Abstract
Abscisic acid (ABA) regulates much important plant physiological and biochemical processes and induces tolerance to different stresses. Here, we studied the regulation of exogenous ABA on adaptation of rice seedlings to simulated acid rain (SAR) stress by measuring biomass dry weight, stomatal conductance, net photosynthesis rate, nutrient elements, and endogenous hormones. The application of 10 μM ABA alleviated the SAR-induced inhibition on growth, stomatal conductance, net photosynthesis rate, and decreases in contents of nutrient (K, Mg, N, and P) and hormone (auxin, gibberellins, and zeatin). Moreover, 10 μM ABA could stimulate the Ca content as signaling molecules under SAR stress. Contrarily, the application of 100 μM ABA aggravated the SAR-induced inhibition on growth, stomatal conductance, net photosynthesis rate, and contents of nutrient and hormone. The results got after a 5-day recovery (without SAR) show that exogenous 10 μM ABA can promote self-restoration process in rice whereas 100 μM ABA hindered the restoration by increasing deficiency of nutrients and disturbing the balance of hormones. These results confirmed that exogenous ABA at proper concentration could enhance the tolerance of rice to SAR stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abscisic acid (ABA) is one of the hormones in plants, playing roles in stress perception and response pathways as a messenger (Cao et al. 2014, Wang et al. 2013a). ABA is involved in many plant physiological processes such as seed maturation, seed dormancy, growth, and developmental regulation, and its role in adaptive responses to abiotic stresses is well established as plant stress hormone (Apel and Hirt 2004, Wasilewska et al. 2008). In addition, ABA plays an important role in plant response to abiotic stresses either as a result of its endogenous concentration or through exogenous application (Haisel et al. 2006, Wang et al. 2007). The application of exogenous ABA can enhance tolerance in plants to extreme temperatures, heavy metals, drought, and salinity by increasing contents of proline and soluble sugar, enhancing water retention, reducing membrane lipid peroxidation, and maintaining membrane integrity and photosynthetic characteristics (Han et al. 2016, Kaur et al. 2015, Li et al. 2014b, Liu et al. 2013, Minardi et al. 2014, Pattanagul 2011, Zheng et al. 2015).
Acid rain, a serious global environmental issue, exerts deleterious effects on the phenotype and physiological characteristics of plants by destructing chloroplast ultrastucture, blocking the synthesis of chlorophyll and decreasing photosynthesis, disturbing cation homeostasis, and accelerating the accumulation of reactive oxygen species (Kováčik et al. 2011, Liang and Wang 2013, Liang et al. 2005, Singh and Agrawal 2008, Sun et al. 2013, Wang et al. 2013b, Zhou and Huang 2002). In addition, acid rain accelerated the leaching of nutrients (Ca, Mg, K, and Na) from leaves which leads to the abnormal or restricted growth (Wang et al. 2014a). In response to acid rain stress, plants have complex sensing and signaling mechanisms to regulate the ion homeostasis. For example, hormones participate in the control of these regulatory networks to improve the transportation and distribution of nutrient (Rubio et al. 2009). ABA increases the vacuole Na+ content but inhibit the xylem transport and the plasma membrane influx in barley roots (Behl and Jeschke 1981). Chen et al. (2001) also found that the increase in Ca2+ uptake may be associated with the increase in ABA to regulate uptake and transport of salt ions (Na+, Cl−) under high levels of external salinity. However, few studies have considered the role of hormones (ABA in particular) in plant response to acid rain stress (Qiu et al. 2004, Wu and Liang 2016), and no evidence is provided for confirming how the application of exogenous ABA affect the tolerance of plants to acid rain stress. Based on the information, finding feasible ways for eliminating the negative effects of acid rain on plants will be possible, and it is meaningful for us to face the challenge of food security.
Therefore, our study is aimed at (1) revealing the tolerance in rice seedlings to simulated acid rain (SAR) with or without exogenous ABA and (2) exploring the role of exogenous ABA in plant tolerance to SAR stress by regulating nutrient contents and endogenous hormone. To achieve these, we studied the effect of exogenous ABA on biomass dry weight, stomatal conductance, net photosynthesis rate, contents of nutrient, and endogenous hormone in rice seedlings exposed to SAR during exposure and recovery periods. These results will provide basic information for alleviating the damage to plants caused by acid rain.
Materials and methods
Plant material and growth conditions
Rice (Oryza sativa) seeds “Huaidao 8” were supplied by Xishan Seed Company (Wuxi, China). The seeds were surface disinfected with HgCl2 (0.1%, w/v) for 10 min and washed three times with deionized water. After soaking in distilled water for 12 h, the seeds were placed in a dish (90 mm) underlaid with three layers of filter paper and germinated in an incubator at 25 ± 1 °C. After 2 days, germinated rice seeds were cultured in plastic box (6.88 L) filled with vermiculite for 25 days. When two leaves appeared, rice seedlings were cultivated in routine nutrition solution (pH 5.5) prepared by the method provided by the International Rice Research Institute (Zhu et al. 2009) in a growth chamber with irradiance of 300 μmol m−2 s−1 photosynthetically active radiation (400 to 700 nm) at 25 °C and 70% relative humidity during day time and at 20 °C and 80% relative humanity at night.
Simulated acid rain and exogenous ABA treatment
The solution of simulated acid rain (Yang et al. 2014) at pH values of 4.5 and 3.5 was prepared by adjusting the pH of the control rain with the addition of concentrated H2SO4 and HNO3 at a ratio of 3:1 (v/v, by chemical equivalents) (Chen et al. 2010). Simulated acid rain solution (pH 4.5 and 3.5) was sprayed at 24-h intervals on the leaves of rice seedlings till drops began to fall. In our preliminary experiments (data not shown in the manuscript), we studied the effect of different concentrations of exogenous ABA (0.1, 1, 10 and 100 μM) on growth of rice seedlings. We found that 10 μM ABA could promote obviously the growth of rice seedlings whereas the application of 100 μM ABA inhibited the growth of rice seedlings. Based on the results, we used the exogenous ABA at the concentration of 10 and 100 to study effects of the combination ABA and SAR on growth and physiology in rice seedlings in this paper. At the seedling stage (the fourth leaf of rice developed completely), the rice seedlings were separated to nine groups, and treated as described in Table 1. (1) For the control group, rice seedlings were cultured with the nutrition solution at pH 5.5 and sprayed with distilled water (pH 7.0) on leaves. (2) For the single SAR group, rice seedlings were cultured with the nutrition solution at pH 4.5 or 3.5 and sprayed with SAR at pH 4.5 or 3.5 on leaves. (3) For the single exogenous ABA group, rice seedlings were cultured with the nutrition solution at pH 5.5 and sprayed with exogenous ABA at different concentrations (10 or 100 μM) on leaves. (4) For the combined treatment of SAR and exogenous ABA groups, rice seedlings were cultured with the nutrition solution at pH 4.5 or 3.5, sprayed with SAR at pH 4.5 or 3.5 on the leaves in the morning and then sprayed with exogenous ABA at different concentrations (10 and 100 μM) on the leaves in the afternoon. The application of exogenous ABA by leaf spray was better because the addition of exogenous ABA in the nutrition solution could be influenced by the pH of the nutrition solution adjusted by acid rain. The nutrition solution was renewed every day to stabilize pH. All of the treatments were performed in triplicates. For each replication, three rice seedlings were analyzed. After the treatment with SAR and exogenous ABA for 5 days, half of the rice seedlings were collected to determine the indices. The rest of the rice seedlings were moved to be cultured under the control conditions for another 5 days and then were collected for analysis.
Biomass dry weight determination
Fresh rice seedlings were collected and washed three times with distilled water, and the water on the leaf surface was absorbed by filter paper. Biomass dry weight was determined after at 60 °C for 24 h (Rusak et al. 2009).
Determination of gas exchange parameters
Stomatal conductance (G s) and net photosynthesis rate (P n) were measured with a portable gas exchange system (CIRAS-1, PP Systems International Ltd., UK) under the cultured condition of rice seedling at 25 ± 2 °C, CO2 concentration 340 μmol mol−1 and a photosynthetically active photon flux density of 500 μmol·m−2 s−1 (Liang et al. 2011).
Determination of mineral element content
Fresh plants were collected, cleaned, and washed three times with distilled water. The leaves were dried in an oven and crushed into pieces of 1 mm in diameter. For each sample, 0.1 g crushed leaves was then digested in 8 mL oxidizing solution (15 M HNO3 and 9 M H2O2, v/v) for 30 min at 2600 kPa (80 psi) in a MDS-2000 microwave oven (CEM Corp., Matthews, NC). The samples were diluted to a final volume of 25 mL with deionized water for further analysis (Wang et al. 2008). The contents of mineral elements (K, Ca, Mg, and P) in each sample were determined by AAS (atomic absorption spectroscopy, 3110, Perkin Elmer, Norwalk, CT, USA) (Hermans et al. 2010).
Nitrate content determination
Nitrate content was measured according to the method described by Miranda et al. (2001). Rice leaves were collected and washed three times with distilled water, and the water on the surface of the leaves was absorbed by filter paper. Fresh leaves (1 g) were put into graduated test tube with 20 mL deionized water. Test tubes blocked with plugs were put into boiling water for 30 min, and then cooled with flowing water. Extraction was filtered and diluted to 25 mL with deionized water, and then the extracting solution (0.1 mL) was transferred to test tubes with 0.4 mL 5% (w/v) salicylic acid–H2SO4 solution. The mixture was shaken for 20 min at ambient temperature. After adding 9.5 mL 8% NaOH, the mixture was shaken, cooling to room temperature. The absorbance was measured at 410 nm by a spectrophotometer (JINGHRI, Shanghai, China).
Hormone content determination
Hormone contents in plants were extracted according to the method provided by Hou et al. (2008). The leaves of rice were quickly preserved in liquid nitrogen and kept in an ultra-low temperature freezer (−80 °C). Approximately 3 g of tissue was weighed and homogenized in small volumes of pre-cooled 80% methanol with trace amount of sliver diethyldithiocarbamate. The samples were extracted with 4–5 mL pre-cooled 80% methanol for 12 h at 4 °C. After centrifugation at 20,000×g for 20 min at 4 °C, the supernatant was collected and the residue re-extracted with 4–5 mL of pre-cooled 80% methanol. Following a further 12 h at 4 °C, the extract was centrifuged and the supernatants pooled. This process of extraction with 4–5 mL of pre-cooled 80% methanol was three times, and finally, all the supernatants pooled. The supernatants were concentrated to placing in a 40 °C water bath with Rotary Evaporator (RE-100, Bibby Sterlin LTD. Stone Stafford-shire, England). The organic phase was treated with 0.2–0.3 g of polyvinylpolypyrrolidone. After centrifugation again at 20,000×g for 15 min at 4 °C, the supernatant was collected and evaporated to dryness using a vacuum freeze-drying equipment. The dried samples containing ABA, IAA (indol-3-ylacetic acid), GA3 (gibberellins), and ZT (zeatin) were re-dissolved with 10 mL methanol. Then, the endogenous hormone contents were determined using high-performance liquid chromatography (HPLC) according to Yang et al. (2014) with some modifications. The flow rate for all analyses was adjusted to 1 mL min−1. Twenty microliters of samples was injected for HPLC analysis, and the detection wavelength was 254 nm. HPLC analysis was performed with an HP100 (Agilent, Palo Alto, CA, USA) coupled with a Diode array Detector. An Agilent ZORBAX SB-C18 column (5 μm, 4.6 mm × 250 mm) was used in analysis and the column temperature was set at 35 °C. Mobile phases were 100% methanol (A) and 0.6% acetic acid (B). The gradient elution performed as follows: 0–10 min, A 90% and B 10%; 10–20 min, A 50% and B 50%; after 20 min, A 10% and B 90%.
Statistical analysis
The significant differences between the different treatments were analyzed by one-way analysis of variance (ANOVA) using SPSS16.0. The error analysis for the parameters also used SPSS 16.0. All the data are presented as means ± standard deviation. Student’s t tests were applied to determine the significance between different treatments (P < 0.05) (Ke et al. 2003).
Results
Effects of exogenous ABA on the growth of rice seedlings under SAR stress
Figure 1 shows the effect of exogenous ABA on the dry weight of the rice seedlings under SAR stress. After a 5-day exposure, SAR at pH 4.5 or 3.5 decreased dry weight by 24.5 and 38.9% compared with that of the control. A low concentration of ABA (10 μM) had no effect on dry weight whereas a high concentration of ABA (100 μM) decreased dry weight. The dry weight treated with the combination of SAR (pH 4.5 or 3.5) and 10 μM ABA was still lower than those of the control, but higher than those treated with the single SAR. The decrease in the dry weight treated with the combination of pH 3.5 SAR and 10 μM ABA was larger than those treated with the combination of pH 4.5 SAR and 10 μM ABA. However, the decrease in the dry weight of seedlings treated with the combination of SAR (pH 4.5 or 3.5) and 100 μM ABA was higher than those treated with the single SAR. After a 5-day recovery, the dry weight of leaves treated with the single SAR (pH 4.5 or 3.5) was still lower than those of the control. The dry weight of leaves treated with pH 4.5 SAR and 10 μM ABA was close to the control. The dry weight of leaves treated with pH 3.5 SAR and 10 μM ABA was higher than those treated with the single pH 3.5 SAR, although it was still lower than those of the control. However, the dry weight in rice seedlings treated with SAR (pH 4.5 or 3.5) and 100 μM ABA was even lower than those measured during the exposure period.
Effects of exogenous ABA on the G s and P n of rice seedlings under SAR stress
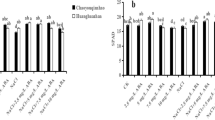
The G s and P n in rice leaves exposed to SAR and exogenous ABA were shown in Fig. 2. SAR at pH 4.5 or 3.5 decreased the G s and P n by 31.0/32.3% and 41.0/43.1% compared with those of the control. A low concentration of ABA (10 μM) had no obvious effect on the G s and P n whereas a high concentration of ABA (100 μM) caused decreases in the G s and P n. When rice seedlings were treated with the combination of SAR and 10 μM ABA, the G s and P n were lower than those of the control, but higher than those treated with the single SAR. In addition, the decrease in the G s and P n of leaves treated with the combination of pH 3.5 SAR and 10 μM ABA was larger than those treated with the combination of pH 4.5 SAR and 10 μM ABA. Contrarily, the decrease in the G s and P n of leaves treated with the combination of SAR and 100 μM ABA was higher than those treated with the single SAR. After a 5-day recovery, the G s and P n in leaves treated with the single SAR were still lower than those of the control. The G s and P n in leaves treated with pH 4.5 SAR and 10 μM ABA were recovered at the control level. Moreover, the G s and P n in leaves treated with pH 3.5 SAR and 10 μM ABA were higher than those treated with the single pH 3.5 SAR although they were lower than those of the control. However, the G s and P n of leaves treated with the combination of SAR and 100 μM ABA were even lower than those measured during the exposure period.
Effects of exogenous ABA on nutrient contents of rice seedlings under SAR stress
Table 2 shows changes in nutrient element contents (K, Ca, Mg, nitrate, and phosphorus) in rice leaves treated with SAR and exogenous ABA measured during exposure and recovery periods. SAR at pH 4.5 or pH 3.5 decreased contents of K, Ca, Mg, nitrate and phosphorus compared with those of the control. A low concentration of ABA (10 μM) alone decreased K content and increased Ca content, but had no effects on Mg, nitrate, and phosphorus contents. However, a high concentration of ABA (100 μM) decreased K, Mg, nitrate, and phosphorus contents and increased Ca content. When rice seedlings were treated with the combination of pH 4.5 SAR and 10 μM ABA, K and Mg contents were lower whereas Ca, nitrate, and phosphorus contents were higher compared with those of the control, and all were higher than those treated with pH 4.5 SAR. When rice seedlings were treated with the combination of pH 3.5 SAR and 10 μM ABA, contents of K, Mg, nitrate, and phosphorus in leaves were lower than those of the control whereas Ca content increased, and all of them were higher than those treated with the single pH 3.5 SAR. However, contents of K, Mg, nitrate and phosphorus in leaves treated with the combination of SAR and 100 μM ABA were lower than those treated with the single SAR, with the exception that Ca content in leaves was higher than those of the control. After a 5-day recovery, nutrient element contents in leaves treated with the single SAR were still lower than those of the control. Contents of K, Ca, Mg, nitrate, and phosphorus in leaves treated with pH 4.5 SAR and10 μM ABA were recovered at the control level, and better than those treated with pH 3.5 SAR and 10 μM ABA. Only the Ca content in leaves treated with pH 3.5 SAR and 10 μM ABA was higher than those of the control. However, contents of all nutrient elements in leaves treated with SAR and 100 μM ABA were still lower than those treated with the single SAR, and even lower than those measured during the exposure period.
Effects of exogenous ABA on endogenous hormone contents in rice seedlings under SAR stress
As shown in Table 3, SAR at pH 4.5 or 3.5 caused an increase in the ABA content and decreases in IAA, GA3, and ZT contents in rice seedlings compared with those of the control. A low concentration of ABA (10 μM) alone had no effects on contents of ABA, IAA, GA3, and ZT whereas a high concentration of ABA (100 μM) alone caused an increase in ABA content and a decrease in contents of IAA, GA3, and ZT in rice leaves compared with those of the control. When rice leaves were treated with the combination of SAR (pH 4.5 or 3.5) and 10 μM ABA, the ABA content in leaves was higher than those of the control, but lower than those treated with the single SAR, whereas contents of IAA, GA3, and ZT were lower than those of the control, but higher than those treated with the single SAR. Compared to the single SAR and the control, the ABA content in leaves treated with SAR and 100 μM ABA was the highest, whereas contents of IAA, GA3 and ZT in leaves were the lowest. After a 5-day recovery, the ABA content in leaves treated with pH 4.5 and 3.5 SAR was higher whereas contents of IAA, GA3 and ZT were lower compared to the control. Differing from changes in leaves treated with the single pH 4.5 SAR, contents of ABA, IAA, GA3, and ZT in leaves treated with pH 4.5 SAR and 10 μM ABA were recovered at the control level, better than those in leaves treated with pH 3.5 SAR and 10 μM ABA. However, the ABA content in leaves treated with SAR and 100 μM ABA was still higher than those of the control. In addition, contents of IAA, GA3, and ZT in leaves treated with the combination of SAR and 100 μM ABA were still lower than those treated with the single SAR and even lower than those measured during the exposure period.
Discussion
Effects of exogenous ABA on the growth of rice seedlings under SAR stress
Biomass dry weight of plants is a prominent indicator to reflect the growth under environmental stresses. SAR at pH 4.5 or 3.5 caused a decrease in the dry weight of rice seedlings (Fig. 1a). However, the dry weight in rice seedlings treated with the combination of SAR and 10 μM ABA was higher than those treated with the single SAR. And the dry weight treated with the combination of pH 4.5 SAR and 10 μM ABA were higher than those treated with the combination pH 3.5 SAR and 10 μM ABA. These results indicate that exogenous ABA at the concentration of 10 μM can alleviate the inhibition caused by SAR on plant growth, and the regulating effect depended on the acidity of SAR. A similar result was also reported by Khadri et al. (2006) who found that the inhibition on dry weight of common beans treated with 100 mM NaCl stress can be alleviated by supplying exogenous 10 μM ABA. However, the dry weight in rice seedlings treated with the combination of SAR and 100 μM ABA was obviously lower than those treated with the single SAR (Fig. 1a) indicates that the application of ABA at a higher concentration (100 μM) aggravated the SAR-induced inhibition on plant growth. After a 5-day recovery, the dry weight of the rice seedlings treated with the combination of pH 4.5 SAR and 10 μM ABA was recovered at the control levels. However, the decrease in the dry weight of the rice seedlings treated with SAR and 100 μM ABA was still lower than those treated with the single SAR, even lower than those measured during the exposure period. The phenomena indicate that the application of 10 μM ABA can promote the recovery of the rice seedling leaves following the withdrawal of SAR stress whereas the application of 100 μM ABA can hinder the recovery process.
Effects of exogenous ABA on the G s and P n of rice seedlings under SAR stress
Plant growth and development depend on synthesis and accumulation of organic substances that are produced by photosynthesis (Chia and He 1999, Li et al. 2014a). Therefore, the decrease in photosynthetic capacity of plants under environmental stresses is one of the key factors limiting plant growth (Kummerová et al. 2006). In the present work, we found that SAR at pH 4.5 or 3.5 caused inhibition on the G s and P n in leaves, and the decreased degree in rice treated with pH 4.5 SAR was lower (Fig. 2a, c). Wang et al. (2014b) also proved that stomatal conductance and photosynthetic rate were decreased with the increase in acidity of acid rain. With the application of 10 μM ABA, the G s and P n in leaves exposed to SAR were higher than those treated with the single SAR, and the alleviating effects of 10 μM ABA on the G s and P n under pH 4.5 SAR stress were better than those under pH 3.5 SAR stress. These results show that the application of 10 μM ABA can induce the stomatal opening to maintain CO2 supply and then alleviate the decrease in photosynthetic rate caused by SAR. Teng et al. (2014) also found that the application of exogenous ABA (60 μM) alleviates P n, G s, and transpiration rate in leaves under PEG stress and revealed that photosynthetic system II is repaired by the upregulation of genes related to chlorophyll and ABA biosynthesis. However, the G s and P n in leaves treated with the combination of SAR and 100 μM ABA were lower than those treated with the single SAR. It means that higher concentration of ABA aggravated the SAR-induced inhibition on the G s by inducing the stomatal closure and then a heavier decrease in photosynthesis, leading to more loss in the dry weight. Hejnák and Kykalová (2009) also reported that foliar spraying with 100 μM ABA accelerated chlorophyll degradation in maize seedlings under water deficit conditions. After a 5-day recovery, the G s and P n in leaves treated with pH 4.5 SAR and 10 μM ABA was recovered at the control levels, indicating that 10 μM ABA promoted the recovery of the G s and P n. However, the decrease in the G s and P n of leaves treated with SAR and 100 μM ABA was even lower than those measured during the exposure period, indicating that 100 μM ABA hindered the recovery process above.
Effects of exogenous ABA on nutrient contents of rice seedlings under SAR stress
Nutrient elements are necessary for plant growth and development, and the deficiency of nutrient elements in plants are used as an indicator for revealing the environmental stress-induced damage to plants (Klimenko and Klimenko 2001, Maathuis 2009). In our experiments, contents of K, Ca, Mg, nitrate, and phosphorus in leaves treated with the single SAR were decreased (Table 2), indicating that acid rain led to nutrient deficiency, and the degree of deficiency depended on the acidity of acid rain. The decrease in K, Ca, and Mg was also found in other plant species under SAR stress (Zhou et al. 2003). Compared with those treated with the single SAR, the decrease in contents of nutrient elements in leaves under SAR stress was alleviated by exogenous 10 μM ABA (Table 2), indicating that ABA was involved in regulating nutrient element contents in rice seedlings under SAR stress. Usually, acid rain inhibits photosynthesis in plants partly because the SAR-induced increase in extracellular H+ can replace the Mg2+ in Mg protoporphyrin IX, resulting in suppression of chlorophyll biosynthesis (Mohapatra and Tripathy 2007, Zhou and Huang 2002). Thus, the alleviating effects of 10 μM ABA on the decrease in Mg content in leaves under SAR stress can contribute to maintain photosynthetic capacity (Table 2) by alleviating SAR-induced inhibition on chlorophyll synthesis (Basak et al. 2012). In addition, the application of 10 μM ABA increased contents of nitrate and phosphorus in leaves treated with SAR to maintain protein or carbohydrate metabolisms, benefiting to accumulating dry matter in plants (Fig. 1). However, the application of 100 μM ABA aggravated the deleterious effects on nutrient element contents in leaves under SAR stress (Table 2). Although an increase in Ca is necessary for signal transduction, a continuous increase in Ca is lethal (Pan et al. 2008). The higher content of Ca could be one of the factors leading to the heavier decrease in growth of rice treated with SAR and 100 μM ABA. Hirschi (2004) also reported that a marked increase in Ca is harmful to cells because it leads to activation of particular Ca2+-dependent enzymes, which have a potentially adverse effect. Even in some mammalian cells, Ca overload can also cause mitochondrial failure; if this becomes irreversible, it leads to cell death (Duchen 2000). On the other hand, the higher decrease in contents of K, Mg, nitrate, and phosphorus in leaves treated with SAR and 100 μM ABA destroyed ion homeostasis in cells and nutritional balance in leaves and then caused a heavy decrease in the dry weight (Fig. 1). After a 5-day recovery, nutrient element contents in leaves treated with pH 4.5 SAR and 10 μM ABA were recovered at the control level whereas those in leaves treated with the single pH 4.5 SAR were still worse than those of the control. This suggests that application of 10 μM ABA could alleviate the decrease in nutrient elements in leaves induced by acid rain, and promote sel-restoration in rice seedlings during the recovery period. However, the decrease in nutrient element contents in leaves treated with SAR and 100 μM ABA was even worse than those measured during the exposure period. These phenomena indicate that the application of 100 μM ABA aggravated the SAR-induced nutrient deficiency, and hinder self-restoration in the rice seedling during the recovery period.
Effects of exogenous ABA on endogenous hormone contents of rice seedlings under SAR stress
ABA, IAA, GA3, and ZT are pivotal endogenous hormones regulating plant growth and development, and changes in their concentrations affect cell growth (Zhao et al. 2012). SAR at pH 4.5 or 3.5 elevated the ABA content in rice leaves, being consistent with the accumulation of ABA in plants under abiotic stresses usually. ABA, as a signal molecule, is involved in plant response to abiotic stresses such as water deficit and salinity (Ghanaatiyan and Sadeghi 2015, Milosavljević et al. 2012). In addition, the decrease in IAA, GA3, and ZT in leaves treated with the single pH 4.5 or 3.5 SAR (Table 3) also could be one of the factors inhibiting plant growth (Fig. 1) because IAA, GA3, and ZT can regulate plant growth by participating in cell division, expansion and differentiation (David and Machteld 2001, Rademacher 2000, Woodward and Bartel 2005). We found that the ABA content in leaves treated with the combination of SAR and 10 μM ABA was higher than those of the control, but lower than those treated with the single SAR. The increase in ABA in leaves could be induced by exogenous ABA, and be transported by phloem to roots to enhance root tolerance to acid rain stress (Ikegami et al. 2009), thus lowered ABA in leaves compared to the single SAR. In addition, Ca has been identified as a possible mediator of ABA-induced stimulus–response coupling (Netting 2000). Therefore, we inferred that exogenous ABA induced the Ca content in leaves to regulate the stomatal conductance (Table 2), which might lead to the decrease in the G s (Fig. 2a), prevent acid rain injury to the cell. Consequently, the application of exogenous ABA can regulate endogenous ABA in leaves to enhance rice tolerance to acid rain stress. Meanwhile, the alleviating effects of exogenous ABA on IAA, GA3, and ZT in leaves under SAR stress (Table 3) also contribute to enhance rice tolerance because the higher IAA can stimulate the acquirement of plants to nutrition and the translocation of nutrient elements by enhancing cell elongation and division (López et al. 2007). These findings indicate that the application of 10 μM ABA can trigger the signaling network in rice response to pH 4.5 or 3.5 SAR by regulating endogenous ABA and other phytohormones (IAA, GA3, and ZT) as well. When rice seedlings were treated with the combination of SAR and 100 μM ABA, the ABA content was increased whereas contents of IAA, GA3, and ZT were decreased compared with the single SAR (Table 3). The excess accumulation of endogenous ABA caused by the application of 100 μM ABA can induce the stomata closure (decrease in G s) (Fig. 2a) to block CO2 entering into cells (Li et al. 2015), thus the P n was decreased (Fig. 2c). The decrease in IAA, GA3, and ZT contents in leaves treated with SAR and 100 μM ABA inhibited cell growth and division (Fahad et al. 2015) and then led to the decrease in the dry weight of rice (Fig. 1). After a 5-day recovery, the ABA content in leaves treated with SAR and 10 μM ABA was lower than those treated with the single SAR whereas contents of IAA, GA3, and ZT were higher. In addition, endogenous hormones in leaves treated with pH 4.5 SAR and 10 μM ABA were recovered at the control level, indicating that 10 μM ABA can promote the self-restoration by regulating endogenous hormones in leaves during the recovery period, and the recovery degree was dependent on the acidity of acid rain. Contrarily, contents of IAA, GA3, and ZT in leaves treated with SAR and 100 μM ABA were still the lowest compared to the single SAR, and even lower than those measured during the exposure period. It indicates that the application of 100 μM ABA not only aggravated the SAR-induced inhibition on endogenous hormones in leaves, but also hindered the self-restoration of rice seedlings. Meanwhile, the higher increase in the ABA content in leaves treated with SAR and 100 μM ABA still induced the stomatal closure, causing the heavier decrease in the P n and dry weight (Fig. 2b, d) in rice seedlings.
Conclusions
SAR at pH 4.5 or 3.5 exerted deleterious effect on growth of rice by decreasing contents of nutrient element, IAA, GA3, and ZT, as well as increasing the ABA content in leaves. The application of 10 μM ABA can alleviate SAR-induced inhibition on growth by maintaining the G s and P n contents of nutrient elements (K, Mg, nitrate, and phosphorus) and the balance of endogenous hormones in leaves under SAR stress. In addition, the application of 10 μM ABA could stimulate the Ca content as signaling molecules under SAR stress. However, the application of 100 μM ABA aggravated the deleterious effect on growth of rice seedlings caused by SAR through increasing deficiency of nutrients and disturbing the balance of hormones. After a 5-day recovery, exogenous 10 μM ABA can promote self-restoration process in rice whereas 100 μM ABA can hinder the restoration. In addition, the more treatments (an intermediate treatment of ABA between 10 and 100 μM) could also be informative to reveal the improving effect of exogenous ABA on the tolerance of rice seedlings to acid rain stress.
These results could enrich our understanding on the role of exogenous ABA in regulating the adaptation of plants to acid rain and provide a new direction to find ways for alleviating the damage to plants cause by acid rain. Based on the information got under the laboratory conditions, the regulating effect of exogenous ABA on tolerance of plants to acid rain needs to be identified by different crop species in field experiments before its application in agriculture.
References
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Basak H, Demir K, Doganlar ZB (2012) The effect of abscisic acid application on root-shoot length and some antioxidant enzyme activities of two different tomato seedlings. J Anim Plant Sci 22:243–257
Behl R, Jeschke WD (1981) Influence of abscisic acid on unidirectional fluxes and intracellular compartmentation of K+ and Na+ in excised barley root segments. Physiol Plant 53:95–100
Cao X, Jia JB, Zhang C, Li H, Liu TX, Jiang XN, Polle A, Peng CH, Luo ZB (2014) Anatomical, physiological and transcriptional responses of two contrasting poplar genotypes to drought and re-watering. Physiol Plant 151:480–494
Chen SL, Li JK, Wang SS, Hüttermann A, Altman A (2001) Salt, nutrient uptake and transport, and ABA of Populus euphratica; a hybrid in response to increasing soil NaCl. Trees 15:186–194
Chen J, Li W, Gao F (2010) Biogeochemical effects of forest vegetation on acid precipitation-related water chemistry: a case study in Southwest China. J Environ Monit 12:1799–1806
Chia TF, He J (1999) Photosynthetic capacity in Oncidium (Orchidaceae) plants after virus eradication. Environ Exp Bot 42:11–16
David WM, Machteld CM (2001) Cytokinin metabolism and action. Annu Rev Plant Physiol Plant Mol Biol 52:89–118
Duchen MR (2000) Mitochondria and Ca2+ in cell physiology and pathophysiology. Cell Calcium 28:339–348
Fahad S, Hussain S, Matloob A, Khan FA, Khaliq A, Saud S, Hassan S, Shan D, Khan F, Ullah N, Faiq M, Khan MR, Tareen AK, Khan A, Ullah A, Ullah N, Huang JL (2015) Phytohormones and plant responses to salinity stress: a review. Plant Growth Regul 75:391–404
Ghanaatiyan K, Sadeghi H (2015) Divergences in hormonal and enzymatic antioxidant responses of two Chicory ecotypes to salt stress. Plant Signal Behav 34:199–207
Haisel D, Pospíšilová J, Synková H, Schnablová R, Baková P (2006) Effects of abscisic acid or benzyladenine on pigment contents, chlorophyll fluorescence, and chloroplast ultrastructure during water stress and after rehydration. Photosynthetica 44:606–614
Han YS, Wang SJ, Zhao N, Deng SR, Zhao CJ, Li NF, Sun J, Zhao R, Yi HL, Shen X, Chen SL (2016) Exogenous abscisic acid alleviates cadmium toxicity by restricting Cd2+ influx in Populus euphratica cells. J Plant Growth Regul 35:827–837
Hejnák V, Kykalová K (2009) The effect of abscisic acid and benzylaminopurine on production of dry matter and chlorophyll content in maize (Zea mays L.) under water stress. Cereal Res Commun 37:125–128
Hermans C, Vuylsteke M, Coppens F, Craciun A, Inzé D, Verbruggen N (2010) Early transcriptomic changes induced by magnesium deficiency in Arabidopsis thaliana reveal the alteration of circadian clock gene expression in roots and the triggering of abscisic acid-responsive genes. New Phytol 187:119–131
Hirschi KD (2004) The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol 136:2438–2442
Hou SJ, Zhu J, Ding MY, Lv GH (2008) Simultaneous determination of gibberellic acid, indole-3-acetic acid and abscisic acid in wheat extracts by solid-phase extraction and liquid chromatography-electrospray tandem mass spectrometry. Talanta 76:798–802
Ikegami K, Okamoto M, Seo M, Koshiba T (2009) Activation of abscisic acid biosynthesis in the leaves of Arabidopsis thaliana in response to water deficit. J Plant Res 122:235–243
Kaur S, Gupta AK, Zhawar VK (2015) ABA-dependent sucrose regulation of antioxidant metabolism in wheat cultivars varying in ABA-sensitivity. Biologia 70:165–173
Ke L, Wong TWY, Wong AHY, Wong YS, Tam NFY (2003) Negative effects of humic acid addition on phytoremediation of pyrene-contaminated sediments by mangrove seedlings. Chemosphere 52:1581–1591
Khadri M, Tejera NA, Lluch C (2006) Alleviation of salt stress in common bean (Phaseolus vulgaris) by exogenous abscisic acid supply. J Plant Growth Regul 25:110–119
Klimenko OE, Klimenko NI (2001) Nutrient concentrations in leaves of peach trees subjected to acid rain. Springer, Netherlands
Kováčik J, Klejdus B, Bačkor M, Štork F, Hedbavny J (2011) Physiological responses of root-less epiphytic plants to acid rain. Ecotoxicology 20:348–357
Kummerová M, Krulová J, Zezulka Š, Tříska J (2006) Evaluation of fluoranthene phytotoxicity in pea plants by Hill reaction and chlorophyll fluorescence. Chemosphere 65:489–496
Li H, Rao BQ, Wang GH, Shen S, Li DH, Hu CX, Liu YD (2014a) Spatial heterogeneity of cyanobacteria-inoculated sand dunes significantly influences artificial biological soil crusts in the Hopq Desert China. Environ Earth Sci 71:245–253
Li SW, Leng Y, Feng L, Zeng XY (2014b) Involvement of abscisic acid in regulating antioxidative defense systems and IAA-oxidase activity and improving adventitious rooting in mung bean [Vigna radiata (L.) Wilczek] seedlings under cadmium stress. Environ Sci Pollut Res 21:525–537
Li HD, Wang Y, Xiao J, Xu K (2015) Reduced photosynthetic dark reaction triggered by ABA application increases intercellular CO2 concentration, generates H2O2 and promotes closure of stomata in ginger leaves. Environ Exp Bot 113:11–17
Liang CJ, Wang WM (2013) Antioxidant response of soybean seedlings to joint stress of lanthanum and acid rain. Environ Sci Pollut Res 20:8182–8191
Liang CJ, Huang XH, Tao WY, Zhou Q (2005) Responses of antioxidant enzyme and photosynthesis in rape seedling to the combined stresses of acid rain and ultraviolet-B radiation. J Environ Sci 17:1038–1041
Liang CJ, Zhang GS, Zhou Q (2011) Effect of cerium on photosynthetic pigments and photochemical reaction activity in soybean seedling under ultraviolet-B radiation stress. Biol Trace Elem Res 142:796–806
Liu Y, Ji X, Zheng L, Nie X, Wang Y (2013) Microarray analysis of transcriptional responses to abscisic acid and salt stress in Arabidopsis thaliana. Int J Mol Sci 14:9979–9998
López ML, Peralta-Videa JR, Benitez T, Duarte-Gardea M, Gardea-Torresdey JL (2007) Effects of lead, EDTA, and IAA on nutrient uptake by alfalfa plants. J Plant Nutr 30:1247–1261
Maathuis FJ (2009) Physiological functions of mineral macronutrients. Curr Opin Plant Biol 12:250–258
Milosavljević A, Prokić L, Marjanovic M, Stikic R, Sabovljevic A (2012) The effects of drought on the expression of TAO1, NCED and EIL1 genes and ABA content in tomato wild-type and flacca mutant. Arch Biol Sci 64:297–306
Minardi BD, Voytena APL, Santos M, Randi ÁM (2014) Water stress and abscisic acid treatments induce the CAM pathway in the epiphytic fern Vittaria lineata (L.) Smith. Photosynthetica 52:404–412
Miranda KM, Espey MG, Wink DA (2001) A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5:62–71
Mohapatra A, Tripathy BC (2007) Differential distribution of chlorophyll biosynthetic intermediates in stroma, envelope and thylakoid membranes in Beta vulgaris. Photosynth Res 94:401–410
Netting AG (2000) pH, abscisic acid and the integration of metabolism in plants under stressed and non-stressed conditions: cellular responses to stress and their implication for plant water relations. J Exp Bot 51:147–158
Pan TF, Li YY, Zheng S, Ma CL, Qiu DL (2008) The activation and regulation of Ca-CaM signaling system on the activity of Ca2 + -ATPase and H + -ATPase in leaves of longan under stimulated acid rain stress. Dissertation, Shanghai
Pattanagul W (2011) Exogenous abscisic acid enhances sugar accumulation in rice (Oryza sativa L.) under drought stress. Asian J Plant Sci 10:212–219
Qiu DL, Liu XH, Guo SZ (2004) Effects of simulated acid rain on cellulase activity and contents of endogenous hormones. Chin J Appl Environ Biol 10:35–38
Rademacher W (2000) Growth retardants: effects on gibberellin biosynthesis and other metabolic pathways. Plant Biol 51:501–531
Rubio V, Bustos R, Irigoyen ML, Cardona-López X, Rojas-Triana M, Paz-Ares J (2009) Plant hormones and nutrient signaling. Plant Mol Biol 69:361–373
Rusak G, Piantanida I, Bretschneider S, Ludwig-Müller J (2009) Complex formation of quercetin with lanthanum enhances binding to plant viral satellite double stranded RNA. J Inorg Biochem 103:1597–1601
Singh A, Agrawal M (2008) Acid rain and its ecological consequences. J Environ Biol 29:15–24
Sun ZG, Wang LH, Zhou Q, Huang XH (2013) Effects and mechanisms of the combined pollution of lanthanum and acid rain on the root phenotype of soybean seedlings. Chemosphere 93:344–352
Teng KQ, Li JZ, Liu L, Han YC, Du YX, Zhang J, Sun HZ, Zhao QZ (2014) Exogenous ABA induces drought tolerance in upland rice: the role of chloroplast and ABA biosynthesis-related gene expression on photosystem II during PEG stress. Acta Physiol Plant 36:2219–2227
Wang T, Zhang X, Li C (2007) Growth, abscisic acid content, and carbon isotope composition in wheat cultivars grown under different soil moisture. Biol Plant 51:181–184
Wang LH, Huang XH, Zhou Q (2008) Effects of rare earth elements on the distribution of mineral elements and heavy metals in horseradish. Chemosphere 73:314–319
Wang JC, Chen J, Pan KW (2013a) Effect of exogenous abscisic acid on the level of antioxidants in Atractylodes macrocephala Koidz under lead stress. Environ Sci Pollut Res 20:1441–1449
Wang XQ, Liu Z, Li N, Fu B (2013b) Long-term effects of simulated acid rain stress on a staple forest plant, Pinus massoniana Lamb: a proteomic analysis. Trees 27:297–309
Wang L, Chen Z, Shang H, Wang J, Zhang PY (2014a) Impact of simulated acid rain on soil microbial community function in Masson pine seedlings. Electron J Biotechnol 17:199–203
Wang LH, Wang W, Zhou Q, Huang XH (2014b) Combined effects of lanthanum (III) chloride and acid rain on photosynthetic parameters in rice. Chemosphere 112:355–361
Wasilewska A, Vlad F, Sirichandra C, Redko Y, Jammes F, Valon C, Frey NFD, Leung J (2008) An update on abscisic acid signaling in plants and more. Mol Plant 1:198–217
Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot 5:707–735
Wu X, Liang CJ (2016) Effects of simulated acid rain on hormone concentration and growth of rice roots. Environ Chem 3:568–574
Yang RC, Yang T, Zhang HJ, Qi Y, Xing YX, Zhang N, Li R, Weeda S, Ren SX, Ouyang B, Guo YD (2014) Hormone profiling and transcription analysis reveal a major role of ABA in tomato salt tolerance. Plant Physiol Biochem 77:23–34
Zhao MR, Han YY, Feng YN, Li F, Wang W (2012) Expansins are involved in cell growth mediated by abscisic acid and indole-3-acetic acid under drought stress in wheat. Plant Cell Rep 31:671–685
Zheng YL, Li WQ, Sun WB (2015) Effects of acclimation and pretreatment with abscisic acid or salicylic acid on tolerance of Trigonobalanus doichangensis to extreme temperatures. Biol Plant 59:382–388
Zhou Q, Huang XH (2002) Injury mechanism of acid rain on plant and its rare earths control. J Chin Rare Earth Soc 12:116–118
Zhou Q, Huang XH, Zeng QL, Liang CJ, Ye YX (2003) Injury mechanism of acid rain on vegetation and its chemicals control. J Agro-Environ Sci 22:632–635
Zhu YY, Di TJ, GH X, Chen X, Zeng HQ, Yan F, Shen QR (2009) Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant Cell Environ 32:1428–1440
Acknowledgements
The authors are grateful for the financial support from the Natural Science Foundation of Jiangsu Province (No.BK20161131) and the National Natural Science Foundation of China (31000245, 31370517).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Philippe Garrigues
Rights and permissions
About this article
Cite this article
Wu, X., Liang, C. Enhancing tolerance of rice (Oryza sativa) to simulated acid rain by exogenous abscisic acid. Environ Sci Pollut Res 24, 4860–4870 (2017). https://doi.org/10.1007/s11356-016-8219-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-8219-3