Abstract
Background and aims
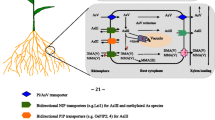
Efficient accumulation of arsenic (As) in rice (Oryza sativa L.) poses a potential health risk to rice consumers. The aim of this study was to investigate the mechanisms of uptake, transport and distribution of inorganic arsenic (Asi) and dimethylarsinic acid (DMA) in rice plants.
Methods
Rice was exposed to Asi (As(V)) and DMA in hydroponics. High-performance liquid chromatography inductively coupled plasma mass spectrometry (HPLC-ICP-MS) and synchrotron X-ray fluorescence (SXRF) microprobe were used to determine As concentration and the in situ As distribution.
Results
DMA induced abnormal florets before flowering and caused a sharp decline in the seed setting rate after flowering compared to Asi. Rice grains accumulated 2-fold higher DMA than Asi. The distribution of Asi concentration (root > leaf > husk > caryopsis) in As(V) treatments was different from that of the DMA concentration (caryopsis > husk > root ≥ leaf) in DMA treatments. SXRF showed that Asi mainly accumulated in the vascular trace of caryopsis with limited distribution to the endosperm, whereas DMA was observed in both tissues.
Conclusions
DMA tended to accumulate in caryopsis and induced higher toxicity to the reproductive tissues resulting in markedly reduced grain yield, whereas Asi mainly remained in the vegetative tissues and had no significant effect on yield. DMA is more toxic than Asi to the reproductive tissues when both of them are at similar concentrations in nutrient solution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic (As), a well-known first-class carcinogen, is introduced into the environment by geogenically and/or anthropogenically derived activities and is considered as a global contaminant (Mandal and Suzuki 2002). As contamination in soil can cause phytotoxicity in rice and consequently yield losses (Panaullah et al. 2009). In addition, long-term As exposure in humans is associated with a number of diseases, such as skin and bladder cancers and diabetes (Tchounwou et al. 2004; Smith and Steinmaus 2009). Millions of people have been affected directly by As elevation in drinking water in South and Southeast Asia (Brammer and Ravenscroft 2009; Fendorf et al. 2010). More people may face a potential health risk from consumption of rice elevated in As concentration due to the irrigation of As-contaminated groundwater (Rahman and Hasegawa 2011). Among various cereal crops, rice is an important exposure route for As to populations dependent on a rice diet (Kile et al. 2007; Mondal and Polya 2008; Li et al. 2011), because rice shows a higher efficiency in accumulating As in shoots and grain than other cereals (Williams et al. 2007). Even when grown in soils with background As levels, rice can contain higher As concentrations (Lu et al. 2009; Meharg et al. 2009). Additionally, the use of rice straw as cattle feed in many countries might increase the As exposure to humans via the plant-animal-human pathway. Therefore, there is an urgent need to understand how rice absorbs, transports and metabolizes As in order to develop effective mitigation strategies against the widespread contamination in the food chain (Zhao et al. 2010).
Arsenic may be present in soil in various chemical forms, including arsenate [As(V)], arsenite [As(III)], and methylated As species such as monomethylarsonic acid (MMA) and dimethylarsinic acid (DMA). Usually, inorganic arsenic species [Asi: As(V) and As(III)] predominate in soil, while small quantities of methylated As species have also been found in some soils, most likely from either microbial methylation or the past application of As-based pesticides/herbicides (Takamatsu et al. 1982). Asi and DMA are the main As species in rice grain, with MMA occasionally found at minor levels (Zhao et al. 2010). The mechanism of Asi uptake into plant roots has been extensively studied recently (Zhao et al. 2009). It is well established that As(V) is taken up by rice roots through the phosphate transport pathway (Abedin et al. 2002a; Wu et al. 2011), and As(III) through the silicic acid transport system (Ma et al. 2008). Once taken up into the cells, As(V) is reduced to As(III) by arsenate reductases (Duan et al. 2005), and then may be complexed with phytochelatins followed by sequestration in the vacuole (Bleeker et al. 2006; Zhao et al. 2009; Liu et al. 2011; Moore et al. 2011), or enter the xylem via a silicic acid/arsenite effluxer (Ma et al. 2008). In contrast, the mechanism of DMA uptake and metabolism in plant is less well understood.
The relative proportions of Asi and DMA in rice grain vary widely. The proportion of DMA ranged from 10 % to 90 %, depending on various factors such as the rice cultivars (Norton et al. 2009), the soil environments in which rice was grown (Williams et al. 2006; Zavala et al. 2008; Meharg et al. 2009) and water management (Xu et al. 2008; Li et al. 2009; Arao et al. 2009). There have been a great number of studies on the uptake and translocation of methylated As in plant (Marin et al. 1993; Li et al. 2009; Ye et al. 2010; Carey et al. 2010, 2011). Li et al. (2009) showed that the rice lsi1 mutant defective in the silicon/arsenite transporter Lsi1 lost about 80 % and 50 % of the uptake capacity for MMA(V) and DMA(V), respectively, compared with the wild-type rice, whereas Lsi2 mutation exhibited little difference. DMA/MMA is more mobile in both xylem and phloem transport than Asi (Marin et al. 1993; Li et al. 2009; Ye et al. 2010). Furthermore, DMA is extremely efficiently translocated to rice grain (Carey et al. 2010, 2011). However, few studies have examined DMA uptake and translocation under long time treatment with DMA (> 4 d) (Marin et al. 1993). Another long-term experiment on investigation of varying translocation of DMA into rice grain during different growth periods is still insufficient (Lomax et al. 2012).
In order to identify the difference between inorganic As and DMA in phytotoxicity and their accumulation in rice grain cultivated in hydroponics, the effects of DMA and Asi at different growth periods on the floret development, As concentrations in leaf, straw, and caryopsis, and As distribution in mature caryopsis were compared.
Materials and methods
Hydroponic experiments
Hydroponic experiments, with three or six replications for each treatment, were performed in 2010 in a greenhouse, Xiamen, China, at ambient temperatures (14-38 °C) under sunlight. Rice seeds (Oryza sativa L., cv Zhe-704) were germinated in perlite. Eight days after germination, uniform seedlings were selected. The perlite was washed from the root system with distilled deionized water and seedlings transferred to 4.3 L PVC pot containing 4 L nutrient solution, with one seedling per pot, on 13 April 2010. The basal nutrient solution was modified from that of Hewitt (1966) and contained 1.26 mM NH4NO3, 0.25 mM K2SO4, 1.0 mM CaCl2, 0.4 mM MgSO4·7H2O, 0.3 mM KH2PO4, 50 μM Fe(ii) EDTA, 1 μM ZnSO4·7H2O, 1 μM CuSO4·5H2O, 5 μM MnSO4·H2O,10 μM H3BO3, 0.5 μM Na2MoO4·2H2O, 100 μM NaCl, and 0.2 μM CoSO4·7H2O. Seedlings were passed through a hole in a black PVC plate (18 cm diameter and 8 mm thick), which covered the pot to shield the culture solution from sunlight to prevent algal growth.
After 7 days of acclimatization plants were subjected to 5 μM As treatments by using Na2HAsO4 for As (V) and cacodylic acid sodium salt (C2H6AsO2Na·3H2O, Sigma-Aldrich) for DMA. The As exposure were conducted during the whole growth period, before and after heading stage, which is marked by the emergence of the panicle tip from the flag leaf sheath. The detailed treatments are indicated in Table 1. According to the previous study (Carbonell-Barrachina et al. 1998), nutrient solutions containing specific As forms were replaced every 4 days in order to maintain the desired treatments.
The heading occurred between June 9th and June 15th. As treatment was changed at the beginning of the last heading day of each treatment of the pot experiments. Half of plants in the C-A and D-C treatments were harvested at the heading stage; the remaining plants were harvested at grain maturity stage. The harvested plants were washed with deionized water and separated into husk, caryopsis, flag leaves, and roots, which were incubated in 50 ml dithionite-citrate-bicarbonate solution (containing 0.03 M Na3C6H5O7·2H2O, 0.125 M NaHCO3 and 0.06 M Na2S2O4) for 1 h at the room temperature (Chen et al. 2005) to remove iron plaque and the adsorbed As from the root surface. These tissues were blotted and preserved in liquid nitrogen. Seed setting rate was analyzed when the seeds were harvested for each treatment, which was the ratio of full-size grain number to the total grain number.
Guttation collection
Rice is known to produce guttation fluid during the night, a phenomenon in which xylem sap exudes through specialized pores called hydatholdes that are associated with vein endings at the leaf margin (Yamaji et al. 2008). Guttation samples were collected using sterilized pipettes within 3 h at night. Each guttation sample consisted of the sap collected from six rice plants under the same treatment. All collections were finished during 1 week before flowering.
Images
Micrographs of flower organs were take on a Leica digital camera DC500.
Determination of As species
For the analysis of As species, liquid nitrogen-preserved samples were pulverized; 0.2 g was weighed into a 50 mL microwave tube, and extracted with 10 mL of 1 % nitric acid as described by Zhu et al. (2008). The extraction solutions were centrifuged and passed through a 0.45 μm membrane filter. To minimize potential further transformation of As species, samples were kept on ice and in the dark and analyzed within a few hours after extraction. As species in the extracts, nutrient solutions and guttations were assayed by high-performance liquid chromatography inductively coupled to plasma mass spectrometry (HPLC-ICP-MS) (7500cx, Agilent Technologies, Inc., USA)). Chromatographic columns consisted of a Hamilton precolumn (11.2 mm, 12-20 mm) and a Hamilton PRP-X100 10 μm anion-exchange column (240 × 4.1 mm). The mobile phase consisted of 6.66 mM NH4NO3 and 6.66 mM NH4H2PO4 (pH 6.2), and ran isocratically at 1 mL/min. Standard compounds of As(V), As(III), DMA, and MMA were used to obtain retention times. Matrix-matched DMA standards were used to calibrate the instrument. As species in samples were identified by comparisons with the retention times of the standard compounds and quantified by external calibration curves with peak areas. The As recovery from the rice certified reference material (CRM, GBW10010 rice flour) ranged from 95 % to 110 %.
In situ imaging by synchrotron X-ray fluorescence (SXRF) microprobe
Oven dried mature rice grains were cut with a disposable stainless steel blade with hand. Longitudinal sections along its longitudinal axis, and cross sections of middle-third grains were used for SXRF. For each treatment, 3 samples were imaged by SXRF microprobe. These experiments were performed at the beamline BL15U1 at Shanghai Synchrotron Radiation Facility. Incident X-rays of 14.0 keV energy were used to excite elements of samples. The SXRF signals were collected up to 2 s for each point with a 7-element detector, using a spot size of 50 μm and step size of 50 μm. The fluorescence intensities of As and Compton scattering were recorded, respectively. In order to correct for the effect of the SR beam flux variation on the signal intensity, the fluorescence intensity was normalized to the incident X-ray intensity, which was monitored by an ionization chamber located in the front of the K-B mirror. The corrected fluorescence intensity was used to estimate the relative elemental content.
Results
Effects of Asi and DMA on the development of rice florets and grain filling rate
Compared with the control (Fig. 1a), DMA treatment before flowering induced abnormal flowers of rice plant Fig. 1c-l), whereas Asi treatment did not (Fig. 1b). Microscopic examination showed that the DMA treatment induced a variety of defects in florets (Fig. 1c-l), with the most pronounced symptom being the distorted hull (Fig. 1c and f). Occasionally, DMA also caused the absence of floral organs (Fig. 1d and l), or more than one floret per spiklet (Fig. 1e). When hulls were removed, increased numbers of ovaries and pistils and irregular ‘lumps’ in the surface of ovaries were observed (Fig. 1g-k).
DMA treatment before flowering induced abnormal development of florets. a, a floret from a rice plant grown in normal culture solution. b, a floret from a rice plant treated with As (V) before flowering. c to l, florets from rice plants treated with DMA before flowering. Note the crescent-shaped hull (c and f), absence of floral organs (d and l) and more than one floret per spikelet (e). Furthermore, note the increased numbers of ovaries (arrow head) and pistils (red arrow) (g-k), and irregular ‘lumps (star)’ in some surface of ovaries (g and h). le, lemma; pa, palea; st, stamen; pi, pistil; ov, ovary; gl, glume; lo, lodicule
In addition, the effects of Asi and DMA on seed setting rate were also investigated (Fig. 2). Compared with rice growing in the control medium, As(V) treatments resulted in only a slight reduction (6.5-6.7 %) in the seed setting rate, while DMA treatments decreased seed setting rate considerably (27.1-52.6 %). ANOVA results indicated that there was no significant difference in the seed setting rates of Asi treatments, irrespective of the exposure and duration time, while distinct difference was observed in DMA treatments. The mean seed setting rate decreased in the order of C-D > D-C > D-D among the DMA treatments.
Effects of treatments with Asi and DMA at different stages on seed setting rate. C-C, the normal nutrient solution in the whole growth period; A-C, As(V) treatment till heading; C-A, As(V) treatment from heading to fully ripen; A-A, As(V) treatment in the whole growth period; D-C, DMA treatment till heading; C-D, DMA treatment from heading to fully ripen; D-D, DMA treatment in the whole growth period. The same letters are not significant at the 5 % level. Data are mean values ± SE (n = 3)
Accumulation of Asi and DMA in rice plant
Accumulation of As species and their concentrations in different tissues were shown in Fig. 3. It was observed that Asi dominated when rice plants were treated with As(V), although small amounts of DMA and MMA were found in some samples. In contrast, DMA was the predominant As species in rice plants treated with DMA. Asi and DMA had a similar temporal accumulation pattern, i.e. rice plants treated with As after flowering accumulated three-fold higher As concentrations than those treated with As before flowering.
However, ANOVA revealed significant differences in the accumulation of Asi and DMA in rice plants under similar exposure level. When As(V) and DMA were added to the nutrient solution during the whole growth period, As concentrations in roots (Fig. 3a), leaves (Fig. 3b) and husk (Fig. 3c) of the rice plants treated with As(V) were approximate 112.0, 89.4, and 4.1 times as much as those treated with DMA. In contrast, the DMA-treated caryopsis accumulated about twice As concentration compared with the Asi-treated one (Fig. 3d).
Translocation of Asi and DMA between neighbor tissues
In order to explain the different accumulation patterns of Asi and DMA in rice plants, the As translocation efficiency, defined as the As concentration ratios between neighboring tissues, was calculated (Fig. 4). The result showed that the Asi concentration decreased sharp in the order of root > leaf > husk > caryopsis in As(V) treatments (Fig. 4a). The Asi ratios between tissues were all lower than 1.0 (Fig. 4b). Although these ratios varied considerably between treatments, there was a general pattern that they were affected by both the application time and the duration of As(V) treatment. On the contrary, the DMA concentration decreased in the order of caryopsis > husk > root ≥ leaf in DMA treatments (Fig. 4c). Except for the leaf-to-root ratios, the transfer ratios between different tissues of DMA concentration were all higher than 1.0, which is distinct from the Asi concentration ratios (less than 1.0) (Fig. 4d). In addition, the DMA concentration ratios were affected by the timing of treatment more than the Asi ratios.
As species in guttation
To investigate other possible reasons explaining different accumulation of Asi and DMA in rice plants, As species in guttation of rice plants treated with Asi and DMA before flowering were examined. The results showed that Asi was the predominant As forms in the guttation of rice plants treated with As(V), whereas DMA dominated in the guttation of rice plants treated with DMA (Fig. 5a). Moreover, the DMA concentration in the guttation from the rice plants treated with DMA was approximate threefold of the Asi concentration in guttation from those treated with As(V) (Fig. 5b).
Efflux of As from guttation. a, HPLC-ICP-MS chromatograms for the guttation samples collected from rice plants in treatments A-C and D-C at the end of the As treatment. Note that DMA was mainly detected in D-C treatment, and Asi mainly in A-C treatment. b, As concentrations in guttation samples collected from rice plants in treatments A-C and D-C at the end of the As treatment. Data are mean values ± SE (n = 3)
Redistribution of Asi and DMA in rice plant after flowering
To investigate the redistribution of Asi and DMA in rice plants exposed to As before flowering, the As concentrations in different tissues at flowering were compared with those in the mature plants. In the rice plants treated with As(V), root Asi concentration decreased significantly after flowering (Fig. 6a), accompanied with a marked increase of Asi concentration in the above-ground tissues (Fig. 6b-d). In contrast, DMA concentration declined in all rice tissues of rice plant treated with DMA (Fig. 6a-d).
Different distribution of DMA and Asi in mature caryopsis
As localization in the caryopsis was determined using SXRF. The As signals in grains treated with As(V) and DMA during the whole growth period represented Asi and DMA, respectively, because HPLC-ICP-MS detected that they were the predominant form (97 – 98 %) of As in these samples. The SXRF results showed that the As signal in grains treated with As(V) mainly accumulated in the ovular vascular trace, especially in ventral vascular trace (Fig. 7a, b and e), but less in the dorsal and lateral stylar vascular trace, whereas the As signal was very low in the endosperm (Fig. 7f). In contrast, the As signal in grains treated with DMA was observed in all of vascular traces as well as the endosperm (Fig. 7c, d and g).
Synchrotron x-ray fluorescence images of As distribution in mature caryopses from rice plants in treatments D-D and A-A. a and b, Cross-sections of caryopsis samples from rice plants in treatment A-A. Note that the highest fluorescence intensity of As accumulated at ovular vascular trace. c and d, Cross-sections of caryopsis samples from rice plants in treatment D-D. Note the accumulation of As signal at ovular vascular trace, dorsal stylar vascular trace and lateral stylar vascular trace. e, A longitudinal section of caryopsis from a rice plant in treatment A-A. Note that As signal accumulated at ovular vascular trace. f, Fluorescence densities along a central longitudinal section through the fluorescence image in E. g, A longitudinal section of caryopsis from rice plant in treatment D-D. Note that As signal dispersed into endosperm. h, Fluorescence densities along a central longitudinal section through the fluorescence image in G. Inserts represent corresponding light micrographs. Bars = 500 μm
Discussion
Similar patterns of growth stage-dependent accumulation of Asi and DMA
Arsenic uptake into rice plants is highly reliant on its growth stage. Arao et al. (2009) has reported that an aerobic treatment for 3 weeks before and after heading was most effective for reducing the As concentration in grain. Lomax et al. (2012) further reported that, compared to tillering, stem extension and heading stages, exposure to As (Asi and DMA) for 1 week at flowering caused more As accumulation in rice plant. In this study, it was observed that, in both Asi (As (V)) and DMA treatments, As concentration was considerably higher in rice plants exposed to As after flowering than that before flowering, suggesting that uptake of DMA and Asi by rice plant have a similar growth stage-dependent pattern. This kind of similarity was consistent with our prediction that increasing uptake of nutrients by rice plants during the flowering and grain filling stages increased the uptake of Asi and DMA. However, DMA absorbed by rice plants could be due to soil microbial activity as it was shown that plants are unable to methylate Asi (Lomax et al. 2012). Therefore, the different accumulation of DMA and Asi into caryopsis were frequently detected in soil-grown rice. Our previous study found that the DMA concentration in rice grain decreased during grain filling, whereas inorganic As concentration remained stable in soil pot experiments (Zheng et al. 2011).
Different transport of Asi and DMA into rice grain
A previous study revealed that at the substrate concentration of 53.3 μM, the DMA uptake rate in rice seedlings was 25-fold and 22-fold lower than that of As(III) and As(V), respectively (Abedin et al. 2002b). In the present study, rice grains accumulated twice more DMA than Asi when plants were exposed to similar concentrations of the two As species, whereas there was much more Asi than DMA in the vegetative tissues. Apparently, DMA tends to accumulate in the caryopsis, while Asi in the vegetative tissues.
Xylem plays an important role in Asi transport (Ma et al. 2008). The distribution ratio of the xylem sap for each vasculature depends on transpiration rate of each peripheral organ. If the transport of Asi by the phloem is limited, the distribution ratio of Asi with xylem sap would be diluted with the increase of split-flow vasculature along the direction of mass flow. Recently, Zhao et al. (2012) demonstrated that Asi has a relatively low mobility within rice plants by using radioactive 73As tracer. Therefore, it can be speculated that Asi would be more likely to accumulate in the vegetative tissues which have higher transpiration rate, and once sequestrated in these tissues, only a limited amount of As is remobilized into caryopsis. Our founding that the Asi concentration decreased sharp in the order of root > leaf > husk > caryopsis in As(V) treatments provided an evidence for this hypothesis.
DMA can be transported by the xylem (Ye et al. 2010), which was demonstrated by the observation of DMA in guttations which are from the xylem sap (Yamaji et al. 2008). Besides, DMA is more easily remobilized through phloem from the flag leaves to the rice grain (Carey et al. 2011). The observation using SXRF mapping showed that DMA dispersed into the endosperm whereas Asi is more restricted to the vascular trace. Phloem in plant is developed for transport from leaves to roots, apex, and reproductive organs. After flowering, phloem transport contributes substantially to nutrient accumulation in fruits and seeds (Wardlaw 1990). Therefore, the preferred accumulation of DMA in caryopsis can be explained by its high translocation efficiency through the phloem.
Phytotoxicity of DMA on development of reproductive tissues
In the pentavalent oxidation state, organic arsenic species are generally considered to be less toxic than inorganic species to a wide range of organisms including aquatic plants, animals and humans (Tamaki and Frankenberger 1992). In the present study, we found that exposure to 5 μM DMA before flowering induced abnormal development of rice florets, while exporue to As(V) did not, suggesting that either DMA was more toxic than Asi to the development of rice florets, or DMA was more efficiently translocated to the florets. In addition, DMA treatment after flowering also markedly decreased the seed setting rate, which may be due to the malfunction of phloem translocation damaged by the changed water potential caused by DMA (Carey et al. 2011). Therefore, the presence of DMA in paddy soil might pose higher risk to rice yield than Asi. In fact, the distorted hulls and marked decline in grain filling found in the present study was similar to straighthead disease, which has been frequently observed when rice is grown in soils treated with arsenical herbicides (Azizur Rahman et al. 2012) and causes severe yield reduction in some rice paddy fields in the United States, South America, Japan, and elsewhere (Belefant-Miller and Beaty 2007).
The higher toxicity of DMA to the development of rice florets than Asi can partly, but not wholly, be explained by its higher accumulation and more efficient transfer into grain that has been widely reported in previous studies (Li et al. 2009; Carey et al. 2010, 2011). Significantly higher toxicity with lower accumulation of DMA than Asi (As(V) was shown in two As hyperaccumulators, Pteris vittata and Pteris cretica and an As-tolerant plant Boehmeria nivea (Huang et al. 2008). The mechanism underlying the abnormal development of rice florets induced by DMA remains unknown.
In a previous study, Sun and Zhou (2008) reported similar defects of florets in JMJ706 (loss-of-function) rice mutation, which led to increased di- and trimethylations of lysine 9 of histone H3. Given the possible demethylation of DMA by rice plant (Huang et al. 2008) and the possible methyl transfer to DNA resulting in DNA damage, one of the possible explanations on the abnormal development of rice florets induced by DMA is that DMA can damage the DNA regulating the development of flower just like in mice (Yamanaka et al. 1991). In addition, other possible mechanism like overexpression of microRNA172 that is related to floral organ development cannot be excluded (Zhu et al. 2009). The alternative possibility is that the suppressed transport of sucrose by DMA affected the developmental processes of florets (Lalonde et al. 2004). Given that the experiments were conducted under controlled hydroponic conditions, we can conclude that DMA can affect the development of rice reproductive tissues while Asi cannot when they are available in the same concentration (5 μM). To decipher the precise molecular mechanism underlying this phenomenon, further investigations using molecular technology are needed.
References
Abedin MJ, Cresser MS, Meharg AA, Feldmann J, Cotter-Howells J (2002a) Arsenic accumulation and metabolism in rice (Oryza sativa L.). Environ Sci Technol 36:962–968
Abedin MJ, Feldmann J, Meharg AA (2002b) Uptake kinetics of arsenic species in rice plants. Plant Physiol 128:1120–1128
Arao T, Kawasaki A, Baba K, Mori S, Matsumoto S (2009) Effects of water management on cadmium and arsenic accumulation and dimethylarsinic Acid concentrations in Japanese rice. Environ Sci Technol 43:9361–9367
Azizur Rahman M, Mamunur Rahman M, Hasegawa H (2012) Arsenic-induced straighthead: an impending threat to sustainable rice production in South and South-East Asia. Bull Environ Contam Toxicol 88:311–315
Belefant-Miller H, Beaty T (2007) Distribution of arsenic and other minerals in rice plants affected by natural straighthead. Agronomy J 99:1675–1681
Bleeker PM, Hakvoort HW, Bliek M, Souer E, Schat H (2006) Enhanced arsenate reduction by a CDC25-like tyrosine phosphatase explains increased phytochelatin accumulation in arsenate-tolerant Holcus lanatus. Plant J 45:917–929
Brammer H, Ravenscroft P (2009) Arsenic in groundwater: a threat to sustainable agriculture in South and South-east Asia. Environ Int 35:647–654
Carbonell-Barrachina AA, Aarabi MA, DeLaune RD, Gambrell RP, Patrick WH (1998) The influence of arsenic chemical form and concentration on Spartina patens and Spartina alterniflora growth and tissue arsenic concentration. Plant Soil 198:33–43
Carey AM, Scheckel KG, Lombi E, Newville M, Choi Y, Norton GJ, Charnock JM, Feldmann J, Price AH, Meharg AA (2010) Grain unloading of arsenic species in rice (Oryza sativa L.). Plant Physiol 152:309–319
Carey AM, Norton GJ, Deacon C, Scheckel KG, Lomb E, Punshon T, Guerinot ML, Lanzirotti A, Newville M, Choi Y, Price AH, Meharg AA (2011) Phloem transport of arsenic species from flag leaf to grain during grain filling. New Phytol 192:87–98
Chen Z, Zhu YG, Liu WJ, Meharg AA (2005) Direct evidence showing the effect of root surface iron plaque on arsenite and arsenate uptake into rice (Oryza sativa) roots. New Phytol 165:91–97
Duan GL, Zhu YG, Tong YP, Cai C, Kneer R (2005) Characterization of arsenate reductase in the extract of roots and fronds of Chinese brake fern, an arsenic hyperaccumulator. Plant Physiol 138:461–469
Fendorf S, Michael HA, van Geen A (2010) Spatial and temporal variations of groundwater arsenic in South and Southeast Asia. Science 328:1123–1127
Hewitt EJ (1966) Sand and waterculture methods used in the study of plant nutrition, 2 ed. Communication No. 22. Commonwealth Agriculture Bureau Technical, Farnham Royal, UK.
Huang Z, Chen T, Lei M, Liu Y, Hu T (2008) Difference of toxicity and accumulation of methylated and inorganic arsenic in arsenic-hyperaccumulating and –hypertolerant plants. Environ Sci Technol 42:5106–5111
Kile ML, Houseman EA, Breton CV, Smith T, Quamruzzaman O (2007) Dietary arsenic exposure in Bangladesh. Environ Health Perspect 115:889–893
Lalonde S, Wipf D, Frommer WB (2004) Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu Rev Plant Biol 55:341–372
Li RY, Ago Y, Liu WJ, Mitani N, Feldmann J, McGrath SP, Ma JF, Zhao FJ (2009) The rice aquaporin Lsi1 mediates uptake of methylated arsenic species. Plant Physiol 150:2071–2080
Li G, Sun GX, Williams PN, Nunes L, Zhu YG (2011) Inorganic arsenic in Chinese food and its cancer risk. Environ Int 37:1219–1225
Liu WJ, Wood BA, Raab A, McGrath SP, Zhao FJ, Feldmann J (2011) Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in Arabidopsis. Plant Physiol 152:2211–2221
Lomax C, Liu W, Wu L, Xue K, Xiong J, Zhou J, McGrath SP, Meharg AA, Miller AJ, Zhao FJ (2012) Methylated arsenic species in plants originate from soil microorganisms. New Phytol 193:665–672
Lu Y, Adomako EE, Solaiman AR, Islam MR, Deacon C, Williams PN, Rahman GK, Meharg AA (2009) Baseline soil variation is a major factor in arsenic accumulation in Bengal Delta paddy rice. Environ Sci Technol 43:1724–1729
Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105:9931–9935
Mandal BK, Suzuki KT (2002) Arsenic round the world: a review. Talanta 58:201–235
Marin AR, Masscheleyn PH, Patrick WH (1993) Soil redox-pH stability of arsenic species and its influence on arsenic uptake by rice. Plant Soil 52:245–253
Meharg AA, Williams PN, Adomako E, Lawgali YY, Deacon C, Villada A, Cambell RC, Sun G, Zhu YG, Feldmann J, Raab A, Zhao FJ, Islam R, Hossain S, Yanai J (2009) Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ Sci Technol 43:1612–1617
Mondal D, Polya DA (2008) Rice is a major exposure route for arsenic in Chakadaha block, Nadia district, West Bengal, India: A probabilistic risk assessment. Appl Geochem 23:2987–2998
Moore KL, Schröder M, Wu Z, Martin BG, Hawes CR, McGrath SP, Hawkesford MJ, Feng Ma J, Zhao FJ, Grovenor CR (2011) High-resolution secondary ion mass spectrometry reveals the contrasting subcellular distribution of arsenic and silicon in rice roots. Plant Physiol 156:913–924
Norton GJ, Duan GL, Dasgupta T, Islam MR, Lei M, Zhu YG, Deacon CM, Moran AC, Islam S, Zhao FJ (2009) Environmental and genetic control of arsenic accumulation and speciation in rice grain: comparing a range of common cultivars grown in contaminated sites across Bangladesh, China, and India. Environ Sci Technol 43:8381–8386
Panaullah GM, Alam T, Hossain MB, Loeppert RH, Lauren JG, Meisner CA, Ahmed ZU, Duxbury JM (2009) Arsenic toxicity to rice (Oryza sativa L.) in Bangladesh. Plant Soil 317:31–39
Rahman MA, Hasegawa H (2011) High levels of inorganic arsenic in rice in areas where arsenic-contaminated water is used for irrigation and cooking. Sci Total Environ 409:4645–4655
Smith AH, Steinmaus CM (2009) Health effects of arsenic and chromium in drinking water: recent human findings. Ann Rev Public Health 30:107–122
Sun Q, Zhou DX (2008) Rice jmjC domain-containing gene JMJ706 encodes H3K9 demethylase required for floral organ development. Proc Natl Acad Sci USA 105:13679–13684
Takamatsu T, Aoki H, Yoshida T (1982) Determination of arsenate, arsenite, monomethylarsonate, and dimethylarsinate in soil polluted with arsenic. Soil Sci 133:239–246
Tamaki S, Frankenberger WT Jr (1992) Environmental biochemistry of arsenic. Rev Environ Contam Toxicol 124:79–110
Tchounwou PB, Centeno JA, Patlolla AK (2004) Arsenic toxicity, mutagenesis, and carcinogenesis–a health risk assessment and management approach. Mol Cell Biochem 255:47–55
Wardlaw IF (1990) The control of carbon partitioning in plants. New Phytol 116:341–381
Williams PN, Islam MR, Adomako EE, Raab A, Hossain SA, Zhu YG, Feldmann J, Meharg AA (2006) Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in groundwaters. Environ Sci Technol 40:4903–8
Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA (2007) Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol 41:6854–6859
Wu Z, Ren H, McGrath SP, Wu P, Zhao FJ (2011) Investigating the contribution of the phosphate transport pathway to arsenic accumulation in rice. Plant Physiol 157:498–508
Xu XY, McGrath SP, Meharg AA, Zhao FJ (2008) Growing rice aerobically markedly decreases arsenic accumulation. Environ Sci Technol 42:5574–5579
Yamaji N, Mitatni N, Ma JF (2008) A transporter regulating silicon distribution in rice shoots. Plant Cell 20:1381–1389
Yamanaka K, Ohaba H, Hasegawa A, Sawamura R, Okada S (1991) Cellular response to oxidative damage in lung induced by the administration of dimethylarsinic acid, a major metabolite of inorganic arsenic, in mice. Toxicol Appl Pharm 108:205–213
Ye WL, Wood BA, Stroud JL, Andralojc PJ, Raab A, McGrath SP, Feldmann J, Zhao FJ (2010) Arsenic speciation in phloem and xylem exudates of castor bean. Plant Physiol 154:1505–1513
Zavala YJ, Gerads R, Gürleyük H, Duxbury JM (2008) Arsenic in rice: II. Arsenic speciation in USA grain and implications for human health Environ Sci Technol 42:3861–3866
Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181:777–794
Zhao FJ, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559
Zhao FJ, Stroud JL, Asaduzzaman Khan M, McGrath SP (2012) Arsenic translocation in rice investigated using radioactive 73As tracer. Plant Soil 350:413–420
Zheng MZ, Cai C, Hu Y, Sun GX, Williams PN, Cui HJ, Li G, Zhao FJ, Zhu YG (2011) Spatial distribution of arsenic and temporal variation of its concentration in rice. New Phytol 189:200–209
Zhu YG, Sun GX, Lei M, Teng M, Liu YX, Chen NC, Wang LH, Carey AM, Deacon C, Raab A, Meharg AA, Williams PN (2008) High percentage inorganic arsenic content of mining impacted and nonimpacted Chinese rice. Environ Sci Technol 42:5008–5013
Zhu QH, Upadhyaya NM, Gubler F, Helliwell CA (2009) Over-expression of miR172 causes loss of spikelet determinacy and floral organ abnormalities in rice (Oryza sativa). BMC Plant Biol 9:149
Acknowledgments
This work was supported by Knowledge Innovation Program of the Chinese Academy of Sciences (KZCX2-YW-Q02-04), The National Environmental Protection Public Welfare Industry Targeted Research Fund (201109052), the foundation of Macau University (MYRG204(Y1-L4)-FST11-SHJ)) and Social Development of Yunnan Province Science and Technology Plan (Special social development fund) (2010CA001). We thank the Shanghai Synchrotron Radiation Facility for providing technical support in the synchrotron x-ray fluorescent microprobe. We also thank Prof. Fang-Jie Zhao for his critical comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mao-Zhong Zheng and Gang Li contributed equally to this work.
Responsible Editor: Juan Barcelo.
Rights and permissions
About this article
Cite this article
Zheng, MZ., Li, G., Sun, GX. et al. Differential toxicity and accumulation of inorganic and methylated arsenic in rice. Plant Soil 365, 227–238 (2013). https://doi.org/10.1007/s11104-012-1376-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1376-3