stress perception and further amplification of the signals resulting in modulation of gene expression that ultimately imparts protection against the metalloid (Thakur et al. 2020).
ROS and RNS signaling
Plants produce various ROS in response to As stress such as H2O2, O2•−or •OH. Often the tenor has been that ROS are only harmful but it is now believed that ROS has a very critical role in stress responses. They primarily act as signaling molecules and can initiate diverse signaling cascades upon stress perception. These compounds are easily scavenged by plants’ antioxidant machinery. The fine-tuning between ROS production and its scavenging by antioxidants in the onset of multifarious signaling cascades determines a plant’s response to heavy metal stress. Respiratory burst homolog genes (RBOH) genes that encode NADPH oxidases associated with plasma membranes are key elements involved in ROS signal transduction. NADPH oxidases sense stress stimuli and get triggered by calcium and phosphorylation and produce ROS which in turn is perceived by either receptor, redox-sensitive molecules like TFs, or by inhibition of phosphatases (Singh et al. 2019). The interaction of ROS with G protein and protein phosphorylation events serve as evidence of their role in cellular stress signaling. They supposedly work as sensors of signaling cascades which leads to gene expression and targeting TFs. Alternatively, they can directly oxidize the signaling pathway components. Cell responses like tropisms, cell division, cell differentiation, and cell death are controlled by ROS signaling (Islam et al. 2015). ROS can participate in stress signaling through the transduction of signals from MAPKs (Nadarajah 2020). The critical factor in ROS-mediated cellular and intracellular signaling is the spatiotemporal production of ROS. The systemic signaling upon ROS generation works like an auto-propagating wave to an adjacent cell and confers stress tolerance in a spatiotemporal manner. For this, plants deploy phytohormones and/or amino acids for the specific signal transmission in a stress situation. For stress regulation, ROS operates signaling in a highly coordinated fashion. It stimulates antioxidants, defense genes, kinases, influx of Ca2+ ions, protein phosphorylation, induction of phytohormones like jasmonic acid, salicylic acid, ethylene, etc. (Sachdev et al. 2021). It is now believed that ROS generation in plants performs the role of a second messenger like that of highly regulated Ca2+ (Raja et al. 2017). The expression profile of ROS-associated genes on microarray analysis exhibited regulation of GR, GST, and class-III peroxidases upon As(V) stress (5–200 μM) (Huang et al. 2012). The JA-related genes, MYB TF, and terminal deoxynucleotidyl transferase (TDT) transporters may regulate the downstream genes meant for As(V) stress and, therefore, play pivotal roles against oxidative stress induced by As(V) stress but not against general oxidative stress. Similar expression patterns were observed for GARP-G2-like and C3H TFs. These TF families may be regulated particularly by As(V) stress which leads to downregulation of downstream genes (Huang et al. 2012). Among many potential candidates, H2O2 appears to be the one that plays the central role. It is the most stable and easily disseminated form of ROS and functions as a molecular switch. Moreover, the affinity of H2O2 towards thiol groups implies its role in stress modulation (Nadarajah 2020). On one hand, it induces oxidative damage, on the other hand, it affects target molecules in a signaling cascade or transcription, oxidizes bio-molecules that further act as a second messenger and changes cellular redox equilibrium towards a more oxidized state (Cuypers et al. 2016). The most accepted mechanism of perceiving stress-induced H2O2 is through redox-sensitive TFs (e.g., heat shock factors or HSFs) that are oxidized by H2O2 which in turn directly activates downstream signaling (Miller et al. 2008; Cuypers et al. 2016). According to a model for ROS signaling, HSFs act as H2O2 sensors in upstream of TFs like Zn finger protein Zat family and proteins of WRKY family (Miller et al. 2008). H2O2 is further interconnected with other signaling pathways/molecules such as MAPK, calcium, phytohormones, miRNA, nitric oxide, oxylipins, etc. (Jonak et al. 2002; Petrov and Van Breusegem 2012; Cuypers et al. 2016).
Nitric oxide (NO) is a gaseous free radical that belongs to the family of RNS found to accumulate within plants upon As stress. The other family members of RNS include peroxynitrite (ONOO−), dinitrogen trioxide (N2O3), S-nitrosoglutathione (GSNO), nitrogen dioxide (NO2), etc. (Mishra et al. 2019). NO also acts as a double-edged sword like H2O2. It simultaneously works as a signaling molecule and also proves to be injurious upon a certain threshold level. The major protective role of NO includes prevention of Fenton reaction, activation of antioxidants like SOD, CAT, APX, and POX, conversion of O2•− into less toxic ONOO−, reduction in radical-mediated lipid peroxidation, and contribution as a signaling molecule that leads to gene expression (Tripathi and Tripathi 2019). NO carry out its diverse regulatory functions through post-translational modifications of proteins and interaction with various regulatory pathways including those involving the interplay of phytohormones (Sharma et al. 2021). NO can work in coordination with several phytohormones (like abscisic acid, auxins, cytokinins, brassinosteroids, ethylene, jasmonic acid, and salicylic acid) and secondary messengers to regulate innumerable metabolic and physiological plant processes showing synergistic or antagonistic interactions during As-induced stress (Bhat et al. 2021). The level of internal NO was found to be elevated in H. verticillata [100 and 500 μM As(V)], O. sativa [0–50 μM As(III) and As(V)], and A. thaliana [100–1000 μM As(V)] upon As exposure (Srivastava et al. 2011; Leterrier et al. 2012; Tripathi et al. 2012, 2015). However, in Pisum sativum downregulation of NO metabolism in roots and up-regulation in leaves was observed in response to different doses (10–200 μM) of As(V) (Rodríguez-Ruiz et al. 2019). External application of NO donors like sodium nitroprusside (SNP), either prior to or concomitantly with As, considerably alleviated As toxicity as observed in multiple plant species like H. vulgare [25–100 μM As(III) and 40 μM SNP], V. faba [100–400 μM As(V) and 100 μM SNP], P. stratoites [1.5 mg L−1 As(V) and 0.1 mg L−1 SNP], O. sativa [0, 25 μM As(III) and 0, 30 μM SNP], Isatis cappadocica [1000 μM As(V) and 200 μM SNP], B. juncea [75 mg kg−1 As(III) and 100 μmol SNP], etc. (Shukla et al. 2015; Mohamed et al. 2016; Farnese et al. 2017; Singh et al. 2017; Souri et al. 2020; Ahmad et al. 2021). Exogenous application of NO was found to reduce chlorosis, improves relative water content, lowers H2O2 content and lipid peroxidation, elevates AsA, GSH, glyoxalase enzyme levels, and increases activities of antioxidant enzymes like APX, MDHAR, DHAR, GR, GST, and CAT, enhances carotenoid synthesis, and augments quantum yield of photosystem-II (PS-II) (Hasanuzzaman and Fujita 2013; Ahmad et al. 2021). A transcriptomic study of NO-arbitrated responses of O. sativa roots exposed to As(III) revealed NO modulate a regulatory network of genes that are associated with multiple transport and metabolic pathways. It found to modulate metal transporters (such as NIPs, NRAMP, ABC, and iron transporters), stress-related genes involved in As detoxification (such as CytP450, GSTs, GRXs, several TFs, amino acid, hormones, signaling, and secondary metabolite genes) (Singh et al. 2017). Altered expression of stress-responsive genes like Aox1 was also reported upon NO pre-treatment (40 μM SNP) under As stress [25–100 μM As(III)] (Shukla et al. 2015). Accumulating reports suggest that As can induce ROS and RNS, thereby altering NO-mediated cell signaling. NO has been shown to regulate the activity of MAPK, NO donors, and recombinant NOS were shown to activate salicylic acid-induced protein kinase (SIPK) (Rao et al. 2011).
Calcium signaling
Calcium ions (Ca2+) act as ubiquitous second messengers and initiate complex signaling cascades in response to As stress. Ca2+channels are frequently found at different locations in the plant cell e.g., plasma membrane, tonoplast, and ER (Kumar and Trivedi 2016). In response to stress stimuli, cytosolic Ca2+concentration changes drastically, either due to the release of Ca2+from intracellular storages or influx of Ca2+from outside the cell (Kumar and Trivedi 2016). A wide array of Ca2+sensors are present in plants such as calmodulins (CaM), calmodulin-like (CML) proteins, calcineurin B-like proteins (CBLs), Ca2+-dependent protein kinases (CDPKs), CBL-interacting protein kinases (CIPK), etc. (Huang et al. 2012; Kumar and Trivedi 2016). These sensors determine the changes in Ca2+ levels resulting in the initiation of signaling pathways. In downstream these Ca2+-binding proteins activate protein kinases and other proteins including enzymatic antioxidants. Ca2+ also reduced the rate of lipid peroxidation keeping membranes intact that further enhance plants' tolerance against abiotic stresses (Parvin et al. 2019). Apart from being a signaling agent, the multifarious functions of Ca2+ include determination cell wall rigidity, stabilization of cell membranes, induction of nutrient uptake, regulation of enzymatic activity and phytohormone functions, regulation of photosynthetic electron transport rate, the enzyme activity of Calvin cycle, maintenance of Na+-K+ ion homeostasis and inhibition of solute leakage from the cytoplasm (Siddiqui et al. 2020; Singh et al. 2020a, b). Calcium is also said to regulate antioxidative enzymes like APX, CAT, GR, and SOD (Singh et al. 2020b). Both ROS and Ca2+ signaling are meant to be positively correlated to each other under environmental stress conditions involving Ca2+ binding protein, such as calmodulin, which interacts with various proteins in the cell serving as an effector molecule or amplifier of Ca2+ signals in diverse cellular functions. The cytosolic concentration of Ca2+ is dependent upon internal and external stimuli which in turn are decoded and translated by Ca2+ sensors like calmodulin and Ca2+ -dependent protein kinases. The CDPKs are widely involved in environmental stress signaling and up-regulation of stress-responsive genes conferring abiotic stress tolerance to plants (Siddiqui et al. 2020). Ca2+ when applied with melatonin found to suppress As-induced programmed cell death (in guard cells, roots, and leaves), improved photosynthesis, biosynthesis of chlorophyll, carbohydrate, and proline; alleviated DNA damage, ROS formation. They were also found to regulate the ascorbate–glutathione pathway and proteins expression under As(V) stress. Moreover, the combined effect of Ca2+ and melatonin upregulated the plasma membrane H+-ATPase activity to enhance the resilience against As toxicity (Siddiqui et al. 2020). The combination of Ca2+ and NO was found to recover As-induced damages in phenotypic appearances, rescued As-induced losses of chlorophylls and carotenoids, recovered photosynthetic rate and PS-II photochemistry, upregulated antioxidative defense system, ascorbate, glutathione contents, thiol compound synthesis, and maintained redox status. These results are indicative of a common signaling network involving Ca2+ and NO (Singh et al. 2020a). The combined effect of sulfur and calcium attenuated As(V) toxicity by regulating the ascorbate–glutathione cycle and sulfur metabolism in B. juncea (Singh et al. 2020b). Li et al. (2006) described the impacts of As on chloroplast ultrastructure and Ca2+ distribution in P. vittata emphasizing the possible function of Ca2+ in As detoxification and accumulation in P. vittata where they found that Ca2+had a close relation with As toxicity in P. vittata. Several genes including CaM, CBL, CDPK, and CIPK genes found to be up-regulated in response to various doses of As(V) in rice roots (Huang et al. 2012). Similarly, calreticulin3 (CRT3), CML38, CML42, CML1, CIPK4, CIPK6, and CBL4 genes were up-regulated or down-regulated (CML38) due to As(V) stress at specific time intervals in B. juncea root and shoot. Among these, CRT3 is a high-affinity Ca2+-binding molecular chaperon that controls calcium homeostasis in the ER lumen. CML42 connects Ca2+ signaling with that of Jasmonic acid (JA) signaling and negatively regulates JA-responsive gene expression. CIPK4 positively regulates calcium-induced abscisic acid (ABA) signaling whereas CBL4 and its interacting kinase CIPK6 regulate ion homeostasis by modulating Na+ and K+ channels (Srivastava et al. 2015). In a recent study, three important components of the calcium signaling pathway- sphingosine kinase (SPHK), mitochondrial calcium uniporter protein (MCU), and CaM encoded by 11 different transcripts were found up-regulated in As treated tissues of B. juncea (Thakur et al. 2019). From the above discussions, it is evident that upon perceiving As stress various Ca2+ sensors got activated and transmit stress signals that cause regulation of genes associated with various stress metabolisms. Furthermore, the Ca2+ signaling was found to be cross-linked with other signaling pathways like ROS, RNS, and phytohormone signaling.
MAPK signaling
MAPKs are serine/threonine kinases that are evolutionary conserved and act as signaling molecules via sequential phosphorylation. MAPKs are quintessential in abiotic stress perception including As stress and crosstalk with other signaling cascades. MAPK cascade consists of three modules viz. MAPKKK, MAPKK/MKK, and MAPK/MPK. MAPKKKs are divergent kinases that may initiate similar MAPK cascades which in turn converge to one MAPK. At the end of the signaling cascade, MAPK is meant to phosphorylate different substrates in the cell such as TFs or proteins. However, the effect of MAPK relies upon the activation time of the MAPK (Ghori et al. 2019). In previous sections, As-induced ROS and RNS production has been discussed in detail. The MAPK signaling cascade is thought to be activated through these species when plants are grown under As stress (Islam et al. 2015). Through activation of MAPK, H2O2 (a prime ROS signaling agent) can control and regulate multiple steps in the MAPK pathway by reacting with phosphatases, kinases, and TFs (Ghori et al. 2019). The MAPK pathway is often regarded as a central signal amplification system whose downstream targets are TFs and stress-responsive acclimation genes (Sharma et al. 2021). In many experiments, the transcripts of MAPK signaling cascade were found to be modulated during As stress. Gupta et al. (2009) reported activation of a 46 kDa MAPK in response to 50 and 150 μM As(III) in two varieties of B. juncea which suggests the involvement of MAPK in As(III)-induced cellular responses. Fast and transient activation of MAPK signaling cascade in response to 150 μM As(III) was also observed where the activation was recorded only after 15 min and subsequently got inactivated at 60 min. This result is indicative of the role of MAPK in transducing As(III) signals for appropriate cellular responses (Gupta et al. 2009). The MAPKKK3–MKK4–MPK3 cascade is thought to be involved in As stress response in B. juncea. However, the exact nature of the MAPK module in this particular response is not analyzed in detail (Srivastava et al. 2015). In O. sativa, two MPKs (OsMPK3 and OsMPK4) were reported to be induced in response to As(III). As(III) also induces MKKs (such as OsMKK4 and OsMKK1) which act upstream of MPKs. In silico homology modeling and docking, the analysis indicated OsMKK4–OsMPK3 interaction, suggesting the role of MAPK module in As stress response (Rao et al. 2011). In a separate study, MAPKKKs, MAPK, and, PP2C genes were found to be instigated by As(V) treatment in rice roots. The study also suggested an interplay between ROS, calcium signaling, and MAPK signaling components to counteract As(III) stress (Fig. 4) (Huang et al. 2012). MAPK regulates gene expression through activation or suppression of TFs such as WRKY (Islam et al. 2015; Ghori et al. 2019). Plant processes such as growth and development, stress resilience, hormonal regulation, cell division, stomatal development, senescence, and ovule development are controlled by MAPK signaling cascade. Moreover, MAPKs are also part of plants’ innate immunity (Islam et al. 2015).
Different signaling pathways and their cross-talk that led to arsenic stress responses in plants. Upon arsenic exposure, various signaling pathways are triggered such as calcium signaling, mitogen-activated protein kinase (MAPK) signaling, phytohormone signaling, reactive oxygen species (ROS), and reactive nitrogen species (RNS) signaling. These signaling cascades are interconnected among themselves and collectively induces several transcription factors such as MYB, AP2/ERF, bZIP, WRKY, NAC, DREB, HSF, Zn finger (C2H2, CCCH), etc. which ultimately led to the expression of defense genes, metal transporter genes, PCs, MTs, antioxidant related genes, MIR genes, sulfur and GSH metabolism genes, etc.
Hormonal signaling
Phytohormones regulate various physiological and developmental processes in plants acting as signaling molecules. Many of them, starting from conventional ones like that of auxins (AUXs), gibberellins (GAs), cytokinins (CKs), ethylene (ET), and ABA to the newly emerged ones such as salicylic acid (SA), brassinosteroids (BRs), strigolactones (SLs) and JA through their endogenous regulatory mechanisms like biosynthesis, transport, redistribution, and conjugation helps in resistance against abiotic stresses including As stress (Huang et al. 2012; Gangwar et al. 2014; Sytar et al. 2019; Thakur et al. 2020). For a long time, growth hormones like AUXs, CKs, and GAs were known for their multifaceted role in plant growth and development. However, recent studies have successfully unfolded their unprecedented role in abiotic stress tolerance that also includes As stress.
The changes in the level of auxins have always been correlated with growth aberrations as a result of hormonal imbalance under stress conditions. As a general rule, heavy metal toxicity leads to a reduction in the endogenous levels of auxins. The levels of three different auxins viz. IAA, indole-3-butyric acid (IBA), and naphthalene acetic acid (NAA) was altered upon As(V) treatments (100–1000 μM) in B. juncea. However, the detrimental effect of the metalloid on auxin metabolism was found to be reduced upon exogenous IAA application (Srivastava et al. 2013). The co-application of Se and auxin altered the morphological and biochemical characteristics in O. sativa seedlings grown under As(III) stress (150 μM) (Pandey and Gupta 2015). Although the external application of auxin or stimulation of internal auxin levels prevents growth inhibition and subsequently increases the endurance against As toxicity, the underlying mechanism is poorly understood. A possible interrelationship between hormones and miRNAs under As exposure cannot be overruled (Srivatava et al. 2013). In O. sativa roots AUX (OsASA2 and OsASB1), GA (GA2ox3), and CK (OsLOG) biosynthesis genes were upregulated in response to As(III). Whereas GA biosynthesis genes GA2ox3 and GA2ox9 were up-regulated in response to As(V) indicating As stress-induced accumulation of growth hormones (Huang et al. 2012; Yu et al. 2012). However, down-regulation of CK biosynthesis (OsIPT4), signaling genes (OsHKL1/OsCRL4, OsRR20), and up-regulation of CK inactivation genes (OsCKX4, OsCKX5) points towards As(V) induced root growth inhibition (Huang et al. 2012). AUX transport was also found to be linked with ROS signaling indicating its involvement in As(III) induced oxidative stress (Krishnamurthy and Rathinasabapathi 2013). Genes encoding AUX-responsive proteins from shoots and roots of B. juncea were found to be upregulated. Whereas AUX transport proteins PIN3 and PIN6 were downregulated in shoot indicating repression of root growth under As stress (Srivastava et al. 2015). The genes involved with AUX signaling pathway (ARF, ARP, AUX/IAA, and SAUR), GA signaling (CIGR, GA2ox, GA20ox, and GID1L2), and CK signaling (CKX, OZG2, and RR) were found to be affected in O. sativa shoot under As(III) and As(V) stresses (Di et al. 2021). Arsenate has been shown to cause severe depletion in endogenous CK levels in A. thaliana. The depleted CK levels in turn found to provoke As(V) tolerance mechanisms which lead to the accumulation of thiol compounds like PCs and glutathione, quintessential for intracellular As sequestration. Therefore, the transcript levels of As(V)/Pi-transporters were found to be similar or even higher than the wild type in CK-depleted plants. Moreover, transgenic lines of CK-deficient A. thaliana and tobacco showed a marked increase in As accumulation further establishing the regulatory role of CK in plant adaptation to As stress (Mohan et al. 2016). From the above discussion, it is evident that phytohormones like AUXs, CKs, and GAs are involved in As-induced stress responses and operate through an individual signaling cascade or signaling crosstalk between different phytohormones and/or different signaling cascades. However, differential expression patterns of transcripts suggest that the responses might be dose dependent, organ, and/or species specific.
JA and its volatile methyl-ester methyl-jasmonate (MeJAs) derived from fatty acid metabolism are key players in plant stress tolerance (Ahmad et al. 2016). They primarily act as a signaling molecule and regulate different physiological, and biochemical processes in plants that also include responses against different biotic and abiotic stresses. The MeJa-mediated As stress mitigation is done via strengthening of antioxidant defense machinery of plants (Farooq et al. 2016b). The application of MeJA was found to minimize As-induced oxidative stress by lowering ROS synthesis and by the maintenance of the high redox status of glutathione and ascorbate. Further, it enhanced enzymatic activity and gene expression of important antioxidants (SOD, APX, CAT, and POD), secondary metabolites (PAL, PPO, CAD), and induces lipoxygenase (LOX) gene in B. napus which suggests MeJa plays an important role in the regulation of multiple transcriptional pathways involved in As-induced oxidative stress responses (Farooq et al. 2016b). A recent study revealed that transcript levels of genes (LOX, OPR, and JAZ) involved in JA signaling are modulated by As stress [100 μM As(III) and As(V)] in O. sativa (Di et al. 2021). Similarly, 11 genes involved in JA biosynthesis and signaling were found upregulated in O. sativa root in response to As(V), whereas in response to As(III), JA biosynthesis genes (OsDAD1;2, OsDAD1;3, OsAOS1, OsAOS2, OsAOC, OsOPCL1, OsKT, etc.), JA signaling genes (OsJAR1, OsJAZ4, and OsJAZ6), and lipoxygenase pathway genes (OsLOX2;1, OsLOX2;2, OsLOX2;3 and OsLOX2;4) were found upregulated (Huang et al. 2012; Yu et al. 2012). Likewise, in As(V) treated B. napus, genes related to JA synthesis and signaling were upregulated (OPR1, OPR2, and OPR3) as well as downregulated (AOC4, JAR1, JAZ1, and JAZ5) for the purpose (Srivastava et al. 2015). Based on these findings, it is evident that jasmonates perform a positive regulatory role during As stress adaptation in plants. However, a detailed understanding of the response mechanisms at the transcriptional level under As toxicity is yet to be explored.
The ABA concentration in plant tissues is known to increase after heavy metal(loid) exposure suggesting its involvement in the induction of protective mechanisms against heavy metal(loid) phytotoxicity. Despite a knowledge gap regarding how ABA signaling pathway operates in response to heavy metal exposure, a series of results indicate a strong correlation between high ABA levels and a reduction in plant stresses (Bücker-Neto et al. 2017). ABA is usually considered as a signaling molecule whose main function is to retain the water potential during drought stress. It is thought to perform a similar role during heavy metal(loid) stress as water potential is also declined during heavy metal(loid) stress. The ABA-induced stomatal closure which causes suppression of transpirational flow might end up restricting-root-to-shoot translocation of heavy metal(loid)s. ABA is also known to activate the transient MAPK activity. Moreover, the ABA-induced growth inhibition is viewed as an acclimation strategy against heavy metal(loid) stressed conditions (Bücker-Neto et al. 2017). Upon As(III) exposure ABA biosynthesis genes, OsNCED1 and OsNCED2 were greatly induced in O. sativa roots (Yu et al. 2012). Transcriptomic study on upland O. sativa also revealed induction of genes of ABA signaling pathway (ASR, LEA, PP2C, and PYL6) on As(III) and As(V) exposure (Di et al. 2021). The induction of ABA biosynthesis (OsNCED2, OsNCED3) and ABA signaling genes (OsPP2C4, OsPP2C5, OsbZIP10, and OsbZIP12) in O. sativa root is thought to evoke root growth inhibition in response to As(V) toxicity (Huang et al. 2012). Numerous ABA-related genes like HAI1, ABI1, and AIP2 were found to be expressed under As(V) stress in B. juncea (Srivastava et al. 2015). The expression of ABA biosynthesis genes under As stress in turn can increase the endogenous ABA levels. ABA transcriptionally regulates up to 10% of protein-encoding genes. It is possible that ABA might act as a trade-off between plant response to heavy metal(loid) stress, triggering a balance between survival and growth of the plants (Sytar et al. 2019). Ethylene is another phytohormone that is considered to be a critical player in the abiotic stress responses of plants. The key response regulated by ethylene in heavy metal stress is the modulation of ROS machinery. The crosstalk between ethylene with ROS network of plants regulates the activity of both ROS producing and ROS scavenging enzymes in response to heavy metal stress (Saini et al. 2021). The transcript levels of ET biosynthesis genes (such as ACS1, ACS2, ACO3, ACO4, ACO5, and ACO6) and signaling genes (such as- EIN3;3, EIN3;4, and EIN3;5) were found to be induced upon As(V) application in O. sativa roots (Huang et al. 2012). Similar results were obtained in a separate study conducted on upland O. sativa where ET signaling genes EIN3 and ACO7 were found to be induced under As(III) and As(V) stress (Di et al. 2021). The enhanced ET production upon As exposure might be implicated in the root growth inhibition process along with other phytohormones like ABA (Saini et al. 2021). Recent reports suggest that the interconnected signaling network of JA, ET, and BRs controls adventitious and lateral rooting that act as stress avoidance responses to As (Betti et al. 2021).
SA is a phenolic compound largely known for its defense-related function in response to biotic stresses. Nonetheless, exogenous application of SA improves growth, reduces total As accumulation by downregulating OsLsi2, decreased As translocation in shoots, reverts As(V)-induced oxidative stress by increasing antioxidant enzymes, enhances vacuolar sequestration of As, and betters iron translocation into shoots (Singh et al. 2015). SA biosynthesis gene (FAMT) was found to be upregulated in both root and shoot of B. juncea under As(V) stress. Therefore, SA can be considered as a key facilitator of As stress-responsive mechanisms in plants (Srivastava et al. 2015).
The roles of other phytohormones like BRs and SLs are now emerging for heavy metalloid stress responses in plants. BRs found to improve the plant defense system, increase photosynthetic capacity (by increasing chlorophyll and carotenoid content), and protect photosynthetic apparatus. It also stimulates the antioxidant system, gene expression, and crosstalk with other phytohormones (like ABA, AUX, CKs, ET, GAs, JA, polyamines, and SA) in a finely tuned signaling cascade. The most pronounced effect of BRs under metalloid toxicity is probably the regulation of metabolic responses and redox homeostasis of plants (Sharma et al. 2022). Exogenously application of BRs like 24-epibrassinolide (Br24) and 28-homobrassinolide (Br28) was found to mitigate As(III) toxicity by lowering As accumulation and also found to stimulate root growth in O. sativa (Xu et al. 2018, 2020). Whereas in T. aestivum, elevated antioxidant enzymes with increased concentration of osmolytes, relative water content, gas exchange parameters along with reduced levels of H2O2, MDA, and electrolyte leakage was reported when Br24 was exogenously applied in combination with SA and Si (Maghsoudi et al. 2020). The external application of 24-epibrassinolide enhanced CAT and SOD activities, improved total antioxidant levels, free proline content, reduced MDA contents along with modulated expression of CAT isoforms (CAT1, CAT2, and CAT3), SOD isoforms (Cu-ZnSODs, FeSODs, and MnSOD), heat shock proteins (Hsp70-4 and Hsp90-1) and proline biosynthesis genes (P5CS1 and P5CS2) genes under As(V) stress in A. thaliana (Surgun-Acar and Zemheri-Navruz 2019). Apart from the transcript level changes, the detailed understanding of the signaling cascade of BRs for As stress mitigation and crosstalk with other signaling cascades for this purpose are still unknown.
Recently, the role of the SLs in As stress mitigation has surfaced. In O. sativa roots, SLs restrict the cellular uptake and accumulation of As(V) by modulating the expression of multiple Pi transporter-encoding genes (like OsPT1, OsPT2, OsPT4, OsPT8, and OsPHO1;2) and efflux transporter-encoding gene OsLsi2. Root cells may enhance the biosynthesis of SLs by inducing the expression of SL-biosynthesis genes like D10 and D17 in response to As stress. Moreover, upon As accumulation in roots SLs, root cells may upregulate GSH biosynthesis (like OsGSH1 and OsGSH2), PCs (OsPCS1), and ABC transporter (OsABCC1) transcripts which indicates efficient vacuolar sequestration of As(V). Furthermore, higher transcript levels of APX (OsAPX1 and OsAPX2), CAT (OsCATA), and SOD (OsCuZnSOD1 and OsCuZnSOD2) indicate that SLs modulate ROS detoxification through activation of key antioxidant enzymes (Mostofa et al. 2021). The combined application of Br24 and SL (Gr24) improved root growth and promoted the accumulation of As(V) in roots thereby reducing root to shoot translocation of the metalloid (Xu et al. 2019). However, the mechanistic understanding of the underlying signaling cascade is yet to uncover.
Role of transcription factors and miRNAs
Transcription factors
The regulation of gene expression is very critical as it determines the fate of the plant development as well as tolerance to As stress. The stressors induce a large number of genes and proteins in order to link signaling pathways that confer stress tolerance. The genes of the regulatory groups encode different TFs which further regulate different stress-responsive genes cooperatively and/or discretely constituting a regulatory gene network (Singh et al. 2016). The expression of downstream target genes of various signaling cascades is tightly controlled by TFs. This TF-mediated regulation also implies signaling cascades induced by As stress imparting protection to plants (Thakur et al. 2020). For example, downstream targets of MAPK signaling contains TFs from different families and sub-families such as myeloblastosis (MYB), WRKY, C2H2- type Zinc finger (ZAT), basic leucine zipper (bZIP), activator protein (AP2), ethylene-responsive factor (ERF), dehydration responsive element (DRE) binding protein, etc. (Jalmi et al. 2018). In response to As stress, numerous TFs were found to be differentially expressed in plants. In A. thaliana, differential expression of genes in response to As(V) with known TF activity includes AP2 domain-containing TF, C2H2 type Zinc finger, WRKY, and NAC domain-containing protein, and DRE binding protein. Among these, NAC domain-containing TF modulates the expression of the FeSOD gene along with several other genes. NAC proteins comprise a large gene family of plant-specific TFs that have roles in development, defense, and abiotic stress responses (Abercrombie et al. 2008). Similarly, Fu et al. (2014) reported As(V)-induced expression of APETALA2/ethylene response element-binding protein (AP2/EREBP), Aux/IAA, heat shock transcription factor (HSF), WRKY TF encoding genes in A. thaliana. Kinases may act as signals on these TFs, leading to the production of As stress-related proteins and secondary metabolites that can act as either damage-causing or stress-countering agents (Fu et al. 2014).
Differential expression of regulatory genes can also be seen in As(V) sensitive (Slavi-1) as well as tolerant (Col-0) accessions of A. thaliana. In As(V) sensitive accession, members of WRKY superfamily, APETALA2/ethylene-responsive factor (AP2/ERF), NAC domain-containing proteins, and MYBs (MYB15 and MYB95) were up-regulated and genes encoding Zinc finger family protein (C2H2 and CCCH type), bHLH, MYBs (MYB 59 and MYB48), and MYB-like 2 (MYBL2) were down-regulated. However, in As(V)-resistant accession, ten TFs including a novel zinc finger (ZF6) involved in trichome development via GA and CK signaling integration and a PLATZ family TF were differentially expressed, and genes encoding members of bHLH, and NAC domain-containing protein (NAC6, NAC19 and NAC47), phytochrome interacting factor 3 (PIF3) and brassinosteroid enhanced expression 3 (BEE3) were exclusively down-regulated (Shukla et al. 2018). The MYB genes are thought to play an important role in As(V) stress tolerance by enhancing antioxidant potential by modulating the phenylpropanoid/flavonoids biosynthesis pathway. Whereas the ERF subfamily has been known for engaging in ET signaling in response to abiotic stresses (Shukla et al. 2018). Previous reports suggest that DREB (a subfamily of AP2/ERF family) maintains osmotic potential in the cell membrane and decreases the inflow of heavy metals in plants under heavy metal stress (Abiri et al. 2017).
Arsenic-responsive TFs that are being reported in O. sativa mostly belonged to AP2/ERF (ERF and DREB subfamilies), HSF, ZIM (Zinc-finger protein expressed in inflorescence meristem), MYB, NAM, ATAF, CUC (NAC), bHLH, ZF/CCCH and WRKY families (Hauang et al. 2012; Yu et al. 2012; Rai et al. 2015). Recently, transcriptomic analyses of upland O. sativa revealed that mostly TFs from AP2-EREBP, bHLH, bZIP, Dof, GRAS, HSF, MYB, NAC, and WRKY families were affected under As(III) and As(V) stress (Di et al. 2021). These TFs are mostly associated with ROS detoxification, phytohormone signaling, and maintenance of cellular homeostasis thereby protecting plants from As toxicity. The As transporters are also regulated by several TFs which is already been discussed in the previous section. Therefore, it can be said that the cellular intake of As is also checked by the specific TFs.
In a study conducted on B. juncea, it was found that a total of 116 TFs were showed changes in expression upon perceiving As stress which include MYB, WRKY, GATA, AP2/ERF, HSFs, G2-like bHLH, homeobox, C2H2 Zn finger, Constans-like, DOF Zn finger, etc. Among them, HSFA2 was up-regulated in the shoot which is a key regulator in inducing defense system in stress. Two C2H2 Zn fingers ZAT6 and ZAT12 were significantly downregulated in root and shoot, respectively. ZAT6 acts as a repressor of primary root length and regulates phosphate homeostasis. On the other hand, ZAT12 is involved in oxidative stress signal transduction and plays a central role in maintaining ROS homeostasis. The early induction of WRKY6 and WRKY75 confirms that As(V) operates as a Pi mimic and affects the expression of genes triggered by Pi starvation. Furthermore, TFs like plant U-box (PUB23) were downregulated under As(V) stress and it is probably to balance the As mediated disruption of water status (Srivastava et al. 2015). Characterization of an artificial microRNAs (amiRNA) line targeting closely homologous CBF and ERF TFs shows that CBF 1, 2, and 3 negatively regulate As(III) and As(V) sensitivity. Generation of CRISPR lines, higher order T-DNA mutants, and gene expression analyses further supports these findings. Besides, ERF TFs differentially regulate As(III) sensitivity and Cd tolerance (Xie et al. 2021).
miRNAs
MicroRNAs (miRNAs) represent a class of 21–24 nucleotide non-coding small RNA molecules involved in silencing their targets through either translational inhibition or direct mRNA cleavage (Sharma et al. 2015). These are not only critical for growth and development but also plants’ responses to toxic heavy metals. In fact, it is now believed that metal uptake, transport, and detoxification including associated physiological responses, phytohormone signaling falls under the master regulation of miRNAs (Srivastava and Suprasanna 2021). Expressions of miRNAs from diverse families were found to be modulated during arsenic stress. By analyzing cis-elements of promoters of various miRNAs that were responsive to As it is also assumed that miRNA mediated regulation is interconnected with SA, JA, GA, ET, and ABA pathways (Sharma et al. 2015). In O. sativa, differential expression (up-regulation- miR396, miR399, miR408, miR528, miR1861, miR2102, miR2907 and downregulation- miR164, miR171, miR395, miR529, miR820, miR1432, miR1846) of members of different miRNA families was observed in response to As stress. The predicted targets of these miRNAs are of various categories such as DNA-binding protein, TFs, metal transport proteins, genes related to sulfur metabolism, and genes related to redox homeostasis maintenance (Sharma et al. 2015). From the putative targets of the miRNA, the exact roles of miRNA in As stress mitigation can be predicted. For example, miR164 targets NAC TFs which transduces AUX signals for the development of lateral roots. Therefore, the emergence of lateral roots under As stressed conditions might be due to modulated transcripts of NAC. Similarly, miR395 regulates low-affinity sulfate transporter (SULTR2;1) and ATP sulfurylases involved in sulfate metabolism. The induction of miR395 during As stress causes enhanced biosynthesis of sulfur-containing amino acids and peptides required for As detoxification (Sharma et al. 2015). Liu and Zhang (2012) also reported several As(III) induced miRNAs in O. sativa that are associated with Ca2+signal transduction (miR1432, miR1318), plant development (miR172c, miR169a), photosynthesis, and metabolism (miR528, miR408, miR397b). From this study, it is evident that miRNAs are involved in developmental processes, metabolism, and signaling cascades during As stress promoting the survival of the plants (Liu and Zhang 2012). Profiling of As-stress induced miRNAs (such as miR159, miR164, miR167, miR319, miR390, miR838, miR854, etc.) in B.napus revealed their multifarious role in developmental processes, sulfur uptake, transport and assimilation, and hormonal biosynthesis and/or function (especially AUX, JAs, and ABA) (Srivastava et al. 2013). External application of JA and IAA altered expressions of miR167, miR319, and miR854 under As stress further suggesting interplay between miRNA and phytohormones under such conditions to ameliorate As toxicity (Srivastava et al. 2013). An O. sativa specific miR156j was found to modulate the metabolic activities in O. sativa plants at different developmental stages under As(III) stress (Pandey et al. 2020). As(V)-responsive miRNAs in Zea mays regulates growth and development (miR156s, miR166e-3p, miR166m, and miR19b-3p), metabolic processes (miR159b-5p.3, zma-460, zma_468, and zma_469), ROS generation (miR167d) and hormone signalling (miR167d, miR159e.2, and miR319b-3p) (Ghosh et al. 2017). The putative targets of the aforementioned miRNAs are suggestive of the defense strategies taken by plants to combat As toxicity which includes modulation of developmental processes, hormonal networks, ROS homeostasis, S metabolism, metal transporters, metal-binding proteins, etc. at the post-transcriptional level.
Conclusion
The hazardous effects of As are a matter of concern for the environment. Due to their sessile nature plants frequently experience As in their growing environment. Several studies indicate that As transporters are pivotal in the uptake, translocation, and detoxification of As. The chemical relatedness between Pi and As(V) enforces As(V) uptake in plants through PHTs located in the plasma membrane of the root epidermal cells under aerobic conditions. In anaerobic environments, such as flooding paddy soil aquaporin channels are being utilized for uptake and translocation of As(III) and a few organic arsenicals. Among such aquaporin channels, the NIP subfamily of the aquaporins is a noteworthy one as they also mediate essential nutrient (like B, Se, Si, etc.) uptake. It is apparent that there is a critical correlation between plant nutrition and As uptake due to their competition for the shared uptake pathway. The nutrient richness in the soil environment helps to lessen As toxicity in plants to a certain extent whereas in nutrient-deprived conditions the metalloid jeopardizes the plant metabolism and the survival becomes cumbersome for plants in such conditions. The uptake of organic arsenicals is generally inefficient when compared against their organic counterparts but when it comes to root to shoot translocation the organic arsenicals perform better than those of inorganic ones. Probably for this reason, oAs content in reproductive tissues was found to be higher than that of iAs as found in some experiments. Apart from PHTs and aquaporins several other transporters such as ABCCs, INTs, MATEs, NRAMPs, and PTRs are also found to be involved in root to shoot translocation of As. After making its way into the plant cell the metalloid exerts a plethora of phytotoxic effects manifested by several morphological, physiological, and biochemical anomalies. It includes reduced growth, yield, biomass, disorganized root structure mainly due to disruption in IAA metabolism, decreased chlorophyll biosynthesis, disarrayed photosynthetic parameters, hampered mineral nutrition, enhanced ROS production, elevated membrane lipid peroxidation, Pi displacement in key metabolic processes, impeded carbohydrate and protein metabolism, and induced genotoxic damages to name a few. Plants counter such oppressive situations through a wide array of defense responses. The efflux of As through various transporters can be viewed as an arsenic stress mitigation strategy. Different transporters such as aquaporins, ACR, and PIN transporters are thought to be involved in this process. Regardless of ambiguity, iron plaque formation can also be considered as a protective mechanism. Arsenic transporters are a critical element in As detoxification strategy and thus are tightly regulated through different TFs, miRNAs, and protein phosphorylation-dephosphorylation. By far, the most talked-about defense response against As stress is the vacuolar sequestration of As mainly by means of ABCC transporters after reduction (through arsenate reductase activity of ACRs or HACs and glutaredoxins) and chelation (through PCs). The As-induced ROS production and its subsequent toxic effects are usually defended under the aegis of antioxidant machinery. The enzymatic antioxidants like APX, CAT, GOPX, GPX, POX, SOD, etc. play a crucial role as evidenced by their level changes as well as changes in the differential expression of genes encoding them found in different biochemical and gene expression studies respectively. Besides, thiol compounds are also indispensable in As stress mitigation responses of plants. Arsenic stress perception triggers multiple signaling cascades in plants that involve the use of Ca2+, hormones, MAPKs, proteins, ROS, RNS, etc. which increases the survival chances of plants exposed to As toxicity by activating multiple lines of defense responses. The expression of downstream targets (stress-responsive genes) of these signaling cascades are regulated by a different family of TFs such as AP2/ERF, bZIP, HSF, MYB, NAC, WRKY, Zn fingers, etc. Moreover, the miRNAs are also involved in the regulatory processes associated with As stress responses. Nonetheless, the deeper understanding of the heavily interconnected signaling cascades and subsequent regulatory processes remain elusive. The thorough analysis of the existing knowledge suggests a molecular network of transporters, enzymes related to transport, detoxification, and antioxidant system which are further regulated via TFs and miRNAs and altogether they play critical roles in defense responses and detoxification of As. Pieces of evidence also suggest that these defense responses are species- and/or genotype-specific. Therefore, it is very hard to outline a common hypothesis to provide a generalized view of As stress responses in plants. To know the overall As defense responses in plants and to improve it further multi-omics approach is required. The current biotechnological tools such as CRISPR/Cas system can also be utilized for the same. This would pave the pathway towards developing safer food crops with lesser or without As for human populations in coming years.
Author contribution statement
SM, KP, and TKM conceptualized the theme of the review. SM wrote the manuscript. SKG, PP, PKG, and AG helped in the data mining of the Table. KP has drawn chemical structures of arsenic species. KP and TKM revised and finalized the manuscript. TKM supervised all authors. All authors finally read and approved the manuscript.
References
Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI, Amjad M, Hussain M, Natasha (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health 15:59. https://doi.org/10.3390/ijerph15010059
Abercrombie JM, Halfhill MD, Ranjan P, Rao MR, Saxton AM, Yuan JS, Stewart CN (2008) Transcriptional responses of Arabidopsis thaliana plants to As (V) stress. BMC Plant Biol 8:87. https://doi.org/10.1186/1471-2229-8-87
Abiri R, Shaharuddin NA, Maziah M, Yusof ZN, Atabaki N, Sahebi M, Valdiani A, Kalhori N, Azizi P, Hanafi MM (2017) Role of ethylene and the APETALA 2/ethylene response factor superfamily in rice under various abiotic and biotic stress conditions. Environ Exp Bot 134:33–44. https://doi.org/10.1016/j.envexpbot.2016.10.015
Adhikary A, Kumar R, Pandir R, Bhardwaj P, Wusirika R, Kumar S (2019) Pseudomonas citronellolis; a multi-metal resistant and potential plant growth promoter against arsenic (V) stress in chickpea. Plant Physiol Biochem 142:179–192. https://doi.org/10.1016/j.plaphy.2019.07.006
Agency for toxic substances and disease registry (2019) https://www.atsdr.cdc.gov/spl/index.html. Accessed on 26.01.2020
Ahmad P, Rasool S, Gul A, Sheikh SA, Akram NA, Ashraf M, Kazi AM, Gucel S (2016) Jasmonates: multifunctional roles in stress tolerance. Front Plant Sci 7:813. https://doi.org/10.3389/fpls.2016.00813
Ahmad P, Alam P, Balawi TH, Altalayan FH, Ahanger MA, Ashraf M (2020) Sodium nitroprusside (SNP) improves tolerance to arsenic (As) toxicity in Vicia faba through the modifications of biochemical attributes, antioxidants, ascorbate-glutathione cycle and glyoxalasecycle. Chemosphere 244:125480. https://doi.org/10.1016/j.chemosphere.2019.125480
Ahmad A, Khan WU, Shah AA, Yasin NA, Naz S, Ali A, Tahir A, Batool AI (2021) Synergistic effects of nitric oxide and silicon on promoting plant growth, oxidative stress tolerance and reduction of arsenic uptake in Brassica juncea. Chemosphere 262:128384. https://doi.org/10.1016/j.chemosphere.2020.128384
Ali W, Isner JC, Isayenkov SV, Liu W, Zhao FJ, Maathuis FJ (2012) Heterologous expression of the yeast arsenite efflux system ACR3 improves Arabidopsis thaliana tolerance to arsenic stress. New Phytol 194:716–723. https://doi.org/10.1111/j.1469-8137.2012.04092.x
Anjum SA, Tanveer M, Hussain S, Ashraf U, Khan I, Wang L (2017) Alteration in growth, leaf gas exchange, and photosynthetic pigments of maize plants under combined cadmium and arsenic stress. Water Air Soil Pollut 228:13. https://doi.org/10.1007/s11270-016-3187-2
Ashraf MA, Umetsu K, Ponomarenko O, Saito M, Aslam M, Antipova O, Dolgova N, Kiani CD, Nezhati S, Tanoi K, Minegishi K, Nagatsu K, Kamiya T, Fujiwara T, Luschnig C, Tanino K, Pickering I, George GN, Rahman A (2020) PIN FORMED 2 modulates the transport of arsenite in Arabidopsis thaliana. Plant Commun 1(3):100009. https://doi.org/10.1016/j.xplc.2019.100009
Awasthi S, Chauhan R, Srivastava S, Tripathi RD (2017) The journey of arsenic from soil to grain in rice. Front Plant Sci 8:1007. https://doi.org/10.3389/fpls.2017.01007
Barozzi F, Papadia P, Stefano G, Renna L, Brandizzi F, Migoni D, Fanizzi FP, Piro G, Di Sansebastiano GP (2019) Variation in membrane trafficking linked to SNARE AtSYP51 interaction with aquaporin NIP1; 1. Front Plant Sci 9:1949. https://doi.org/10.3389/fpls.2018.01949
Baud S, Lepiniec L (2009) Regulation of de novo fatty acid synthesis in maturing oilseeds of Arabidopsis. Plant Physiol Biochem 47(6):448–455. https://doi.org/10.1016/j.plaphy.2008.12.006
Bayle V, Arrighi JF, Creff A, Nespoulous C, Vialaret J, Rossignol M, Gonzalez E, Paz-Ares J, Nussaume L (2011) Arabidopsis thaliana high-affinity phosphate transporters exhibit multiple levels of posttranslational regulation. Plant Cell 23:1523–1535. https://doi.org/10.1105/tpc.110.081067
Betti C, Della Rovere F, Piacentini D, Fattorini L, Falasca G, Altamura MM (2021) Jasmonates, ethylene and brassinosteroids control adventitious and lateral rooting as stress avoidance responses to heavy metals and metalloids. Biomolecules 11(1):77. https://doi.org/10.3390/biom11010077
Bhat JA, Ahmad P, Corpas FJ (2021) Main nitric oxide (NO) hallmarks to relieve arsenic stress in higher plants. J Hazard Mater 406:124289. https://doi.org/10.1016/j.jhazmat.2020.124289
Bianucci E, Godoy A, Furlan A, Peralta JM, Hernández LE, Carpena-Ruiz RO, Castro S (2018) Arsenic toxicity in soybean alleviated by a symbiotic species of Bradyrhizobium. Symbiosis 74:167–176. https://doi.org/10.1007/s13199-017-0499-y
Bienert GP, Thorsen M, Schüssler MD, Nilsson HR, Wagner A, Tamás MJ, Jahn TP (2008) A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH) 3 and Sb(OH) 3 across membranes. BMC Biol 6:26. https://doi.org/10.1186/1741-7007-6-26
Boorboori MR, Gao Y, Wang H, Fang C (2021) Usage of Si, P, Se, and Ca decrease arsenic concentration/toxicity in rice, a review. Appl Sci 11(17):8090. https://doi.org/10.3390/app11178090
Bücker-Neto L, Paiva AL, Machado RD, Arenhart RA, Margis-Pinheiro M (2017) Interactions between plant hormones and heavy metals responses. Genet Mol Biol 40:373–386. https://doi.org/10.1590/1678-4685-GMB-2016-0087
Cai Y, Su J, Ma LQ (2004) Low molecular weight thiols in arsenic hyperaccumulator Pteris vittata upon exposure to arsenic and other trace elements. Environ Pollut 129:69–78. https://doi.org/10.1016/j.envpol.2003.09.020
Cao Y, Sun D, Ai H, Mei H, Liu X, Sun S, Xu G, Liu Y, Ma LQ (2017) Knocking out OsPT4 gene decreases arsenate uptake by rice plants and inorganic arsenic accumulation in rice grains. Environ Sci Technol 51:12131–12138. https://doi.org/10.1021/acs.est.7b03028
Cao Y, Feng H, Sun D, Xu G, Rathinasabapathi B, Chen Y, Ma LQ (2019) Heterologous expression of Pteris vittata phosphate transporter PvPht1;3 enhances arsenic translocation to and accumulation in tobacco shoots. Environ Sci Technol 53:10636–10644. https://doi.org/10.1021/acs.est.9b02082
Castrillo G, Sánchez-Bermejo E, de Lorenzo L, Crevillén P, Fraile-Escanciano A, Mohan TC, Mouriz A, Catarecha P, Sobrino-Plata J, Olsson S, del Puerto YL, Mateos I, Rojo E, Hernández LE, Jarillo JA, Piñeiro M, Paz-Ares J, Leyva A (2013) WRKY6 transcription factor restricts arsenate uptake and transposon activation in Arabidopsis. Plant Cell 25:2944–2957. https://doi.org/10.1105/tpc.113.114009
Catarecha P, Segura MD, Franco-Zorrilla JM, García-Ponce B, Lanza M, Solano R, Paz-Ares J, Leyva A (2007) A mutant of the Arabidopsis phosphate transporter PHT1;1 displays enhanced arsenic accumulation. Plant Cell 19:1123–1133. https://doi.org/10.1105/tpc.106.041871
Chandrakar V, Yadu B, Meena RK, Dubey A, Keshavkant S (2017) Arsenic-induced genotoxic responses and their amelioration by diphenylene iodonium, 24-epibrassinolide and proline in Glycine max L. Plant Physiol Biochem 112:74–86. https://doi.org/10.1016/j.plaphy.2016.12.023
Chandrakar V, Pandey N, Keshavkant S (2018) Plant responses to arsenic toxicity: morphology and physiology. In: Hasanuzzaman M, Nahar K, Fujita M (eds) Mechanisms of arsenic toxicity and tolerance in plants. Springer, Singapore, pp 27–48. https://doi.org/10.1007/978-981-13-1292-2_2
Chao DY, Chen Y, Chen J, Shi S, Chen Z, Wang C, Danku JM, Zha FJ, Salt DE (2014) Genome-wide association mapping identifies a new arsenate reductase enzyme critical for limiting arsenic accumulation in plants. PLoS Biol 12:e1002009. https://doi.org/10.1371/journal.pbio.1002009
Chen J, Liu Y, Ni J, Wang Y, Bai Y, Shi J, Gan J, Wu Z, Wu P (2011) OsPHF1 regulates the plasma membrane localization of low-and high-affinity inorganic phosphate transporters and determines inorganic phosphate uptake and translocation in rice. Plant Physiol 157:269–278. https://doi.org/10.1104/pp.111.181669
Chen YS, Xu WZ, Shen HL, Yan HL, Xu WX, He ZY, Ma M (2013) Engineering arsenic tolerance and hyperaccumulation in plants for phytoremediation by a Pvacr3 transgenic approach. Environ Sci Technol 47:9355–9362. https://doi.org/10.1021/es4012096
Chen Y, Han YH, Cao Y, Zhu YG, Rathinasabapathi B, Ma LQ (2017a) Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front Plant Sci 8:268. https://doi.org/10.3389/fpls.2017.00268
Chen Y, Sun SK, Tang Z, Liu GD, Moore KL, Maathuis FJM, Miller AJ, McGrath SP, Zhao FJ (2017b) The Nodulin 26-like intrinsic membrane protein OsNIP3;2 is involved in arsenite uptake by lateral roots in rice. J Exp Bot 68:3007–3016. https://doi.org/10.1093/jxb/erx165
Chen Y, Hua CY, Jia MR, Fu JW, Liu X, Han YH, Liu Y, Rathinasabapathi B, Cao Y, Ma LQ (2017c) Heterologous expression of Pteris vittata arsenite antiporter PvACR3;1 reduces arsenic accumulation in plant shoots. Environ Sci Technol 51:10387–10395. https://doi.org/10.1021/acs.est.7b03369
Chen C, Li LY, Huang K, Zhang J, Xie WY, Lu Y, Dong XZ, Zhao FJ (2019) Sulfate-reducing bacteria and methanogens are involved in arsenic methylation and demethylation in paddy soils. ISME J 13:2523–2535. https://doi.org/10.1038/s41396-019-0451-7
Costa de Oliveira A, Batista BL, Pegoraro C, Venske E, Viana VE (2020) Mechanisms of arsenic uptake, transport. In: Srivastava S (ed) Arsenic in drinking water and food. Springer, Singapore, pp 371–389. https://doi.org/10.1007/978-981-13-8587-2_14
Cuypers A, Hendrix S, Amaral dos Reis R, De Smet S, Deckers J, Gielen H, Jozefczak M, Loix C, Vercampt H, Vangronsveld J, Keunen E (2016) Hydrogen peroxide, signaling in disguise during metal phytotoxicity. Front Plant Sci 7:470. https://doi.org/10.3389/fpls.2016.00470
Das J, Sarkar P (2018) Remediation of arsenic in mung bean (Vigna radiata) with growth enhancement by unique arsenic-resistant bacterium Acinetobacter lwoffii. Sci Total Environ 624:1106–1118. https://doi.org/10.1016/j.scitotenv.2017.12.157
Das N, Bhattacharya S, Bhattacharyya S, Maiti MK (2018) Expression of rice MATE family transporter OsMATE2 modulates arsenic accumulation in tobacco and rice. Plant Mol Biol 98:101–120. https://doi.org/10.1007/s11103-018-0766-1
Demidchik V (2015) Mechanisms of oxidative stress in plants: from classical chemistry to cell biology. Environ Exp Bot 109:212–228. https://doi.org/10.1016/j.envexpbot.2014.06.021
Devaiah BN, Karthikeyan AS, Raghothama KG (2007) WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol 143:1789–1801. https://doi.org/10.1104/pp.106.093971
Di X, Zheng F, Norton GJ, Beesley L, Zhang Z, Lin H, Zhi S, Liu X, Ding Y (2021) Physiological responses and transcriptome analyses of upland rice following exposure to arsenite and arsenate. Environ Exp Bot 183:104366. https://doi.org/10.1016/j.envexpbot.2020.104366
Dietrich D, Hammes U, Thor K, Suter-Grotemeyer M, Flückiger R, Slusarenko AJ, Ward JM, Rentsch D (2004) AtPTR1, a plasma membrane peptide transporter expressed during seed germination and in vascular tissue of Arabidopsis. Plant J 40:488–499. https://doi.org/10.1111/j.1365-313X.2004.02224.x
DiTusa SF, Fontenot EB, Wallace RW, Silvers MA, Steele TN, Elnagar AH, Dearman KM, Smith AP (2016) A member of the phosphate transporter 1 (Pht1) family from the arsenic-hyperaccumulating fern Pteris vittata is a high-affinity arsenate transporter. New Phytol 209:762–772. https://doi.org/10.1111/nph.13472
Duan G, Kamiya T, Ishikawa S, Arao T, Fujiwara T (2012) Expressing ScACR3 in rice enhanced arsenite efflux and reduced arsenic accumulation in rice grains. Plant Cell Physiol 53:154–163. https://doi.org/10.1093/pcp/pcr161
Duan G, Liu W, Chen X, Hu Y, Zhu Y (2013) Association of arsenic with nutrient elements in rice plants. Metallomics 5(7):784–792. https://doi.org/10.1039/c3mt20277a
Duan GL, Hu Y, Schneider S, McDermott J, Chen J, Sauer N, Rosen BP, Daus B, Liu Z, Zhu YG (2015) Inositol transporters AtINT2 and AtINT4 regulate arsenic accumulation in Arabidopsis seeds. Nat Plants 2:1–6. https://doi.org/10.1038/nplants.2015.202
Duquesnoy I, Champeau GM, Evray G, Ledoigt G, Piquet-Pissaloux A (2010) Enzymatic adaptations to arsenic-induced oxidative stress in Zea mays and genotoxic effect of arsenic in root tips of Vicia faba and Zea mays. CR Biol 333:814–824. https://doi.org/10.1016/j.crvi.2010.07.004
Farmer EE, Mueller MJ (2013) ROS-mediated lipid peroxidation and RES-activated signaling. Annu Rev Plant Biol 64:429–450. https://doi.org/10.1146/annurev-arplant-050312-120132
Farnese FS, Oliveira JA, Gusman GS, Leão GA, Silveira NM, Silva PM, Ribeiro C, Cambraia J (2014) Effects of adding nitroprusside on arsenic stressed response of Pistia stratiotes L. under hydroponic conditions. Int J Phytoremediation 16(2):123–137. https://doi.org/10.1080/15226514.2012.759532
Farnese FS, Oliveira JA, Paiva EA, Menezes-Silva PE, da Silva AA, Campos FV, Ribeiro C (2017) The involvement of nitric oxide in integration of plant physiological and ultrastructural adjustments in response to arsenic. Front Plant Sci 8:516. https://doi.org/10.3389/fpls.2017.00516
Farooq MA, Gill RA, Ali B, Wang J, Islam F, Ali S, Zhou W (2016a) Subcellular distribution, modulation of antioxidant and stress-related genes response to arsenic in Brassica napus L. Ecotoxicology 25:350–366. https://doi.org/10.1007/s10646-015-1594-6
Farooq MA, Gill RA, Islam F, Ali B, Liu H, Xu J, He S, Zhou W (2016b) Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front Plant Sci 7:468. https://doi.org/10.3389/fpls.2016.00468
Finnegan P, Chen W (2012) Arsenic toxicity: the effects on plant metabolism. Front Physiol 3:182. https://doi.org/10.3389/fphys.2012.00182
Fu SF, Chen PY, Nguyen QTT, Huang LY, Zeng GR, Huang TL, Lin CY, Huang HJ (2014) Transcriptome profiling of genes and pathways associated with arsenic toxicity and tolerance in Arabidopsis. BMC Plant Biol 14:94. https://doi.org/10.1186/1471-2229-14-94
Gangwar S, Singh VP, Tripathi DK, Chauhan DK, Prasad SM, Maurya JN (2014) Plant responses to metal stress: the emerging role of plant growth hormones in toxicity alleviation. In: Ahmad P, Rasool S (eds) Emerging technologies and management of crop stress tolerance, a sustainable approach, vol 2. Academic Press, pp 215–248. https://doi.org/10.1016/B978-0-12-800875-1.00010-7
Garg N, Singla P (2011) Arsenic toxicity in crop plants: physiological effects and tolerance mechanisms. Environ Chem Lett 9:303–321. https://doi.org/10.1007/s10311-011-0313-7
Gautam A, Pandey AK, Dubey RS (2020) Unravelling molecular mechanisms for enhancing arsenic tolerance in plants: a review. Plant Gene 23:100240. https://doi.org/10.1016/j.plgene.2020.100240
Ghori NH, Ghori T, Hayat MQ, Imadi SR, Gul A, Altay V, Ozturk M (2019) Heavy metal stress and responses in plants. Int J Environ Sci Technol 16(3):1807–1828. https://doi.org/10.1007/s13762-019-02215-8
Ghosh S, Saha J, Biswas AK (2013) Interactive influence of arsenate and selenate on growth and nitrogen metabolism in wheat (Triticum aestivum L.) seedlings. Acta Physiol Plant 35:1873–1885. https://doi.org/10.1007/s11738-013-1225-x
Ghosh S, Singh K, Shaw AK, Azahar I, Adhikari S, Ghosh U, Basu U, Roy S, Saha S, Sherpa AR, Hossain Z (2017) Insights into the miRNA-mediated response of maize leaf to arsenate stress. Environ Exp Bot 137:96–109. https://doi.org/10.1016/j.envexpbot.2017.01.015
Ghosh PK, Maiti TK, Pramanik K, Ghosh SK, Mitra S, De TK (2018) The role of arsenic resistant Bacillus aryabhattai MCC3374 in promotion of rice seedlings growth and alleviation of arsenic phytotoxicity. Chemosphere 211:407–419. https://doi.org/10.1016/j.chemosphere.2018.07.148
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
González E, Solano R, Rubio V, Leyva A, Paz-Ares J (2005) Phosphate transporter traffic facilitator1 is a plant-specific SEC12-related protein that enables the endoplasmic reticulum exit of a high-affinity phosphate transporter in Arabidopsis. Plant Cell 17:3500–3512. https://doi.org/10.1105/tpc.105.036640
Gratão PL, Polle A, Lea PJ, Azevedo RA (2005) Making the life of heavy metal-stressed plants a little easier. Funct Plant Biol 32:481–494. https://doi.org/10.1071/FP05016
Gunes A, Inal A, Bagci EG, Kadioglu YK (2010) Combined effect of arsenic and phosphorus on mineral element concentrations of sunflower. Commun Soil Sci Plant Anal 41(3):361–372. https://doi.org/10.1080/00103620903462357
Gupta P, Seth CS (2019) Nitrate supplementation attenuates As (V) toxicity in Solanum lycopersicum L. cv Pusa Rohini: Insights into As (V) sub-cellular distribution, photosynthesis, nitrogen assimilation, and DNA damage. Plant Physiol Biochem 139:44–55. https://doi.org/10.1016/j.plaphy.2019.03.007
Gupta M, Sharma P, Sarin NB, Sinha AK (2009) Differential response of arsenic stress in two varieties of Brassica juncea L. Chemosphere 74:1201–1208. https://doi.org/10.1016/j.chemosphere.2008.11.023
Gusman GS, Oliveira JA, Farnese FS, Cambraia J (2013a) Arsenate and arsenite: the toxic effects on photosynthesis and growth of lettuce plants. Acta Physiol Plant 35:1201–1209. https://doi.org/10.1007/s11738-012-1159-8
Gusman GS, Oliveira JA, Farnese FS, Cambraia J (2013b) Mineral nutrition and enzymatic adaptation induced by arsenate and arsenite exposure in lettuce plants. Plant Physiol Biochem 71:307–314. https://doi.org/10.1016/j.plaphy.2013.08.006
Hasanuzzaman M, Fujita M (2013) Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 22:584–596. https://doi.org/10.1007/s10646-013-1050-4
He Z, Yan H, Chen Y, Shen H, Xu W, Zhang H, Shi L, Zhu YG, Ma M (2016) An aquaporin PvTIP4;1 from Pteris vittata may mediate arsenite uptake. New Phytol 209:746–761. https://doi.org/10.1111/nph.13637
Huang TL, Nguyen QTT, Fu SF, Lin CY, Chen YC, Huang HJ (2012) Transcriptomic changes and signalling pathways induced by arsenic stress in rice roots. Plant Mol Biol 80:587–608. https://doi.org/10.1007/s11103-012-9969-z
Hussain MM, Bibi I, Niazi NK, Shahid M, Iqbal J, Shakoor MB, Ahmad A, Shah NS, Bhattacharya P, Mao K, Bundschuh J (2021) Arsenic biogeochemical cycling in paddy soil-rice system: Interaction with various factors, amendments and mineral nutrients. Sci Total Environ 773:145040. https://doi.org/10.1016/j.scitotenv.2021.145040
Indriolo E, Na G, Ellis D, Salt DE, Banks JA (2010) A vacuolar arsenite transporter necessary for arsenic tolerance in the arsenic hyperaccumulating fern Pteris vittata is missing in flowering plants. Plant Cell 22:2045–2057. https://doi.org/10.1105/tpc.109.069773
Isayenkov SV, Maathuis FJ (2008) The Arabidopsis thaliana aquaglyceroporin AtNIP7; 1 is a pathway for arsenite uptake. FEBS Lett 582:1625–1628. https://doi.org/10.1016/j.febslet.2008.04.022
Islam E, Khan MT, Irem S (2015) Biochemical mechanisms of signaling: perspectives in plants under arsenic stress. Ecotoxicol Environ Saf 114:126–133. https://doi.org/10.1016/j.ecoenv.2015.01.017
Jalmi SK, Bhagat PK, Verma D, Noryang S, Tayyeba S, Singh K, Sharma D, Sinha AK (2018) Traversing the links between heavy metal stress and plant signaling. Front Plant Sci 9:12. https://doi.org/10.3389/fpls.2018.00012
Jha AB, Dubey RS (2004a) Carbohydrate metabolism in growing rice seedlings under arsenic toxicity. J Plant Physiol 161(7):867–872. https://doi.org/10.1016/j.jplph.2004.01.004
Jha AB, Dubey RS (2004b) Arsenic exposure alters activity behaviour of key nitrogen assimilatory enzymes in growing rice plants. Plant Growth Regul 43:259–268. https://doi.org/10.1023/B:GROW.0000045995.49365.df
Ji R, Zhou L, Liu J, Wang Y, Yang L, Zheng Q, Zhang C, Zhang B, Ge H, Yang Y, Zhao F (2017) Calcium-dependent protein kinase CPK31 interacts with arsenic transporter AtNIP1; 1 and regulates arsenite uptake in Arabidopsis thaliana. PLoS ONE 12:e0173681. https://doi.org/10.1371/journal.pone.0173681
Jia H, Ren H, Gu M, Zhao J, Sun S, Zhang X, Chen J, Wu P, Xu G (2011) The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol 156:1164–1175. https://doi.org/10.1104/pp.111.175240
Jonak C, Ökrész L, Bögre L, Hirt H (2002) Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol 5:415–424. https://doi.org/10.1016/S1369-5266(02)00285-6
Jung HI, Kong MS, Lee BR, Kim TH, Chae MJ, Lee EJ, Jung GB, Lee CH, Sung JK, Kim YH (2019) Exogenous glutathione increases arsenic translocation into shoots and alleviates arsenic-induced oxidative stress by sustaining ascorbate-glutathione homeostasis in rice seedlings. Front Plant Sci 10:1089. https://doi.org/10.3389/fpls.2019.01089
Kamiya T, Tanaka M, Mitani N, Ma JF, Maeshima M, Fujiwara T (2009) NIP1;1, an aquaporin homolog, determines the arsenite sensitivity of Arabidopsis thaliana. J Biol Chem 284:2114–2120. https://doi.org/10.1074/jbc.M806881200
Kamiya T, Islam R, Duan G, Uraguchi S, Fujiwara T (2013) Phosphate deficiency signaling pathway is a target of arsenate and phosphate transporter OsPT1 is involved in As accumulation in shoots of rice. Soil Sci Plant Nutr 59:580–590. https://doi.org/10.1080/00380768.2013.804390
Katsuhara M, Sasano S, Horie T, Matsumoto T, Rhee J, Shibasaka M (2014) Functional and molecular characteristics of rice and barley NIP aquaporins transporting water, hydrogen peroxide and arsenite. Plant Biotechnol 31:213–219. https://doi.org/10.5511/plantbiotechnology.14.0421a
Kaur S, Singh HP, Batish DR, Negi A, Mahajan P, Rana S, Kohli RK (2012) Arsenic (As) Inhibits radicle emergence and elongation in Phaseolus aureus by altering starch-metabolizing enzymes vis-à-vis disruption of oxidative metabolism. Biol Trace Elem Res 146(3):360–368. https://doi.org/10.1007/s12011-011-9258-8
Kofroňová M, Hrdinová A, Mašková P, Soudek P, Tremlova J, Pinkas D, Lipavská H (2019) Strong antioxidant capacity of horseradish hairy root cultures under arsenic stress indicates the possible use of Armoracia rusticana plants for phytoremediation. Ecotoxicol Environ Saf 174:295–304. https://doi.org/10.1016/j.ecoenv.2019.02.028
Komarova NY, Thor K, Gubler A, Meier S, Dietrich D, Weichert A, Grotemeyer MS, Tegeder M (2008) Rentsch D AtPTR1 and AtPTR5 transport dipeptides in planta. Plant Physiol 148:856–869. https://doi.org/10.1104/pp.108.123844
Krishnamurthy A, Rathinasabapathi B (2013) Auxin and its transport play a role in plant tolerance to arsenite-induced oxidative stress in Arabidopsis thaliana. Plant Cell Environ 36:1838–1849. https://doi.org/10.1111/pce.12093
Kumar S, Trivedi PK (2016) Heavy metal stress signaling in plants. In: Ahmad P (ed) Plant metal interaction. Elsevier, pp 585–603. https://doi.org/10.1016/B978-0-12-803158-2.00025-4
Kumar K, Gupta D, Mosa KA, Ramamoorthy K, Sharma P (2019) Arsenic transport, metabolism, and possible mitigation strategies in plants. In: Srivastava S, Srivastava A, Suprasanna P (eds) Plant-metal interactions. Springer Nature, Switzerland, pp 141–168. https://doi.org/10.1007/978-3-030-20732-8_8
Kumari A, Pandey N, Pandey-Rai S (2018) Exogenous salicylic acid-mediated modulation of arsenic stress tolerance with enhanced accumulation of secondary metabolites and improved size of glandular trichomes in Artemisia annua L. Protoplasma 255:139–152. https://doi.org/10.1007/s00709-017-1136-6
Lakshmanan V, Shantharaj D, Li G, Seyfferth AL, Sherrier DJ, Bais HP (2015) A natural rice rhizospheric bacterium abates arsenic accumulation in rice (Oryza sativa L.). Planta 242:1037–1050. https://doi.org/10.1007/s00425-015-2340-2
LeBlanc MS, McKinney EC, Meagher RB, Smith AP (2013) Hijacking membrane transporters for arsenic phytoextraction. J Biotechnol 163:1–9. https://doi.org/10.1016/j.jbiotec.2012.10.013
Leterrier M, Airaki M, Palma JM, Chaki M, Barroso JB, Corpas FJ (2012) Arsenic triggers the nitric oxide (NO) and S-nitrosoglutathione (GSNO) metabolism in Arabidopsis. Environ Pollut 166:136–143. https://doi.org/10.1016/j.envpol.2012.03.012
Li WX, Chen TB, Huang ZC, Lei M, Liao XY (2006) Effect of arsenic on chloroplast ultrastructure and calcium distribution in arsenic hyperaccumulator Pteris vittata L. Chemosphere 62:803–809. https://doi.org/10.1016/j.chemosphere.2005.04.055
Li RY, Ago Y, Liu WJ, Mitani N, Feldmann J, McGrath SP, Ma JF, Zhao FJ (2009) The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol 150:2071–2080. https://doi.org/10.1104/pp.109.140350
Li N, Wang J, Song WY (2016) Arsenic uptake and translocation in plants. Plant Cell Physiol 57:4–13. https://doi.org/10.1093/pcp/pcv143
Li J, Zhao Q, Xue B, Wu H, Song G, Zhang X (2019a) Arsenic and nutrient absorption characteristics and antioxidant response in different leaves of two ryegrass (Lolium perenne) species under arsenic stress. PLoS ONE 14:e0225373. https://doi.org/10.1371/journal.pone.0225373
Li Y, Wang X, Zhang H, Wang S, Ye X, Shi L, Xu F, Ding G (2019b) Molecular identification of the phosphate transporter family 1 (PHT1) genes and their expression profiles in response to phosphorus deprivation and other abiotic stresses in Brassica napus. PLoS ONE 14:e0220374. https://doi.org/10.1371/journal.pone.0220374
Lin A, Zhang X, Zhu YG, Zhao FJ (2007) Arsenate-induced toxicity: Effects on antioxidative enzymes and DNA damage in Vicia faba. Environ Toxicol Chem 27:413–419. https://doi.org/10.1897/07-266R.1
Lindsay ER, Maathuis FJ (2016) Arabidopsis thaliana NIP 7;1 is involved in tissue arsenic distribution and tolerance in response to arsenate. FEBS Lett 590:779–786. https://doi.org/10.1002/1873-3468.12103
Liu Q, Zhang H (2012) Molecular identification and analysis of arsenite stress-responsive miRNAs in rice. J Agric Food Chem 60:6524–6536. https://doi.org/10.1021/jf300724t
Lomax C, Liu WJ, Wu L, Xue K, Xiong J, Zhou J, McGrath SP, Meharg AA, Miller AJ, Zhao FJ (2012) Methylated arsenic species in plants originate from soil microorganisms. New Phytol 193:665–672. https://doi.org/10.1111/j.1469-8137.2011.03956.x
Luan M, Zhao F, Han X, Sun G, Yang Y, Liu J, Shi J, Fu A, Lan W, Luan S (2019) Vacuolar phosphate transporters contribute to systemic phosphate homeostasis vital for reproductive development in Arabidopsis. Plant Physiol 179:640–655. https://doi.org/10.1104/pp.18.01424
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105:9931–9935. https://doi.org/10.1073/pnas.0802361105
Maghsoudi K, Arvin MJ, Ashraf M (2020) Mitigation of arsenic toxicity in wheat by the exogenously applied salicylic acid, 24-epi-brassinolide and silicon. J Soil Sci Plant Nutr 20(2):577–588. https://doi.org/10.1007/s42729-019-00147-3
Maglovski M, Gerši Z, Rybanský Ľ, Bardáčová M, Moravčíková J, Bujdoš M, Dobrikova A, Apostolova E, Kraic J, Blehová A, Matušíková I (2019) Effects of nutrition on wheat photosynthetic pigment responses to arsenic stress. Pol J Environ Stud 28:1821–1829. https://doi.org/10.15244/pjoes/89584
Malik JA, Goel S, Sandhir R, Nayyar H (2011) Uptake and distribution of arsenic in chickpea: effects on seed yield and seed composition. Commun Soil Sci Plant Anal 42(14):1728–1738. https://doi.org/10.1080/00103624.2011.584593
Mano JI (2012) Reactive carbonyl species: their production from lipid peroxides, action in environmental stress, and the detoxification mechanism. Plant Physiol Biochem 59:90–97. https://doi.org/10.1016/j.plaphy.2012.03.010
Mano JI, Biswas M, Sugimoto K (2019) Reactive carbonyl species: a missing link in ROS signaling. Plants 8:391. https://doi.org/10.3390/plants8100391
Mansour NM, Sawhney M, Tamang DG, Vogl C, Saier MH Jr (2007) The bile/arsenite/riboflavin transporter (BART) superfamily. FEBS J 274:612–629. https://doi.org/10.1111/j.1742-4658.2006.05627.x
Maurel C, Boursiac Y, Luu DT, Santoni V, Shahzad Z, Verdoucq L (2015) Aquaporins in plants. Physiol Rev 95:1321–1358. https://doi.org/10.1152/physrev.00008.2015
Meng YL, Liu ZJ, Rosen BP (2004) As(III) and Sb(III) uptake by GlpF and efflux by ArsB in Escherichia coli. J Biol Chem 279:18334–18341. https://doi.org/10.1074/jbc.M400037200
Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133:481–489. https://doi.org/10.1111/j.1399-3054.2008.01090.x
Mishra S, Srivastava S, Tripathi RD, Trivedi PK (2008) Thiol metabolism and antioxidant systems complement each other during arsenate detoxification in Ceratophyllum demersum L. Aquat Toxicol 86:205–215. https://doi.org/10.1016/j.aquatox.2007.11.001
Mishra S, Srivastava S, Dwivedi S, Tripathi RD (2013) Investigation of biochemical responses of Bacopa monnieri L. upon exposure to arsenate. Environ Toxicol 28:419–430. https://doi.org/10.1002/tox.20733
Mishra S, Alfeld M, Sobotka R, Andresen E, Falkenberg G, Küpper H (2016) Analysis of sublethal arsenic toxicity to Ceratophyllum demersum: subcellular distribution of arsenic and inhibition of chlorophyll biosynthesis. J Exp Bot 67(15):4639–4646. https://doi.org/10.1093/jxb/erw238
Mishra S, Dwivedi S, Mallick S, Tripathi RD (2019) Redox homeostasis in plants under arsenic stress. In: Panda S, Yamamoto Y (eds) Redox homeostasis in plants. Signaling and communication in plants, Springer Nature, Switzerland, pp 179–198. https://doi.org/10.1007/978-3-319-95315-1_9
Mitani-Ueno N, Yamaji N, Zhao FJ, Ma JF (2011) The aromatic/arginine selectivity filter of NIP aquaporins plays a critical role in substrate selectivity for silicon, boron, and arsenic. J Exp Bot 62(12):4391–4398. https://doi.org/10.1093/jxb/err158
Mohamed HI, Latif HH, Hanafy RS (2016) Influence of nitric oxide application on some biochemical aspects, endogenous hormones, minerals and phenolic compounds of Vicia faba plant grown under arsenic stress. Gesunde Pflanzen 68(2):99–107. https://doi.org/10.1007/s10343-016-0363-7
Mohan TC, Castrillo G, Navarro C, Zarco-Fernández S, Ramireddy E, Mateo C, Zamarreño AM, Paz-Ares J, Muñoz R, García-Mina JM, Hernández LE (2016) Cytokinin determines thiol-mediated arsenic tolerance and accumulation. Plant Physiol 171(2):1418–1426. https://doi.org/10.1104/pp.16.00372
Mondal S, Pramanik K, Ghosh SK, Pal P, Mondal T, Soren T, Maiti TK (2021) Unraveling the role of plant growth-promoting rhizobacteria in the alleviation of arsenic phytotoxicity: a review. Microbiol Res 250:126809. https://doi.org/10.1016/j.micres.2021.126809
Mosa KA, Kumar K, Chhikara S, Mcdermott J, Liu Z, Musante C, White JC, Dhankher OP (2012) Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Res 21:1265–1277. https://doi.org/10.1007/s11248-012-9600-8
Mostofa MG, Rahman MM, Nguyen KH, Li W, Watanabe Y, Tran CD, Zhang M, Itouga M, Fujita M, Tran LS (2021) Strigolactones regulate arsenate uptake, vacuolar-sequestration and antioxidant defense responses to resist arsenic toxicity in rice roots. J Hazard Mater 415:125589. https://doi.org/10.1016/j.jhazmat.2021.125589
Nabi A, Naeem M, Aftab T, Khan MM, Ahmad P (2021) A comprehensive review of adaptations in plants under arsenic toxicity: Physiological, metabolic and molecular interventions. Environ Pollut 290:118029. https://doi.org/10.1016/j.envpol.2021.118029
Nadarajah KK (2020) ROS homeostasis in abiotic stress tolerance in plants. Int J Mol Sci 21(15):5208. https://doi.org/10.3390/ijms21155208
Nagarajan VK, Jain A, Poling MD, Lewis AJ, Raghothama KG, Smith AP (2011) Arabidopsis Pht1; 5 mobilizes phosphate between source and sink organs and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiol 156:1149–1163. https://doi.org/10.1104/pp.111.174805
Niazi NK, Bibi I, Fatimah A, Shahid M, Javed MT, Wang H, Ok YS, Bashir S, Murtaza B, Saqib ZA, Shakoor MB (2017) Phosphate-assisted phytoremediation of arsenic by Brassica napus and Brassica juncea: morphological and physiological response. Int J Phytoremediat 19:670–678. https://doi.org/10.1080/15226514.2016.1278427
Nussaume L, Kanno S, Javot H, Marin E, Nakanishi TM, Thibaud MC (2011) Phosphate import in plants: focus on the PHT1 transporters. Front Plant Sci 2:83. https://doi.org/10.3389/fpls.2011.00083
Pan D, Liu C, Yi J, Li X, Li F (2021) Different effects of foliar application of silica sol on arsenic translocation in rice under low and high arsenite stress. J Environ Sci 105:22–32. https://doi.org/10.1016/j.jes.2020.12.034
Pandey C, Gupta M (2015) Selenium and auxin mitigates arsenic stress in rice (Oryza sativa L.) by combining the role of stress indicators, modulators and genotoxicity assay. J Hazard Mater 287:384–391. https://doi.org/10.1016/j.jhazmat.2015.01.044
Pandey AK, Gedda MR, Verma AK (2020) Effect of Arsenic stress on expression pattern of a rice specific miR156j at various developmental stages and their allied co-expression target networks. Front Plant Sci 11:752. https://doi.org/10.3389/fpls.2020.00752
Parvin K, Nahar K, Hasanuzzaman M, Bhuyan MHMB, Fujita M (2019) Calcium-mediated growth regulation and abiotic stress tolerance in plants. In: Hasanuzzaman M, Hakeem K, Nahar K, Alharby H (eds) Plant abiotic stress tolerance. Springer Nature, Switzerland, pp 291–331. https://doi.org/10.1007/978-3-030-06118-0_13
Petrov VD, Van Breusegem F (2012) Hydrogen peroxide—a central hub for information flow in plant cells. AoB Plants. https://doi.org/10.1093/aobpla/pls014
Pommerrenig B, Diehn TA, Bienert GP (2015) Metalloido-porins: essentiality of Nodulin 26-like intrinsic proteins in metalloid transport. Plant Sci 238:212–227. https://doi.org/10.1016/j.plantsci.2015.06.002
Quaghebeur M, Rengel Z (2005) Arsenic speciation governs arsenic uptake and transport in terrestrial plants. Microchim Acta 151:141–152. https://doi.org/10.1007/s00604-005-0394-8
Rahman MS, Jamal MA, Biswas PK, Rahman SM, Sharma SP, Saha SK, Hong ST, Islam MR (2020) Arsenic remediation in bangladeshi rice varieties with enhance plant growth by unique arsenic-resistant bacterial isolates. Geomicrobiol J 37:130–142. https://doi.org/10.1080/01490451.2019.1666938
Rai A, Bhardwaj A, Misra P, Bag SK, Adhikari B, Tripathi RD, Trivedi PK, Chakrabarty D (2015) Comparative transcriptional profiling of contrasting rice genotypes shows expression differences during arsenic stress. Plant Genome 8:1–14. https://doi.org/10.3835/plantgenome2014.09.0054
Raja V, Majeed U, Kang H, Andrabi KI, John R (2017) Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ Exp Bot 137:142–157. https://doi.org/10.1016/j.envexpbot.2017.02.010
Rao KP, Vani G, Kumar K, Wankhede DP, Misra M, Gupta M, Sinha AK (2011) Arsenic stress activates MAP kinase in rice roots and leaves. Arch Biochem Biophys 506:73–82. https://doi.org/10.1016/j.abb.2010.11.006
Remy E, Cabrito TR, Batista RA, Teixeira MC, Sá-Correia I, Duque P (2012) The Pht1; 9 and Pht1; 8 transporters mediate inorganic phosphate acquisition by the Arabidopsis thaliana root during phosphorus starvation. New Phytol 195:356–371. https://doi.org/10.1111/j.1469-8137.2012.04167.x
Rodríguez-Ruiz M, Aparicio-Chacón MV, Palma JM, Corpas FJ (2019) Arsenate disrupts ion balance, sulfur and nitric oxide metabolisms in roots and leaves of pea (Pisum sativum L.) plants. Environ Exp Bot 161:143–156. https://doi.org/10.1016/j.envexpbot.2018.06.028
Roitsch T, González MC (2004) Function and regulation of plant invertases: sweet sensations. Trends Plant Sci 9(12):606–613. https://doi.org/10.1016/j.tplants.2004.10.009
Ronzan M, Piacentini D, Fattorini L, Caboni E, Eiche E, Ziegler J, Hause B, Riemann B, Betti C, Altamura MM, Falasca G (2019) Auxin-jasmonate crosstalk in Oryza sativa L. root system formation after cadmium and/or arsenic exposure. Environ Exp Bot 165:59–69. https://doi.org/10.1016/j.envexpbot.2019.05.013
Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M (2021) Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 10(2):277. https://doi.org/10.3390/antiox10020277
Saifullah DS, Naeem A, Iqbal M, Farooq MA, Bibi S, Rengel Z (2018) Opportunities and challenges in the use of mineral nutrition for minimizing arsenic toxicity and accumulation in rice: a critical review. Chemosphere 194:171–188. https://doi.org/10.1016/j.chemosphere.2017.11.149
Saini S, Kaur N, Pati PK (2021) Phytohormones: Key players in the modulation of heavy metal stress tolerance in plants. Ecotoxicol Environ Saf 223:112578. https://doi.org/10.1016/j.ecoenv.2021.112578
Seyfferth AL, Webb SM, Andrews JC, Fendorf S (2010) Arsenic localization, speciation, and co-occurrence with iron on rice (Oryza sativa L.) roots having variable Fe coatings. Environ Sci Technol 44:8108–8113. https://doi.org/10.1021/es101139z
Shaibur MR, Kitajima N, Huq SI, Kawai S (2009a) Arsenic–iron interaction: effect of additional iron on arsenic-induced chlorosis in barley grown in water culture. Soil Sci Plant Nutr 55(6):739–746. https://doi.org/10.1111/j.1747-0765.2009.00414.x
Shaibur MR, Kitajima N, Sugawara R, Kondo T, Huq SI, Kawai S (2009b) Effect of arsenic on phytosiderophores and mineral nutrition of barley seedlings grown in iron-depleted medium. Soil Sci Plant Nutr 55(2):283–293. https://doi.org/10.1111/j.1747-0765.2009.00360.x
Sharma I (2012) Arsenic induced oxidative stress in plants. Biologia 67:447–453. https://doi.org/10.2478/s11756-012-0024-y
Sharma D, Tiwari M, Lakhwani D, Tripathi RD, Trivedi PK (2015) Differential expression of microRNAs by arsenate and arsenite stress in natural accessions of rice. Metallomics 7:174–187. https://doi.org/10.1039/C4MT00264D
Sharma SS, Kumar V, Dietz KJ (2021) Emerging trends in metalloid-dependent signaling in plants. Trends Plant Sci 26(5):452–471. https://doi.org/10.1016/j.tplants.2020.11.003
Sharma A, Ramakrishnan M, Khanna K, Landi M, Prasad R, Bhardwaj R, Zheng B (2022) Brassinosteroids and metalloids: regulation of plant biology. J Hazard Mater 424:127518. https://doi.org/10.1016/j.jhazmat.2021.127518
Shi S, Wang T, Chen Z, Tang Z, Wu Z, Salt DE, Chao DY, Zhao FJ (2016) OsHAC1;1 and OsHAC1;2 function as arsenate reductases and regulate arsenic accumulation. Plant Physiol 172:1708–1719. https://doi.org/10.1104/pp.16.01332
Shin H, Shin HS, Dewbre GR, Harrison MJ (2004) Phosphate transport in Arabidopsis: Pht1; 1 and Pht1;4 play a major role in phosphate acquisition from both low-and high-phosphate environments. Plant J 39:629–642. https://doi.org/10.1111/j.1365-313X.2004.02161.x
Shri M, Kumar S, Chakrabarty D, Trivedi PK, Mallick S, Misra P, Shukla D, Mishra S, Srivastava S, Tripathi RD, Tuli R (2009) Effect of arsenic on growth, oxidative stress, and antioxidant system in rice seedlings. Ecotoxicol Environ Saf 72:1102–1110. https://doi.org/10.1016/j.ecoenv.2008.09.022
Shri M, Singh PK, Kidwai M, Gautam N, Dubey S, Verma G, Chakrabarty D (2019) Recent advances in arsenic metabolism in plants: current status, challenges and highlighted biotechnological intervention to reduce grain arsenic in rice. Metallomics 11:519–532. https://doi.org/10.1039/C8MT00320C
Shukla P, Singh S, Dubey P, Singh A, Singh AK (2015) Nitric oxide mediated amelioration of arsenic toxicity which alters the alternative oxidase (Aox1) gene expression in Hordeum vulgare L. Ecotoxicol Environ Saf 120:59–65. https://doi.org/10.1016/j.ecoenv.2015.05.030
Shukla T, Khare R, Kumar S, Lakhwani D, Sharma D, Asif MH, Trivedi PK (2018) Differential transcriptome modulation leads to variation in arsenic stress response in Arabidopsis thaliana accessions. J Hazard Mater 351:1–10. https://doi.org/10.1016/j.jhazmat.2018.02.031
Siddiqui MH, Alamri S, Khan MN, Corpas FJ, Al-Amri AA, Alsubaie QD, Ali HM, Kalaji HM, Ahmad P (2020) Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J Hazard Mater 398:122882. https://doi.org/10.1016/j.jhazmat.2020.122882
Singh N, Ma LQ, Srivastava M, Rathinasabapathi B (2006) Metabolic adaptations to arsenic-induced oxidative stress in Pteris vittata L. and Pteris ensiformis L. Plant Sci 170:274–282. https://doi.org/10.1016/j.plantsci.2005.08.013
Singh AP, Dixit G, Mishra S, Dwivedi S, Tiwari M, Mallick S, Pandey V, Trivedi PK, Chakrabarty D, Tripathi RD (2015) Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front Plant Sci 6:340. https://doi.org/10.3389/fpls.2015.00340
Singh S, Parihar P, Singh R, Singh VP, Prasad SM (2016) Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci 6:1143. https://doi.org/10.3389/fpls.2015.01143
Singh PK, Indoliya Y, Chauhan AS, Singh SP, Singh AP, Dwivedi S, Tripathi RD, Chakrabarty D (2017) Nitric oxide mediated transcriptional modulation enhances plant adaptive responses to arsenic stress. Sci Rep 7:1–13. https://doi.org/10.1038/srep42530
Singh R, Upadhyay AK, Singh DP (2018) Regulation of oxidative stress and mineral nutrient status by selenium in arsenic treated crop plant Oryza sativa. Ecotoxicol Environ Saf 148:105–113. https://doi.org/10.1016/j.ecoenv.2017.10.008
Singh A, Kumar A, Yadav S, Singh IK (2019) Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 18:100173. https://doi.org/10.1016/j.plgene.2019.100173
Singh R, Parihar P, Prasad SM (2020a) Interplay of calcium and nitric oxide in improvement of growth and arsenic-induced toxicity in mustard seedlings. Sci Rep 10(1):1–2. https://doi.org/10.1038/s41598-020-62831-0
Singh R, Parihar P, Prasad SM (2020b) Sulphur and calcium attenuate arsenic toxicity in Brassica by adjusting ascorbate–glutathione cycle and sulphur metabolism. Plant Growth Regul 91(2):221–235. https://doi.org/10.1007/s10725-020-00601-8
Song WY, Park J, Mendoza-Cózatl DG, Suter-Grotemeyer M, Shim D, Hörtensteiner S, Geisler M, Weder B, Rea PA, Rentsch D, Schroeder JI, Lee Y, Martinoia E (2010) Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc Natl Acad Sci USA 107:21187–21192. https://doi.org/10.1073/pnas.1013964107
Song WY, Yamaki T, Yamaji N, Ko D, Jung K, Fujii-Kashino M, An G, Martinoia E, Lee YS, Ma JF (2014) A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc Natl Acad Sci U S A 111:15699–15704. https://doi.org/10.1073/pnas.1414968111
Souri Z, Karimi N, Farooq MA, Sandalio LM (2020) Nitric oxide improves tolerance to arsenic stress in Isatis cappadocica desv shoots by enhancing antioxidant defenses. Chemosphere 239:124523. https://doi.org/10.1016/j.chemosphere.2019.124523
Srivastava S, Singh N (2014) Mitigation approach of arsenic toxicity in chickpea grown in arsenic amended soil with arsenic tolerant plant growth promoting Acinetobacter sp. Ecol Eng 70:146–153. https://doi.org/10.1016/j.ecoleng.2014.05.008
Srivastava S, Suprasanna P (2021) MicroRNAs: tiny, powerful players of metal stress responses in plants. Plant Physiol Biochem 166:928–938. https://doi.org/10.1016/j.plaphy.2021.07.004
Srivastava S, Mishra S, Tripathi RD, Dwivedi S, Trivedi PK, Tandon PK (2007) Phytochelatins and antioxidant systems respond differentially during arsenite and arsenate stress in Hydrilla verticillata (Lf) Royle. Environ Sci Technol 41:2930–2936. https://doi.org/10.1021/es062167j
Srivastava S, Suprasanna P, D’Souza SF (2011) Redox state and energetic equilibrium determine the magnitude of stress in Hydrilla verticillata upon exposure to arsenate. Protoplasma 248:805–815. https://doi.org/10.1007/s00709-010-0256-z
Srivastava S, Srivastava AK, Sablok G, Deshpande TU, Suprasanna P (2015) Transcriptomics profiling of Indian mustard (Brassica juncea) under arsenate stress identifies key candidate genes and regulatory pathways. Front Plant Sci 6:646. https://doi.org/10.3389/fpls.2015.00646
Srivastava S, Sinha P, Sharma YK (2017) Status of photosynthetic pigments, lipid peroxidation and anti-oxidative enzymes in Vigna mungo in presence of arsenic. J Plant Nutr 40:298–306. https://doi.org/10.1080/01904167.2016.1240189
Sun S, Gu M, Cao Y, Huang X, Zhang X, Ai P, Zhao J, Fan X, Xu G (2012) A constitutive expressed phosphate transporter, OsPht1;1, modulates phosphate uptake and translocation in phosphate-replete rice. Plant Physiol 159:1571–1581. https://doi.org/10.1104/pp.112.196345
Sun SK, Chen Y, Che J, Konishi N, Tang Z, Miller AJ, Ma JF, Zhao FJ (2018) Decreasing arsenic accumulation in rice by overexpressing OsNIP 1;1 and OsNIP 3;3 through disrupting arsenite radial transport in roots. New Phytol 219:641–653. https://doi.org/10.1111/nph.15190
Sun D, Feng H, Li X, Ai H, Sun S, Chen Y, Xu G, Rathinasabapathi B, Cao Y, Ma LQ (2019) Expression of new Pteris vittata phosphate transporter PvPht1;4 reduces arsenic translocation from the roots to shoots in tobacco plants. Environ Sci Technol 54:1045–1053. https://doi.org/10.1021/acs.est.9b05486
Surgun-Acar Y, Zemheri-Navruz F (2019) 24-Epibrassinolide promotes arsenic tolerance in Arabidopsis thaliana L. by altering stress responses at biochemical and molecular level. J Plant Physiol 238:12–19. https://doi.org/10.1016/j.jplph.2019.05.002
Sytar O, Kumari P, Yadav S, Brestic M, Rastogi A (2019) Phytohormone priming: regulator for heavy metal stress in plants. J Plant Growth Regul 38(2):739–752. https://doi.org/10.1007/s00344-018-9886-8
Takano J, Wada M, Ludewig U, Schaaf G, Von Wirén N, Fujiwara T (2006) The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell 18:1498–1509. https://doi.org/10.1105/tpc.106.041640
Tang Z, Zhao FJ (2021) The roles of membrane transporters in arsenic uptake, translocation and detoxification in plants. Crit Rev Env Sci Tec 51(21):2449–2484. https://doi.org/10.1080/10643389.2020.1795053
Tang Z, Chen Y, Chen F, Ji Y, Zhao FJ (2017) OsPTR7 (OsNPF8. 1), a putative peptide transporter in rice, is involved in dimethylarsenate accumulation in rice grain. Plant Cell Physiol 58:904–913. https://doi.org/10.1093/pcp/pcx029
Tang Z, Chen Y, Miller AJ, Zhao FJ (2019) The C-type ATP-binding cassette transporter OsABCC7 is involved in the root-to-shoot translocation of arsenic in rice. Plant Cell Physiol 60:1525–1535. https://doi.org/10.1093/pcp/pcz054
Tang Z, Wang Y, Gao A, Ji Y, Yang B, Wang P, Tang Z, Zhao FJ (2020) Dimethylarsinic acid is the causal agent inducing rice straighthead disease. J Exp Bot 71:5631–5644. https://doi.org/10.1093/jxb/eraa253
Thakur S, Choudhary S, Dubey P, Bhardwaj P (2019) Comparative transcriptome profiling reveals the reprogramming of gene networks under arsenic stress in Indian mustard. Genome 62:833–847. https://doi.org/10.1139/gen-2018-0152
Thakur S, Choudhary S, Majeed A, Singh A, Bhardwaj P (2020) Insights into the molecular mechanism of arsenic phytoremediation. J Plant Growth Regul 39:532–543. https://doi.org/10.1007/s00344-019-10019-w
Tiwari M, Sharma D, Dwivedi S, Singh M, Tripathi RD, Trivedi PK (2014) Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ 37:140–152. https://doi.org/10.1111/pce.12138
Tripathi P, Tripathi RD (2019) Metabolome modulation during arsenic stress in plants. In: Srivastava S, Srivastava A, Suprasanna P (eds) Plant-metal interactions. Springer Nature, Switzerland, pp 119–140. https://doi.org/10.1007/978-3-030-20732-8_7
Tripathi RD, Srivastava S, Mishra S, Singh N, Tuli R, Gupta DK, Maathuis FJ (2007) Arsenic hazards: strategies for tolerance and remediation by plants. Trends Biotechnol 25:158–165. https://doi.org/10.1016/j.tibtech.2007.02.003
Tripathi P, Mishra A, Dwivedi S, Chakrabarty D, Trivedi PK, Singh RP, Tripathi RD (2012) Differential response of oxidative stress and thiol metabolism in contrasting rice genotypes for arsenic tolerance. Ecotoxicol Environ Saf 79:189–198. https://doi.org/10.1016/j.ecoenv.2011.12.019
Tripathi P, Singh RP, Sharma YK, Tripathi RD (2015) Arsenite stress variably stimulates pro-oxidant enzymes, anatomical deformities, photosynthetic pigment reduction, and antioxidants in arsenic-tolerant and sensitive rice seedlings. Environ Toxicol Chem 34:1562–1571. https://doi.org/10.1002/etc.2937
Verma PK, Verma S, Meher AK, Pande V, Mallick S, Bansiwal AK, Tripathi RD, Dhankher OP, Chakrabarty D (2016) Overexpression of rice glutaredoxins (OsGrxs) significantly reduces arsenite accumulation by maintaining glutathione pool and modulating aquaporins in yeast. Plant Physiol Biochem 106:208–217. https://doi.org/10.1016/j.plaphy.2016.04.052
Vithanage M, Dabrowska BB, Mukherjee AB, Sandhi A, Bhattacharya P (2012) Arsenic uptake by plants and possible phytoremediation applications: a brief overview. Environ Chem Lett 10:217–224. https://doi.org/10.1007/s10311-011-0349-8
Wang H, Xu Q, Kong YH, Chen Y, Duan JY, Wu WH, Chen YF (2014) Arabidopsis WRKY45 transcription factor activates phosphate transporter1;1 expression in response to phosphate starvation. Plant Physiol 164:2020–2029. https://doi.org/10.1104/pp.113.235077
Wang P, Zhang W, Mao C, Xu G, Zhao FJ (2016) The role of OsPT8 in arsenate uptake and varietal difference in arsenate tolerance in rice. J Exp Bot 67:6051–6059. https://doi.org/10.1093/jxb/erw362
Wang FZ, Chen MX, Yu LJ, Xie LJ, Yuan LB, Qi H, Xiao M, Guo W, Chen Z, Yi K, Zhang J, Qiu R, Shu W, Xiao S, Chen QF (2017) OsARM1, an R2R3 MYB transcription factor, is involved in regulation of the response to arsenic stress in rice. Front Plant Sci 8:1868. https://doi.org/10.3389/fpls.2017.01868
Wang C, Na G, Bermejo ES, Chen Y, Banks JA, Salt DE, Zhao FJ (2018a) Dissecting the components controlling root-to-shoot arsenic translocation in Arabidopsis thaliana. New Phytol 217(1):206–218. https://doi.org/10.1111/nph.14761
Wang P, Xu X, Tang Z, Zhang W, Huang XY, Zhao FJ (2018b) OsWRKY28 regulates phosphate and arsenate accumulation, root system architecture and fertility in rice. Front Plant Sci 9:1330. https://doi.org/10.3389/fpls.2018.01330
Wang J, Kerl CF, Hu P, Martin M, Mu T, Brüggenwirth L, Wu G, Said-Pullicino D, Romani M, Wu L, Planer-Friedrich B (2020) Thiolated arsenic species observed in rice paddy pore waters. Nat Geosci 13:282–287. https://doi.org/10.1038/s41561-020-0533-1
Wanke D, Üner Kolukisaoglu H (2010) An update on the ABCC transporter family in plants: many genes, many proteins, but how many functions? Plant Biol 12:15–25. https://doi.org/10.1111/j.1438-8677.2010.00380.x
Williams PN, Islam S, Islam R, Jahiruddin M, Adomako E, Soliaman AR, Rahman GK, Lu Y, Deacon C, Zhu YG, Meharg AA (2009) Arsenic limits trace mineral nutrition (selenium, zinc, and nickel) in Bangladesh rice grain. Environ Sci Technol 43(21):8430–8436. https://doi.org/10.1021/es901825t
Wu Z, Ren H, McGrath SP, Wu P, Zhao FJ (2011) Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiol 157:498–508. https://doi.org/10.1104/pp.111.178921
Xie MY, Tian ZH, Yang XL, Liu BH, Yang J, Lin HH (2019) The role of OsNLA1 in regulating arsenate uptake and tolerance in rice. J Plant Physiol 236:15–22. https://doi.org/10.1016/j.jplph.2019.02.013
Xie Q, Yu Q, Jobe TO, Pham A, Ge C, Guo Q, Liu J, Liu H, Zhang H, Zhao Y, Xue S (2021) An amiRNA screen uncovers redundant CBF and ERF34/35 transcription factors that differentially regulate arsenite and cadmium responses. Plant Cell Environ 44(5):1692–1706. https://doi.org/10.1111/pce.14023
Xu W, Dai W, Yan H, Li S, Shen H, Chen Y, Xu H, Sun Y, He Z, Ma M (2015) Arabidopsis NIP3;1 plays an important role in arsenic uptake and root-to-shoot translocation under arsenite stress conditions. Mol Plant 8:722–733. https://doi.org/10.1016/j.molp.2015.01.005
Xu J, Shi S, Wang L, Tang Z, Lv T, Zhu X, Ding X, Wang Y, Zhao FJ, Wu Z (2017) OsHAC4 is critical for arsenate tolerance and regulates arsenic accumulation in rice. New Phytol 215:1090–1101. https://doi.org/10.1111/nph.14572
Xu B, Yu JY, Xie T, Li YL, Liu MJ, Guo JX, Li HL, Yu Y, Zheng CY, Chen YH, Wang G (2018) Brassinosteroids and iron plaque affect arsenic and cadmium uptake by rice seedlings grown in hydroponic solution. Biol Plant 62:362–368. https://doi.org/10.1007/s10535-018-0784-5
Xu B, Yu J, Zhong Y, Guo Y, Ding J, Chen Y, Wang G (2019) Influence of Br 24 and Gr24 on the accumulation and uptake of Cd and As by rice seedlings grown in nutrient solution. Pol J Environ Stud 28(5):3951–3958. https://doi.org/10.15244/pjoes/95036
Xu B, Chen J, Yu J, Guo Y, Cai Q, Wu Y, Li Y, Xie T, Chen Y, Wang G (2020) Effects of 24-epibrassinolide and 28-homobrassinolide on iron plaque formation and the uptake of As and Cd by rice seedlings (Oryza sativa L.) in solution culture. Environ Technol Innov 19:100802. https://doi.org/10.1016/j.eti.2020.100802
Yadav G, Srivastava PK, Parihar P, Tiwari S, Prasad SM (2016) Oxygen toxicity and antioxidative responses in arsenic stressed Helianthus annuus L. seedlings against UV-B. J Photochem Photobiol B 165:58–70. https://doi.org/10.1016/j.jphotobiol.2016.10.011
Yamaji N, Ma JF (2021) Metalloid transporters and their regulation in plants. Plant Physiol 187(4):1929–1939. https://doi.org/10.1093/plphys/kiab326
Yan G, Chen X, Du S, Deng Z, Wang L, Chen S (2019) Genetic mechanisms of arsenic detoxification and metabolism in bacteria. Curr Genet 65:329–338. https://doi.org/10.1007/s00294-018-0894-9
Yang J, Gao MX, Hu H, Ding XM, Lin HW, Wang L, Xu JM, Mao CZ, Zhao FJ, Wu ZC (2016) OsCLT1, a CRT-like transporter 1, is required for glutathione homeostasis and arsenic tolerance in rice. New Phytol 211:658–670. https://doi.org/10.1111/nph.13908
Yang J, Wang L, Mao C, Lin H (2017) Characterization of the rice NLA family reveals a key role for OsNLA1 in phosphate homeostasis. Rice 10:1–6. https://doi.org/10.1186/s12284-017-0193-y
Yao L, Huang L, He Z, Zhou C, Lu W, Bai C (2016) Delivery of roxarsone via chicken diet→ chicken→ chicken manure→ soil→ rice plant. SciTotal Environ 566:1152–1158. https://doi.org/10.1016/j.scitotenv.2016.05.157
Ye Y, Li P, Xu T, Zeng L, Cheng D, Yang M, Luo J, Lian X (2017) OsPT4 contributes to arsenate uptake and transport in rice. Front Plant Sci 8:2197. https://doi.org/10.3389/fpls.2017.02197
Yoshinari A, Hosokawa T, Amano T, Beier MP, Kunieda T, Shimada T, Hara-Nishimura I, Naito S, Takano J (2019) Polar localization of the borate exporter BOR1 requires AP2-dependent endocytosis. Plant Physiol 179(4):1569–1580. https://doi.org/10.1104/pp.18.01017
Yu LJ, Luo YF, Liao B, Xie LJ, Chen L, Xiao S, Li JT, Hu SN, Shu WS (2012) Comparative transcriptome analysis of transporters, phytohormone and lipid metabolism pathways in response to arsenic stress in rice (Oryza sativa). New Phytol 195:97–112. https://doi.org/10.1111/j.1469-8137.2012.04154.x
Yue W, Ying Y, Wang C, Zhao Y, Dong C, Whelan J, Shou H (2017) OsNLA1, a RING-type ubiquitin ligase, maintains phosphate homeostasis in Oryza sativa via degradation of phosphate transporters. Plant J 90:1040–1051. https://doi.org/10.1111/tpj.13516
Zhao FJ, Wang P (2020) Arsenic and cadmium accumulation in rice and mitigation strategies. Plant Soil 446(1):1–21. https://doi.org/10.1007/s11104-019-04374-6
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794. https://doi.org/10.1111/j.1469-8137.2008.02716.x
Zhao FJ, McGrath SP, Meharg AA (2010a) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559. https://doi.org/10.1146/annurev-arplant-042809-112152
Zhao FJ, Ago Y, Mitani N, Li RY, Su YH, Yamaji N, McGrath SP, Ma JF (2010b) The role of the rice aquaporin Lsi1 in arsenite efflux from roots. The New Phytol 186:392–399. https://doi.org/10.1111/j.1469-8137.2010.03192.x
Zheng MZ, Li G, Sun GX, Shim H, Cai C (2013) Differential toxicity and accumulation of inorganic and methylated arsenic in rice. Plant Soil 365:227–238. https://doi.org/10.1007/s11104-012-1376-3
Acknowledgements
Authors are thankful to the Council of Scientific and Industrial Research (CSIR), India, for financial assistance [vide letter No. 38 (1469)/18/EMR-II, dated 04.04.2018]. KP gratefully acknowledges University Grants Commission (UGC), New Delhi, for UGC—Dr. D. S. Kothari Post-Doctoral Fellowship [No.F.4-2/2006 (BSR)/BL/19-20/0072 dated October 21, 2019].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Gerhard Leubner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mondal, S., Pramanik, K., Ghosh, S.K. et al. Molecular insight into arsenic uptake, transport, phytotoxicity, and defense responses in plants: a critical review. Planta 255, 87 (2022). https://doi.org/10.1007/s00425-022-03869-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00425-022-03869-4