Abstract
Present study was performed in order to explicate whether added sulphur (S; 60 mg S kg−1 sand) and calcium (Ca; 250 mg Ca kg−1 sand) alone and in combination could modulate arsenic-induced (As1; 15 mg As kg−1 sand and As2; 30 mg As kg−1 sand) toxicity in Brassica juncea L. seedlings. To study this, growth and growth regulating processes i.e. status of oxidative stress biomarkers (H2O2 generation and lipid peroxidation), enzymes and metabolites of AsA-GSH cycle and S-metabolism were examined. Both the doses of As significantly reduced the growth as evident from diminishing dry weight and increased lipid peroxidation as a consequence of excess H2O2 accumulation. Arsenic also altered the redox status of the cell thereby depleting the AsA and GSH pool that consequently decreased AsA/DHA, AsA/H2O2 and GSH/GSSG ratios. Neverthless, APX, DHAR and GR activities were enhanced under similar conditions. Contrary to this, additional S and/ or Ca maintained the redox status of the cell that improved AsA/DHA and GSH/GSSG ratios, and further enhanced the enzymatic activities in both root and leaves of the test seedlings. Upon As exposure, test seedlings exhibited an increase in S assimilation as a result of increased enzyme activities of ATPS, OASTL and γ-ECS, which were further enhanced upon S and/ or Ca addition to stressed seedlings. Due to increment in S assimilation, PCs synthesis was also increased that restricted As translocation from root to shoot. Collectively, our result provides an insight for protective role of S and Ca alone and more efficiently in combination (S+Ca) to As-stressed Brassica seedlings suggesting that S and Ca together could be a promising candidates in managing As toxicity in crops.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Everyday about 200,000 individuals are being added to the world population and it is expected that by 2050 the world population will surpass 9+ billion. To meet the demand of food for over-increasing population, enormous pressure is being put on world’s agricultural resources that cause mineral nutrients’ deficiency in soils. On the other hand, atmospheric sulphur (S) emissions caused by industrial practices have been reduced by ~ 80% hence, limiting the deposition of soil S (McNeill et al. 2005). This reduction in anthropic S inputs along with the repeated cropping practices decreases S availability in soils, thereby it is detrimental for S-demanding crops such as Brassica (Grant et al. 2012). Sulphur is present in all the soil and is mainly derived from the atmosphere (through deposition of S on soil from marine aerosols, industrial gases and particulates from volcanic eruption) and parent rocks (sulfides and sulphates, are common mineral in earth’s crust). Sulphur assimilation starts with the uptake of sulphate ion which then transforms into S containing compounds: cysteine (Cys) and methionine (met) through the cascade of reactions catalyze by the activities of ATP sulfurylase (ATPS), O-acetylserine (thiol)lyase (OASTL) and γ-glutamylcysteine synthetase (γ-ECS) (Rausch and Wachter 2005; Noctor et al. 2012; Capaldi et al. 2015; Liang et al. 2016). These enzymes are crucial in bridging disulfide (S–S) and sulfhydryl (-SH) bonds, which make functional to enzymes and proteins having thiol group in their active sites (Saito et al. 1994). Glutathione (GSH), a S containing compound scavenges ROS through the AsA-GSH cycle and it is also used as a substrate in the biosynthesis of phytochelatins (PCs) (Rausch and Wachter 2005), which chelate heavy metals in cellular system.
On the other hand, calcium (Ca) is also receiving attention as it is not only a signalling agent but also participates in regulating a variety of cellular activities like cytoplasmic streaming, cell division and elongation, pH of cells and prevents solute leakage from cytoplasm (Kader and Lindberg 2010; Xu et al. 2013). It has been reported that exogenous Ca2+ is capable of improving growth, ion homeostasis by inhibiting Na+ influx and K+ leakage and ROS detoxification in plants exposed to abiotic stresses (Tan et al. 2011; Huang et al. 2012; Rahman et al. 2016). Calcium has also been reported to regulate the superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR) to defend plants against different environmental stresses (Tan et al. 2011; Ahmad et al. 2016; Singh et al. 2018a). The major sources of calcium in the soil are gypsum, lime (CaCO3), calcium nitrate, calcium chloride, eggshells, etc.
In current scenario, As contamination in South and South-East Asia especially in India and Bangladesh have become the matter of great concern (Singh et al. 2015). Arsenic concentration in these countries exceeds (up to 3200 μg As L−1) the safe limit (10 μg As L−1) in drinking water, and when this water is used for irrigation of crops it enters into the food chain and causes serious injuries to the living beings (McCarty et al. 2011). The major sources of As in the environment are synthetic chemicals (herbicides, insecticides, phosphate fertilizers), timber preservatives and semi-conductor industries, coal combustion, burning of fossil fuels, mining and smelting, etc. (Bundschuh et al. 2011). Of the two inorganic forms of As i.e. arsenate (AsV) and arsenite (AsIII); AsV predominates in aerobic environment and rapidly reduces into AsIII by arsenate reductase activity in root cells (Dhankher et al. 2002; Dixit et al. 2015). Inside the cell, AsV generates reactive oxygen species (ROS), which damage cell membranes and biomolecules, photosynthetic pigments, affects PS II photochemistry and inhibits activities of important enzymes (Ahsan et al. 2010; Gupta and Ahmad 2014; Singh et al. 2018a, b). Being a chemical analogue of inorganic phosphate (iP), AsV disturbs energy metabolism by replacing inorganic phosphate in ATP and forms adenosine-5-diphosphate-arsenate (ADP-As).
Brassica with 47 million ton production worldwide in 2007 (FAO), occupied top rank amongst major agro-economic crops. It is considered as an excellent rotation crop for cereals as it facilitates the suppression of soil-borne pathogens either by improving subsoil macro porosity through its deep tap rooting system or by releasing biocidal compounds (Kirkegaard et al. 1997). But presently in India and Bangladesh, this commonly grown Brassica is facing As challenges when irrigated with As laden water. Therefore, keeping in mind the persuasive role of S and Ca under stress conditions, present study was conducted to investigate whether the two important mineral nutrients i.e. S (its deficiency is fairly recent in agriculture) and Ca (an important signalling agent), when applied alone and in combination (as researches on the combined effect of plant nutrients still lags far behind) can help the Brassica seedlings to withstand against As toxicity. Further, to form a clear view on above argued facts, different objectives: growth, cellular accumulation of As, oxidative biomarkers and As detoxification by coordinated functioning of different components of AsA-GSH cycle and enzyme activities of S metabolism, were set forth.
Materials and methods
Experimental design and As, S and/ or Ca treatments
Healthy seeds of Brassica juncea L. were soaked in 2% (v/v) sodium hypochlorite solution for 15 min for surface sterilization and kept in darkness. After 48 h, equal sized germinated seeds were sown in plastic cups having 150 g acid washed sand and kept in dark for germination at 25 ± 2 °C. After that, germinated seedlings were transferred to growth chamber (CDR model GRW-300 DGe, Athens) with photosynthetically active radiation (PAR): 150 μmol photons m−2 s−1, day-night regime: 16:8 h at 22 ± 2 °C and relative humidity: 65–70%. During growth phase (30 days from the day of sowing), seedlings were irrigated with half strength (50%) Hoagland solution (Hoagland and Arnon 1950) alternating with distilled water. Thereafter, seedlings at secondary leaf stage were treated with varying concentrations of As (Na2HAsO4·7H2O) dissolved in nutrient solution containing additional S (K2SO4) and/ or Ca (CaCl2). It is noticeable that solubility level of K2SO4 and CaCl2 is 12 g/100 ml and 74.5 g/100 ml at 25 °C, respectively. To carry out the experimental work, two doses of As i.e. As1; 15 mg As kg−1 sand and As2; 30 mg As kg−1 sand and single doses of S i.e. 60 mg S kg−1 sand and Ca i.e. 250 mg Ca kg−1 sand were selected on the basis of screening experiments. Following combinations were made for the present study: control (C)-nutrient solution alone; C+ additional S, C+ additional Ca and C+ additional S+Ca with and without As (As1 and As2), and the experiments were performed in quintuplicates (n = 5). After 7 days of treatment, untreated and treated seedlings were harvested and different parameters were analyzed.
Effect of S and/ or Ca on growth attributes of As-challenged Brassica seedlings
Growth of Brassica seedlings was measured in terms of dry weight (DW), root and shoot length and sensitivity index (SI). The sensitivity index was calculated as:
Estimation of cellular accumulation of As, S and Ca
The contents of As, S and Ca in untreated and treated samples of Brassica plant were assayed following the method of Allen et al. (1986).
Effect of S and/ or Ca on oxidative stress biomarkers in As-challenged Brassica seedlings
The contents of hydrogen peroxide (H2O2) and lipid peroxidation [malondialdehyde (MDA) equivalents] in root and leaves were assayed following the methods of Velikova et al. (2000) and Heath and Packer (1968), respectively.
Effect of S and/ or Ca on contents of ascorbate of As-challenged Brassica seedlings
The contents of reduced ascorbate (AsA) and oxidized ascorbate (DHA) were assayed as described by Gossett et al. (1994).
Effect of S and/ or Ca on contents of thiol compounds of As-challenged Brassica seedlings
The contents of reduced glutathione (GSH) and oxidized glutathione (GSSG) were determined by Brehe and Burch (1976). The contents of cysteine (Cys) and non-protein thiols (NPTs) were estimated by the methods of Gaitonde (1967) and Ellman's (1959), respectively. The content of phytochelatins (PCs) was calculated as PCs = NPTs–total GSH as per the equation of Hartley-Whitaker et al. (2001).
Effect of S and/ or Ca on enzyme activities of ascorbate and glutathione metabolism of As-challenged Brassica seedlings
The activities of ascorbate peroxidase (APX; EC 1.11.1.11) and dehydroascorbate reductase (DHAR; EC 2.5.1.18) were assayed following the methods of Nakano and Asada (1981) while glutathione reductase (GR; EC 1.6.4.2) activity was assayed according to Schaedle and Bassham (1977). One unit (U) of APX, DHAR and GR activities have been defined as 1 nmol ascorbate oxidized min−1, 1 nmol DHA reduced min−1 and 1 nmol NADPH oxidized min−1, respectively.
Further, the isoenzymes detection for APX, DHAR and GR was performed on irregular polyacrylamide gels (PAGE) having 4.5 and 10% polyacrylamide in stacking and separating gels, respectively. Proteins from each sample were loaded in gels for their electrophoretic separation exceptionally for APX; where gels were pre-runned in 2.0 mM ascorbate for 30 min. The bands of APX and GR isoenzymes were stained following the methods of Mittler and Zinlinkas (1993) and Ye et al. (1997), respectively; while that of DHAR isoenzymes were stained as per De Tullio et al. (1999) and Tommasi et al. (2001).
Effect of S and/ or Ca on enzyme activities of sulphur assimilation pathway of As-challenged Brassica seedlings
The activities of ATP sulfurylase (ATPS; EC 2.7.7.4), O-acetylserine (thiol)lyase (OASTL; EC 4.2.99.8) and γ-glutamylcysteine synthetase (γ-ECS; EC 6.3.2.2) were determined by the methods of Lappartient and Touraine (1996), Riemenschneider et al. (2005) and Nagalakshmi and Prasad (2001), and the activities were expressed in terms of nmol PPi mg−1 protein min−1, nmol cysteine mg−1 protein min−1 and nmol Pi mg−1 protein min−1, respectively.
Statistical analyses
The independent experiments were carried out in quintuplicates (n = 5) and results presented as figures and tables are the mean ± standard error. Since results showed normal distribution, therefore comparison between the mean of control and treatment along with the comparison among the treatments was performed by using a one-way ANOVA to test significance level (Tukey alpha test) at P < 0.05. The SPSS-16 software was used for Tukey test. Before Tukey test, a multivariate test was used for the assumptions of homogeneity of variances. All the data sets satisfied assumptions of ANOVA based on homogeneity of variances, normality of errors and independence of errors.
Results
Growth
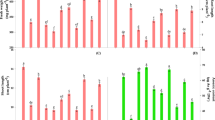
Results showed that both the doses of As i.e. As1 and As2, notably declined the DW of root by 15 and 35% and length by 22 and 47%, and the corresponding decrease in the DW of the shoots was 10 and 23% while in length, it was 10 and 26%, respectively as compared to control (Fig. 1). Contrastingly, exogenous application of S, Ca and S+Ca, counteracted the negative effect of As on growth attributes; where S+Ca supplementation found to be more efficient. The results for sensitivity index (indicates the sensitivity of Brassica seedlings to As showing highly sensitive in absence of additional S and/ or Ca and less sensitive in presence of S and/ or Ca) also showed similar trend to that of growth parameters.
Impact of exogenous sulphur and calcium applied alone and in combination on dry weight and length of root and shoot and sensitivity index of Brassica juncea L. seedlings grown under arsenic (As; As1:15 mg As Kg−1 sand and As2: 30 mg As Kg−1 sand) stress. Data represent the mean value ± standard error from quintuplicates (n = 5). Bars followed by different letters showing significant difference at P < 0.05 significance level among the treatments according to Tukey test
Cellular accumulation of arsenic
Arsenic accumulation in Brassica seedlings was increased in As dose dependent manner (Table 1). Contrary to this, upon exogenous supplementation of S, Ca and S+Ca to As stressed seedlings, a decrease in cellular accumulation of As was noticed. Amongst, S+Ca was found to be more efficient in reducing As accumulation in test seedlings, being 12.94 ± 0.26 and 27.23 ± 0.61 μg As g−1 DW, respectively, under As1 and As2 stress as compared to the values of As treated samples.
Mineral nutrient status
Cellular accumulation of S and Ca in test seedlings was increased with increasing doses of As (Table 1). Under As (As1 and As2) stressed condition, test seedling exhibited an increment in the contents of S by 47 and 97% and Ca by 40 and 77%, respectively over the values of control. Furthermore, upon exogenous application of S, Ca and S+Ca to As1 stressed seedlings, a sharp rise in the contents of S (176, 172 and 190%) and Ca (135, 142 and 152%), respectively as compare to untreated control was found and under similar treatment the contents of S and Ca were further increased in As2 treated seedlings.
Oxidative stress markers: H2O2 and MDA equivalents contents
The results showed that the content of H2O2 was raised by 36 and 73% and MDA equivalents by 13 and 29%, respectively under As1 and As2 stress in root as compared to control and the corresponding increase was also observed in leaves but with lesser extent in comparison to roots (Table 1). On the other hand, exogenous S and Ca alone and in combination (S+Ca) to As1 and As2 stressed seedlings lowered the levels of H2O2 and MDA equivalents contents as compared to those observed in As stressed seedlings alone (without additional S and Ca). Though, the application of additional S and Ca (alone and together) caused decreasing trend in H2O2 and MDA equivalents contents; however with Ca and S+Ca combination the levels of MDA equivalents content in As1 stressed root and leaves was even less than that of control.
Metabolites of AsA-GSH cycle
The metabolites of AsA-GSH cycle i.e. ascorbate (AsA, DHA, AsA+DHA and AsA/DHA), and glutathione (GSH, GSSG, GSH+GSSG and GSH/GSSG) in both root and leaves showed varied results under As stress (Tables 2 and 3). Both the doses of As i.e. As1 and As2 sharply declined the content of AsA by 14 and 29% and GSH by 30 and 49% in root and the corresponding decrease in AsA of leaves was 12 and 26% while in case of GSH it was by 21 and 35%, respectively with respect to the control values. Under similar conditions, the contents of DHA and GSSG were increased thereby decreasing the ratios of AsA/DHA, AsA/H2O2 and GSH/GSSG. On the other hand, exogenous supplementation of S, Ca and S+Ca, markedly improved the AsA and GSH contents and thus the values for AsA/DHA, AsA/H2O2 and GSH/GSSG under both the doses of As were found to be improved.
Enzyme activities of AsA-GSH cycle
The enzymes of ascorbate–glutathione cycle i.e. APX, DHAR and GR responded differentially to As challenged Brassica seedlings (Fig. 2a). Under As1 stress, the activities of APX, GR and DHAR were increased by 26, 52 and 24% in root and by 25, 42 and 14% in leaves, respectively over the values of control. Further increase in APX and GR activities was also noticed under As2 treatment, except DHAR activity which was decreased by 9 and 5% in root and leaves, respectively as compared to control. Upon S, Ca and S+Ca application, a further rise in the activities of these enzymes was noticed with more pronounced effect under S+Ca treatments.
a Impact of exogenous sulphur and calcium applied alone and in combination on enzyme activities of AsA-GSH cycle: ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR) and glutathione reductase (GR) in root and leaves of Brassica juncea L. seedlings grown under arsenic (As; As1:15 mg As Kg−1 sand and As2: 30 mg As Kg−1 sand) stress. Data represent the mean value ± standard error from quintuplicates (n = 5). Bars followed by different letters showing significant difference at P < 0.05 significance level among the treatments according to Tukey test. b Impact of exogenous sulphur and calcium applied alone and in combination on isoenzyme profiling (native-PAGE) of APX (A), DHAR (B) and GR (C) in leaves of Brassica juncea L. seedlings grown under arsenic (As; As1:15 mg As Kg−1 sand and As2: 30 mg As Kg−1 sand) stress. c control; lane 1: As1; lane 2: As2; lane 3: S; lane 4: Ca; lane 5: S+Ca; lane 6: As1+S; lane 7: As1+Ca; lane 8: As1+S+Ca; lane 9: As2+S; lane 10: As2+Ca; lane 11: As2+S+Ca
Furthermore, the isoenzyme profiling of APX, DHAR and GR in leaves confirmed the biochemical results under tested conditions (Fig. 2b). The results regarding isoenzyme profiling of GR, APX and DHAR revealed single, three and five bands, respectively and the intensity of the bands (except in case of DHAR) was increased with the increasing doses of As. Upon S and Ca application either individually or in combination to As stressed seedlings, the expression of these bands (as indicated by intensity) was further increased.
Compounds of thiol pool
The results pertaining to contents of thiol pool i.e. NPTs, Cys and PCs have been presented in Table 4 and all the three contents showed an increasing trend under As stress. Under As1 and As2 stress, the content of NPTs was raised by 128 and 164%, Cys by 93 and 163% and PCs by 179 and 233% in root, and the corresponding increase in the contents of NPTs was 8 and 16%, Cys was 45 and 71% and in PCs it was by 143 and 260% in leaves, respectively over the values of control (Table 4). On the other hand, S, Ca and S+Ca supplementation further raised these contents in both root and leaves of As stressed Brassica seedlings, where maximum rise was observed after S+Ca application.
Enzymes of sulphur assimilation pathway
To find the deeper insight of thiol metabolic response in As stressed Brassica seedlings, the activities of enzymes participating in synthesis and consumption of thiol compounds were also assayed (Fig. 3). Under As1 stress, the activities of ATPS, OASTL and γ-ECS were increased by 21, 13 and 11% in root and by 33, 40 and 20% in leaves, respectively as compare to control. Under As2 stress the corresponding increase in the activities of ATPS, OASTL and γ-ECS was 32, 25 and 19% in root and 54, 73 and 34% in leaves, respectively, and the activities were further accelerated when As stressed Brassica seedlings were treated with exogenous S, Ca and S+Ca.
Impact of exogenous sulphur and calcium applied alone and in combination on enzyme activities of S-assimilation pathway: ATP sulfurylase (ATPS), O-acetylserine (thiol)lyase (OASTL) and γ-glutamyl cysteine synthetase (γ-ECS) in root and leaves of Brassica juncea L. seedlings grown under arsenic (As; As1:15 mg As Kg−1 sand and As2: 30 mg As Kg−1 sand) stress. Data represent the mean value ± standard error from quintuplicates (n = 5). Bars followed by different letters showing significant difference at P < 0.05 significance level among the treatments according to Tukey test
Discussion
Brassica is a strong bioremediant, nutritional supplement of livestock and an excellent source of different phytochemicals of medicinal importance, vitamins, oil, etc. (Xin et al. 2013). Therefore, it becomes mandatory to examine the impact of As, a burning problem of South-East Asia on growth and chief components concerned with the maintenance of cell integrity like oxidative stress markers, AsA-GSH cycle as well as S-assimilation pathway of Brassica seedlings. From the results, it is clear that As at its both the doses (As1 and As2) declined the growth in dose dependent manner (Fig. 1). Growth reduction is an important indicator of As toxicity in test seedlings, which could be attributed to higher As accumulation in tissues (Table 1) that leads to excessive ROS generation (Table 1). These ROS might have imbalanced the delicate equilibrium between ROS (H2O2) generation and antioxidant defense system principally those involved in AsA-GSH cycle and S-assimilation pathway, altered the cell wall structures, damaged the plasma membranes (Table 1) and cellular biomolecules. Our results are in agreement with the earlier findings (Yadav 2010; Sharma 2012; Gupta and Ahmad 2014), where As exhibited severe damaging effect on growth parameters. Furthermore, As induced growth reduction in Brassica seedlings might also be due to the damaging effect on light harvesting pigments and therefore reduction in the performance of PS II (data not shown) (Singh et al. 2018b). Moreover, roots were found to be affected more which could be explained on the basis of: (i) roots being in direct contact with As and (ii) roots might have lowered the rate of As translocation to shoots thereby arresting As in roots (Singh et al. 2018a). The results are in harmony with those recorded by Singh et al. (2017) in Solanum melongena and Ahmad et al. (2016) in Cicer arietinum seedlings.
The exogenous application of S and/ or Ca significantly reduced As accumulation in root and shoots thereby counteracting the inhibitory effects of As stress on the growth performance of test seedlings (Fig. 1) that might be due to: (i) improvement in cell wall thickness of roots thereby hindering the absorption of As by root cells (Ahmad et al. 2016), (ii) improvement in the activities of S assimilating enzymes that are crucial in maintaining cellular redox status by providing reducing power (Sebastian and Prasad 2014) (Fig. 3; Table 4), (iii) favouring cell elongation and expansion (Hernandez and Almansa 2002) that ultimately increases growth and (iv) up-regulation of antioxidant defence system such as AsA-GSH cycle (Figs. 2 and 3; Tables 2, 3 and 4). Sulphur and Ca treatment also highlights low lipid peroxidation (less MDA production) rate in root and leaf tissues as a result of decreased H2O2 accumulation (Table 1), that obviously led towards betterment of growth of As stressed seedlings. Interestingly, S accumulation in As stressed plants was increased with increasing concentration of As (Table 1), which could be linked with its role in As chelation by strong ligands like GSH and PCs conjugation (As-PCs complex) (Tuli et al. 2010). It was also suggested that S might be involved in the arsenic precipitation as As2S3 or FeAsS, which decreases arsenic availability (Newman et al. 1997). The increased Ca accumulation in As stressed condition suggests: (i) its crucial role in free radicals scavenging and (ii) facilitates the complexation of As with Cys-rich proteins and metallothionein (Song et al. 2004).
For the removal of excess level of H2O2 and to mitigate and repair the damage caused by H2O2, a major pathway i.e. AsA-GSH cycle consisting a network of specific enzymes: APX, DHAR and GR and metabolites: ascorbate and glutathione, efficiently operates in chloroplasts. During the present investigation, a noteworthy increase in APX activity was observed in test seedlings exposed to toxic levels of As (Fig. 2). Further, on S and Ca application to stressed seedlings, a sharp rise in APX activity was noticed thereby indicating high efficiency of APX for H2O2 detoxification as it is also evident from decreased level of MDA equivalents content (Table 1). Our results are in harmony with Dixit et al. (2015), where an increase in APX activity after S treatment to As-stressed rice plants have been found. This increment in APX activity might have occurred due to: (i) improvement in AsA and GSH contents (Tables 2 and 3) and (ii) alteration in important enzymes involved in synthesis of amino acids and thiol metabolism (Fig. 3) (Dixit et al. 2015).
Further, APX activity oxidises AsA into MDHA, which rapidly disproportionates into AsA and DHA. This DHA is reduced/ recycled into AsA by the activity of DHAR enzyme in the presence of an electron donor GSH. In the present study, DHAR activity was found to increase under As1 stress while it was decreased under As2 stress (Fig. 2). The decrease in DHAR activity justifies the inadequate regeneration of AsA from DHA under As2 treatment. Hossain and Asada (1984) have reported that under high H2O2 level, DHAR enzyme is very prone to its structural changes; therefore, increased H2O2 level could be a reason for decreased DHAR activity under As2 treatment that might have altered the rate of AsA-GSH cycle; thereby disturbing the redox status of the cell, i.e. decreased AsA/DHA ratio (Table 2). Contrastingly, S and Ca application to As treated test plants improved the level of AsA by keeping higher activity of DHAR enzyme and obviously a higher AsA/DHA ratio was noticed (Table 2). From these findings it can be speculated that S and Ca application either individually or in combination to As stressed Brassica seedlings have effective role in AsA recycling and greater turnover rate of AsA-GSH cycle. Greater AsA recycling accounts more H2O2 detoxification so, increased AsA/H2O2 ratio was observed (Table 2), as was also suggested by Markovska et al. (2009) in earlier finding on Cd stressed B. juncea seedlings. Glutathione (reduced form; GSH) serves as redox buffering agent in protoplast, and indicates the redox status of cell and the degree of oxidative stress experienced (Noctor et al. 2012). As discussed above, that DHA is reduced to AsA at the expense of two molecules of GSH and oxidized to glutathione disulphide (GSSG), which in turn is re-reduced to GSH by NADPH, a reaction catalyzed by GR enzyme (Foyer and Noctor 2005). In the present study, an observable decrease in GSH content and consequent increase in GSSG content was found under both the tested doses of As (As1 and As2), leading to a sharp decline in GSH/GSSG ratio (Table 3). The decrease in GSH content upon As exposure have also been reported in Allium sativum L. seedlings (Ruíz-Torres et al. 2017), which possibly could be due to: (i) its involvement in the PCs (a metal chelator protein) synthesis (Yadav 2010; Ruíz-Torres et al. 2017), (ii) utilization as substrate for GST enzyme, which is directly involved in the removal of endogenous xenobiotics (Marrs 1996), (iii) major reservoir of reduced non-proteinaceous S and (iv) involvement in the membrane protection by maintaining α-tocopherol and zeaxanthin in reduced form (Ruíz-Torres et al. 2017). The increase in GSSG content under As stress might be due to: (i) decline in the rate of GSH recycling and (ii) increase in degradation rate of GSH during stress, as DHA to AsA conversion also needs excessive utilization of GSH (Foyer and Noctor 2005).
Glutathione reductase is an important enzyme involved in the maintenance of GSH pool in AsA-GSH cycle (Foyer and Noctor 2005). It is directly linked with the reduction of GSSG into GSH that’s why any alteration in the GR activity may alters the GSH/GSSG ratio, which is very important in maintaining the redox status of the cell (Dixit et al. 2016; Singh et al. 2016). In the present study, the activity of GR enzyme was found to be improved under As1 stress whereas decreased under As2 stress (Fig. 2). Although, there was increase in GR activity but it was not enough to cope up with the massive GSH utilization [directs metalloid-GSH binding, GSH oxidation, glutathione-S-transferases (GST) and PCs synthesis (As-PCs complex)] for metalloid detoxification (Jozefczak et al. 2012). On the other hand, S and Ca application either alone or in combination to As-stressed Brassica seedlings, further increased the GR activity over the As-stressed seedlings alone, hence keeping GSH level high and consequently improved GSH/GSSG ratio (Fig. 2; Table 3). The results further reveal that addition of S and Ca to As-stressed Brassica seedlings significantly improved the levels of GSH pool, which is in harmony with the earlier findings of Liang et al. (2016) on Cd treated Brassica chinensis L. seedlings. The possibility behind this increment in GSH content could be as GSH plays major role in protecting cells from arsenic either by directly conjugating with the electrophilic arsenic or by providing substrate for the synthesis of PCs (Dixit et al. 2015; Liang et al. 2016), that indirectly will also quench/scavenge the xenobionts. Similarly, Ca induced GSH accumulation have also been reported by Tian et al. (2011) in Cd stressed Sedum alfredii H. The native-PAGE isoenzyme separation for GR, APX and DHAR and detected single, three and five bands, respectively in all the treatments (Fig. 2b), but were greatly expressed under As and S+Ca treatment, which are in conformity with the biochemical findings of the present study.
To survive in a metal(loid) rich environment, a wide range of adaptive mechanisms operate in the cell including sulphate assimilation. Being the final product of assimilatory sulfate reduction; Cys is a source of reduced S and plays crucial role in the biosynthesis of a number of S-containing compounds including GSH and PCs (Anderson 2014). Apart from Cys, NPTs are also known to be involved in the internal detoxification of metal(loids) through PCs formation (Table 4). In the present study, Cys, NPTs and PCs were found to be increased particularly in roots followed by leaves under tested doses of As and further a sharp rise in these contents was noticed following S and Ca application (Table 4), which could be to promote the compartmentalization of As in roots ensuring lesser load (translocation, as reported in earlier finding, Singh et al. 2018a) to the shoots of the test seedlings. The possible explanation for the increment of Cys might be: (i) its involvement in di-sulfide bonding in proteins for their proper functioning as well as for protein synthesis (Rochaix 2011), (ii) its crucial role for the formation of Fe–S cluster in the photosynthetic apparatus and electron transport chain (Rochaix 2011) and (iii) it serves as precursor for a range of S-containing defense compounds such as GSH and PCs (Liang et al. 2016). A further rise in Cys content following S and Ca treatment to As-stressed seedlings suggests that they might have induced the activities of key enzymes of S-assimilation pathway (Fig. 3) (Dhankher et al. 2002).
Phytochelatins are the oligomer of GSH, act as chelator and functions as first fence against As and reduces the detrimental oxidative effects of As on plants (Ruíz-Torres et al. 2017). Under As toxicity, on one side high PCs synthesis demands higher GSH, and on the other side As accumulation induces NPTs to chelate cellular As using most of the available GSH pool therefore; GSH content got lowered under As stress in both root and leaves of test seedlings (Tables 2 and 3). Similar findings regarding the role of Ca in metal detoxification mechanisms have also been reported by Lu et al. (2017), where Ca induced the NPTs accumulation by decreasing the GSH levels in Amaranthus hypochondriacus L. seedlings.
Glutathione (GSH) biosynthesis mainly depends on the rate of S-assimilation. According to Phartiyal et al. (2006), the first enzyme i.e. ATP synthase of S-assimilation pathway involves the formation of adenosine phosphosulfate; while OASTL catalyzes the last step of S-assimilation pathway and resulted into the formation of Cys. In the present study, ATPS and OASTL activities were sharply raised in As stressed test plants, which were more pronounced in root than that of leaves and the activities of these enzymes were further intensified following S and Ca supplementation to As stressed Brassica seedlings (Fig. 3). These finding can be explained on the basis that S and/ or Ca interaction with As might have prompted the assimilation of sulphate in order to fulfill the increasing demand of Cys under As stress, which is necessary for maintaining the pool of GSH, PCs and methionine because Cys acts as a precursor in the synthesis of these compounds as suggested by Khan et al. (2009). Our results are in harmony with the earlier findings on heavy metal stressed B. chinensis L. (Liang et al. 2016) and S. melongena L. (Singh et al. 2017) seedlings. The γ-ECS is a rate-limiting enzyme of GSH synthesis as the activity is regulated by GSH and dependent on Cys availability (feedback-inhibition mechanism). The activity of γ-ECS enzyme was increased with increasing doses of As, which could be linked with the increasing demand for the sulfhydryl group requiring compounds as well as increased utilization of Cys and GSH; and upon S and Ca application, γ-ECS activity was further raised in both root and leaves thereby signifying the enhancement of GSH and PCs synthesis that might have relaxed the plant from being stressed (Fig. 3; Tables 3 and 4). Indeed, Dixit et al. (2015) have also supported this fact in As stressed O. sativa and explained that higher activity of γ-ECS catalyses the ATP-dependent ligation of Cys and GSH to form γ-EC.
Supplementary Table 5 shows correlation between arsenic and mitigating effect of mineral nutrients on growth behavior of B. juncea L. seedlings. Growth, AsA, AsA/DHA, GSH, GSH/GSSG were found to be negatively correlated with arsenic stress while H2O2, APX, DHAR, GR, ATPS, OASTL and γ-ECS were found to be positively correlated. Upon exogenous supplementation of mineral nutrients either alone (S or Ca) or in combination (S+Ca), growth and important growth regulating processes (AsA, GSH and their ratios, APX, DHAR and GR) were found to be positively correlated while Pearson correlation values for H2O2 was found to be lesser in comparison to As. Moreover, upon application of these mineral nutrients to As stressed seedlings further values for all the studied parameters were found to be less negatively correlated in comparison to As stress alone.
Conclusion
The overall study concluded that, arsenic has severe damaging effect on growth of Indian mustard (B. juncea L.) seedlings thereby increasing the oxidative stress biomarkers, and the antioxidant defense system of test plant was inefficient to counteract the As induced oxidative damage, which disturbed the redox status of the cell as manifested by decreased AsA/DHA, AsA/H2O2 and GSH/GSSG ratios. By contrast, exogenously applied S, Ca and their combined exposure i.e. S+Ca, alleviated As-induced toxicity and improved the AsA and GSH contents thereby enhancing the activities of enzyme participating in AsA-GSH cycle and S-assimilation as well as maintain the redox status of the cell as evident by the increased Cys, NPTs and PCs contents. Present study mainly focuses on the ameliorating role of S and Ca simultaneously, that provided significant protection against As induced toxicity suggesting that it could be used as a promising tool for the farmers in managing As-toxicity and perhaps other heavy metals, in future.
References
Ahmad P, Abdel Latef AA, AbdAllah EF, Hashem A, Sarwat M, Anjum NA, Gucel S (2016) Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicer arietinum L.). Front Plant Sci 7:513
Ahsan N, Lee DG, Kim KH, Alam I, Lee SH, Lee KW, Lee H, Lee BH (2010) Analysis of arsenic stress-induced differentially expressed proteins in rice leaves by two-dimensional gel electrophoresis coupled with mass spectrometry. Chemosphere 78:224–231
Allen SE, Grimshaw HM, Rowland AP (1986) Chemical analysis. In: Moore PD, Chapman SB (eds) Methods in plant ecology. Blackwell, Oxford, pp 285–344
Anderson JW (2014) Assimilation of inorganic sulfate into cysteine. Biochem Plants 5:203–223
Brehe JE, Burch HB (1976) Enzymatic assay for glutathione. Anal Biochem 74:189–197
Bundschuh J, Bhattacharya P, Sracek O, Mellano MF, Ramírez AE, Storniolo AR, Martín RA, Cortés J, Litter MI, Jean JS (2011) Arsenic removal from groundwater of the Chaco-Pampean plain (Argentina) using natural geological materials as adsorbents. J Environ Sci Health 46:1297–1310
Capaldi R, Gratão PL, Reis AR, Lima LW, Azevedo RA (2015) Sulfur metabolism and stress defense responses in plants. Tropical Plant Biol 8:60–73
De Tullio MC, Paciolla C, DallaVecchia F, Rascio N, D’Emerico S, De Gara L, Liso R, Arrigoni O (1999) Changes in onion root development induced by the inhibition of peptidyl-proyl hydroxylase and influence of the ascorbate system on cell division and elongation. Planta 209:424–434
Dhankher OP, Li Y, Rosen BP, Shi J, Salt D, Senecoff JF, Meagher RB (2002) Engineering tolerance and hyperaccumulation of arsenic in plants by combining arsenate reductase and γ-glutamylcysteine synthetase expression. Nat Biotechnol 20:1140–1145
Dixit G, Singh AP, Kumar A, Dwivedi S, Deeba F, Kumar S, Suman S, Adhikari B, Shukla Y, Trivedi PK, Pandey V, Tripathi RD (2015) Sulfur alleviates arsenic toxicity by reducing its accumulation and modulating proteome, amino acids and thiol metabolism in rice leaves. Sci Rep 5:16205
Dixit G, Singh AP, Kumar A, Mishra S, Dwivedi S, Kumar S, Trivedi PK, Pandey V, Tripathi RD (2016) Reduced arsenic accumulation in rice (Oryza sativa L.) shoot involves sulfur mediated improved thiol metabolism, antioxidant system and altered arsenic transporters. Plant Physiol Biochem 99:86–96
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82:70–77
Food and Agricultural Organization [FAO] (2009) How to Feed the World in 2050. Food and Agriculture Organization, Rome
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Gaitonde MK (1967) A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J 104:627–633
Gossett DR, Millhollon EP, Cran LM (1994) Antioxidant response to NaCl stress in salt-sensitive cultivars of cotton. Crop Sci 34:706–714
Grant CA, Mahli SS, Karamanos RE (2012) Sulfur management for rapeseed. Field Crops Res 128:119–128
Gupta M, Ahmad MA (2014) Arsenate induced differential response in rice genotypes. Ecotoxicol Environ Saf 107:46–54
Hartley-Whitaker J, Ainsworth G, Vooijs R, Bookum WT, Schat H, Meharg AA (2001) Phytochelatins are involved in differential arsenate tolerance in Holcus lanatus. Plant Physiol 126:299–306
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hernandez JA, Almansa MS (2002) Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves. Physiol Plant 115:251–257
Hoagland DR, Arnon DI (1950) The water culture method for growing plants without soil. Calif Agric Exp Stn Circ 347:1–39
Hossain MA, Asada K (1984) Inactivation of ascorbate peroxidase in spinach chloroplasts on dark addition of hydrogen peroxide: its protection by ascorbate. Plant Cell Physiol 25:1285–1295
Huang J, Zhang J, Yu YM, Liu JX, Li MJ, Zhu KY (2012) Effects of calcium fertilizer on the development, survival, and feeding of B-biotype Bemisia tabaci on Euphorbia pulcherrima. J Appl Ecol 23:2521–2528
Jozefczak M, Remans T, Vangronsveld J, Cuypers A (2012) Glutathione is a key player in metal induced oxidative stress defenses. Int J Mol Sci 13:3145–3175
Kader MA, Lindberg S (2010) Cytosolic calcium and pH signaling in plants under salinity stress. Plant Signal Behav 5:233–238
Khan NA, Nazar R, Anjum NA (2009) Growth, photosynthesis and antioxidant metabolism in mustard (Brassica juncea L.) cultivars differing in ATP-sulfurylase activity under salinity stress. Sci Hort 122:455–460
Kirkegaard JA, Hocking PJ, Angus JF, Howe GN, Gardner PA (1997) Comparison of canola, Indian mustard and Linola in two contrasting environments. II. Break-crop and nitrogen effects on subsequent wheat crops. Field Crops Res 52:179–191
Lappartient AG, Touraine B (1996) Demand-driven control of root ATP sulfurylase activity and SO42־ uptake in intact canola. The role of phloem translocated glutathione. Plant Physiol 111:147–157
Liang T, Ding H, Wang G, Kang J, Pang H, Lv J (2016) Sulfur decreases cadmium translocation and enhances cadmium tolerance by promoting sulphur assimilation and glutathione metabolism in Brassica chinensis L. Ecotoxicol Environ Saf 124:129–137
Lu H, Li Z, Wu J, Shen Y, Li Y, Zou B, Tang Y, Zhuang P (2017) Influences of calcium silicate on chemical forms and subcellular distribution of cadmium in Amaranthus hypochondriacus L. Sci Rep 7:40583
Markovska YK, Gorinova NI, Nedkovska MP, Miteva KM (2009) Cadmium-induced oxidative damage and antioxidant responses in Brassica juncea plants. Biol Plant 53:151–154
Marrs KA (1996) The functions and regulation of glutathione-S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol 47:127–158
McCarty KM, Hanh HT, Kim KW (2011) Arsenic geochemistry and human health in South-East Asia. Rev Environ Health 26:71–78
McNeill A, Eriksen J, Bergström L, Smith K, Marstorp H, Kirchmann H, Nilsson I (2005) Nitrogen and sulphur management: challenges for organic sources in temperate agricultural systems. Soil Use Manag 21:82–93
Mittler R, Zilinskas BA (1993) Detection of ascorbate peroxidase activity in native gels by inhibition of the ascorbate-dependent reduction of nitrobluetetrazolium. Anal Biochem 212:540–546
Nagalakshmi N, Prasad MNV (2001) Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci 160:291–299
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate–specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Newman DK, Beveridge TJ, Morel F (1997) Precipitation of arsenic trisulfide by Desulfotomaculum auripigmentum. Appl Environ Microbiol 63:2022–2028
Noctor G, Mhamdi A, Chaouch S, Han Y, Neukermans J, Marquez-Garcia B, Queval G, Foyer CH (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35:454–484
Phartiyal P, Kim WS, Cahoon RE, Jez JM, Krishnan HB (2006) Soybean ATP sulfurylase, a homodimeric enzyme involved in sulfur assimilation, is abundantly expressed in roots and induced by cold treatment. Arch Biochem Biophys 450:20–29
Rahman H, Xu YP, Zhang XR, Cai XZ (2016) Brassica napus genome possesses extraordinary high number of CAMTA genes and CAMTA3 contributes to PAMP triggered immunity and resistance to Sclerotinia sclerotiorum. Front Plant Sci 7:581
Rausch T, Wachter A (2005) Sulfur metabolism: a versatile platform for launching defence operations. Trends Plant Sci 10:503–509
Riemenschneider A, Riedel K, Hoefgen R, Papenbrock J, Hesse H (2005) Impact of reduced acetylserine (thiol) lyase isoform contents on potato plant metabolism. Plant Physiol 137:892–900
Rochaix JD (2011) Assembly of the photosynthetic apparatus. Plant Physiol 155:1493–1500
Ruíz-Torres C, Feriche-Linares R, Rodríguez-Ruíz M, Palma JM, Corpas FJ (2017) Arsenic induced stress activates sulfur metabolism in different organs of garlic (Allium sativum L.) plants accompanied by a general decline of the NADPH generating systems in roots. J Plant Physiol 211:27–35
Saito K, Kurosawa M, Tatsuguchi K, Takagi Y, Murakoshi I (1994) Modulation of cysteine biosynthesis in chloroplasts of transgenic tobacco over expressing cysteine synthase [O-acetylserine (thiol)-lyase]. Plant Physiol 106:887–895
Schadle M, Bassham JA (1977) Chloroplast glutathione reductase. Plant Physiol 59:1011–1012
Sebastian A, Prasad MNV (2014) Photosynthesis mediated decrease in cadmium translocation protect shoot growth of Oryza sativa seedlings up on ammonium phosphate-sulfur fertilization. Environ Sci Pollut Res 21:986–997
Sharma I (2012) Arsenic induced oxidative stress in plants. Biologia 67:447–453
Singh M, Kushwaha BK, Singh S, Kumar V, Singh VP, Prasad SM (2017) Sulphur alters chromium (VI) toxicity in Solanum melongena seedlings: role of sulphur assimilation and sulphur containing antioxidants. Plant Physiol Biochem 112:183–192
Singh M, Singh VP, Prasad SM (2016) Responses of photosynthesis, nitrogen and proline metabolism to salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physiol Biochem 109:72–83
Singh R, Parihar P, Prasad SM (2018a) Simultaneous exposure of sulphur and calcium hinder As toxicity: up-regulation of growth, mineral nutrients uptake and antioxidants system. Ecotoxicol Environ Saf 161:318–331
Singh R, Parihar P, Prasad SM (2018b) Sulfur and calcium simultaneously regulate photosynthetic performance and nitrogen metabolism status in As-challenged Brassica juncea L. seedlings. Front Plant Sci 9:772
Singh R, Singh S, Parihar P, Singh VP, Prasad SM (2015) Arsenic contamination, consequences and remediation techniques: a review. Ecotoxicol Environ Saf 112:247–270
Song WY, Martinoia E, Lee J, Kim D, Kim DY, Vogt E, Shim D, Choi KS, Hwang I, Lee Y (2004) A novel family of cys-rich membrane proteins mediates cadmium resistance in Arabidopsis. Plant Physiol 135:1027–1039
Tan W, Meng Q, Brestic M, Olsovska K, Yang X (2011) Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J Plant Physiol 168:2063–2071
Tian S, Lu L, Zhang J, Wang K, Brown P, He Z, Liang J, Yang X (2011) Calcium protects roots of Sedum alfredii H. against cadmium-induced oxidative stress. Chemosphere 84:63–69
Tommasi F, Paciolla C, de Pinto MC, De Gara L (2001) A comparative study of glutathione and ascorbate metabolism during germination of Pinus pinea L. seeds. J Exp Bot 52:1647–1654
Tuli R, Chakrabarty D, Trivedi PK, Tripathi RD (2010) Recent advances in arsenic accumulation and metabolism in rice. Mol Breed 26:307–323
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant system in acid rain treated bean plants. Plant Sci 151:59–66
Xin H, Falk CK, Yu P (2013) Studies on Brassica carinata seed. 2. Carbohydrate molecular structure in relation to carbohydrate chemical profile, energy values, and biodegradation characteristics. J Agric Food Chem 61:10127–10134
Xu C, Li X, Zhang L (2013) The effect of calcium chloride on growth, photosynthesis, and antioxidant responses of Zoysia japonica under drought conditions. PLoS ONE 8:e68214
Yadav S (2010) Heavy metals toxicity in plants: an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S Afr J Bot 76:167–179
Ye B, Gitler C, Gressel J (1997) A High-sensitivity, single-gel, polyacrylamide gel electrophoresis method for the quantitative determination of glutathione reductases. Anal Biochem 246:159–165
Acknowledgements
Rachana Singh and Parul Parihar are very grateful to the University Grants Commission, New Delhi for providing financial support (as UGC-AU research scholar) to carry out present work. Authors are also thankful to DST-FIST programme funded by Department of Science and Technology, New Delhi, India to Department of Botany, University of Allahabad, Allahabad for providing necessary facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, R., Parihar, P. & Prasad, S.M. Sulphur and calcium attenuate arsenic toxicity inBrassicaby adjusting ascorbate–glutathione cycle and sulphur metabolism. Plant Growth Regul 91, 221–235 (2020). https://doi.org/10.1007/s10725-020-00601-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-020-00601-8